Abstract
Prismatic crystals of Ag3(4-Amba)2(NO3)2 (I) and Ag(4-AmbaH)NO3 (II) are isolated from the reaction mixture of aqueous solutions of 4-aminomethylbenzoic acid (4-AmbaH) and AgNO3 at Т ≈ 70°C. The crystal structure of compound I (СIF file CCDC no. 1851513) and the luminescence properties of compounds I and II are studied. The structure of compound I contains two crystallographically nonequivalent silver atoms Ag(1) and Ag(2) with the octahedral coordination mode (with allowance for Ag···Ag contacts) with different compositions of the internal spheres of the central atoms. Owing to the contacts, the silver atoms are joined into infinite cluster ribbons (Ag···Ag 3.06−3.27 Å). The carboxylate group of the 4-Amba anion exhibits the bridging properties and binds the silver atoms of both types. The Ag(2) atoms also contact with the oxygen atom of the \({\text{NO}}_{{\text{3}}}^{-}\) ion and coordinate the NH2 group of 4-Amba. The photoluminescence spectra of compounds I and II are of the same type and show the presence of two groups of emission bands with centers at 484 and 543 nm shifted to the red range of the spectrum relative to the band of 4-AmbaH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The synthesis and investigation of the structures and luminescence properties of supramolecular architectures and coordination polymers with multifunctional organic ligands formed by covalent bonds, hydrogen bonds, π–π interactions, and their combination are of great interest in the recent time. Increased interest in this research is related to both the possibility of controlling the self-assembling of supramolecular ensembles and their unusual structures and their potent use in photochemistry, magnetic and nonlinear optical material development, and nanomaterial manufacturing. The design of the coordination polymers involving multifunctional ligands and metal ions is among the active routes for the production of novel materials. It is known that many factors affect the coordination structures. However, the main factors are the nature of the metal center and organic ligand. Organic ligands act as bridges between metal centers and thus play the key role.
The d10–d10 interaction with the short metal–metal contacts was also observed for the coordination polynuclear silver compounds. It was shown [1] that the Ag···Ag distances in the binuclear silver compounds ranged from 2.89 to 3.09 Å. In the coordination polymers the silver atoms exhibit the coordination number from 2 to 6 (linear, trigonal, tetrahedral, and octahedral coordination modes) and a high affinity to hard and soft donors.
Amino acids with nonrigid coordination possibilities are used for the formation of diverse supramolecular architectures [2–4]. The formation of the latter depends substantially on the nature of the metal and ligand, the solvent, the pH of the solution, the reactant ratio, and the nature of counterions. Aromatic carboxylate ligands including amino acids are intensively used in the preparation of supramolecular ensembles [2, 5, 6]. A structural feature of amino acids is the intramolecular protonation of the NH2 group to form \({\text{NH}}_{{\text{3}}}^{ + }\) and COO– groups, i.e., zwitterion formation [2, 7].
The reaction product of amino acids with metal salts was shown to depend on many factors, including the zwitterionic nature of the amino acid, the ability of the metal ions to coordinate O- or N-centers, the stereochemical preference of the counterion, the presence of other ligands, and others.
It is established that the reactions of cadmium chlorides and bromides with 4-aminobenzoic acid lead to the substitution of one of the halide atoms and coordination of the acid residue due to both the COO– groups and amino group [2]. The reaction of silver nitrate with 4-aminobenzoic acid (4-AbaH) gave the compounds containing 4-AbaH, 4-Aba, and nitrate anions in which the 4-Aba anion adds to the Ag+ ion through the COO– and NH2 groups without zwitterion formation. In this case, the 4-Aba anions performs the bridging function in a series of the compounds [4, 8].
4-Aminomethylbenzoic acid, as well as 4-AbaH, is a bifunctional organic ligand. It is shown [9] that 4‑АmbaH in the compound with the H2O molecule exists as a zwitterion with the formation of hydrogen bonds N–H···O(H2O). We synthesized compound [CdI2(4-AmbaH)2] ⋅ H2O [10] in which the acid is coordinated to the cadmium ion by the carboxylate oxygen atoms in the form of a zwitterion.
In this work, we obtained the coordination polymeric ensemble of silver with mixed ligands, 4-(aminomethyl)benzoate and nitrate ions, of the composition Ag3(4-Аmba)2(NO3) (I) and compound Ag(4-AmbaH)NO3 (II).
EXPERIMENTAL
Synthesis of crystals of compounds I and II. A suspension of 4-AmbaH (0.36 g, 2.37 mmol) in water (20 mL) was heated at ~70°С to the nearly complete dissolution of the acid. The obtained solution was added by AgNO3 (0.40 g, 2.35 mmol) in water (5 mL), and the reaction mixture was kept at 35–40°С on stirring for 15 min. Then the blurred solution was filtered and kept for 15 h in the dark. The formed prismatic crystals were decanted, washed with a minor amount of water, and dried in air. According to the elemental analysis data, the obtained crystals corresponded to the composition of compound I.
For С16H16N3O7Ag3 | |||
Anal. calcd., % | C, 28.11 | H, 2.36 | N, 6.15 |
Found, % | C, 29.63 | H, 1.68 | N, 6.72 |
After the crystals were separated, the mother liquor was kept in an open weighing bottle in the dark to a residual volume of ~5 mL. The formed solid phase represented very thin needle-like crystals growing as a fan from one point. Along with the latter, prismatic crystals identical in shape to the crystals of compound I were isolated in an insignificant amount. The mother liquor was shaken to concentrate bunches of light needle-like crystals on the surface of the solution, and they were separated from the prismatic crystals during the subsequent decantation. Then a suspension with the needle-like crystals was transferred onto a glass filter, washed with a minor amount of water, and dried in air in the dark. According to the elemental analysis data, the crystals corresponded to a complex of silver nitrate with the molecular form of compound II.
For C8H9AgN2O5 | |||
Anal. calcd., % | C, 30.00 | H, 2.83 | N, 8.75 |
Found, % | C, 30.74 | H, 3.49 | N, 8.43 |
The photoluminescence spectra of compounds I and II were recorded at room temperature on a PE LS-55 spectrometer (resolution 0.5 nm, slit variation from 7 to 10 nm) using an attachment for solid state samples. Complex I was also studied by X-ray diffraction analysis.
X-ray diffraction analysis. The experimental material for the crystals of compound I was obtained on an Enraf-Nonius CAD-4 automated diffractometer. The structure was solved by a direct method (SHELXL-97) and refined by least squares in the full-matrix anisotropic approximation for all non-hydrogen atoms (SHELXL-97) [11, 12]. The positions of the hydrogen atoms were calculated geometrically and included into refinement by the riding model. The unit cell parameters and the main experimental characteristics are presented in Table 1. Selected bond lengths and bond angles are given in Table 2.
The full crystallographic data for compound I were deposited with the Cambridge Crystallographic Data Centre (CIF file ССDC no. 1851513; http://www. ccdc.cam.ac.uk/deposit/).
RESULTS AND DISCUSSION
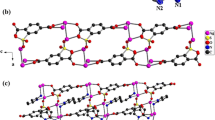
The structure of compound I contains two crystallographically nonequivalent silver atoms Ag(1) and Ag(2) (Fig. 1). The Ag(1) atom lies on the twofold crystallographic axis and has the octahedral coordination mode with allowance for the Ag···Ag contacts. Four Ag(2) atoms are arranged in the equatorial plane of the octahedron, and two oxygen atoms O(1) and O(1A) of the carboxylate group occupy the axial vertices. The coordination polyhedron of the Ag(2) atom occupies the general 8-fold position and also has an octahedron shape with three silver atoms (Ag(1), Ag(1A), and Ag(2B)) and the O(3) atom of the nitrate ion in the equatorial plane and the O(2) and N(1A) atoms in the axial positions. The silver atoms are joined through the Ag···Ag contacts into infinite cluster ribbons along the direction [010]. The Ag…Ag distances (3.06−3.27 Å) are appreciably longer than those in a series of dimeric complexes with the bidentate carboxy group (2.89–3.09 Å) [8]. The silver ions in the cluster coordinate the 4-Amba ligands via the bidentate COO– groups and monodentate amino group; i.e., 4-Amba performs the ditopic bridging function. The carboxylate group exhibits the bridging properties and binds the silver atoms of both types. In addition, the Ag(2) atoms contact with the oxygen atom of the \({\text{NO}}_{{\text{3}}}^{-}\) ion and coordinate the NH2 group of 4-Аmba. A significant distortion of the coordination polyhedron is observed for the Ag(1) and Ag(2) atoms (Fig. 2). The Ag–O distances of 2.137 and 2.135 Å are comparable with the values found for the dimeric structures of silver carboxylates. The length of the Ag–O(NO3) bond (2.587 Å) almost coincides with the published values for the Ag–O(NO3) distances [4]. The Ag–N(NH2) coordination bond in compound I is 2.158 Å. In the silver compounds with 4-aminobenzoic acid, this value ranges from 2.135 to 2.236 Å [4].
The photoluminescence spectra of 4-AmbaH and compound I are presented in Fig. 3. The spectrum of compound II with 4-AmbaH coordinated in the molecular form does not almost differ from the spectrum of compound I. As can be seen from the pre-sented plots, the photoluminescence spectrum of 4-AmbaH is characteristic of the presence of a broad band with the center at 438 nm and a shoulder formed by a lower-intensity band at ~484 nm. Compound I is characteristic of the presence of two groups of emission bands with the centers at 484 and 543 nm. It is of interest that isonicotinic acid manifests no luminescence in the solid state and at room temperature, while the used acid 4-AmbaH has emission at 438 nm. As can be seen from the spectrum of compound I, the coordinated bifunctional ligands show several bands in a range of 480–550 nm shifted to the red spectral range relative to the band of the acid.
As shown above, we synthesized the coordination polymer of silver containing two different anions with the composition Ag3(4-Аmba)2(NO3). The coordination polymer similar in shape was obtained by the reaction of AgNO3 with isonicotinic acid: Ag3(L)2(NO3) (III) (L is the isonicotinate anion) [13]. As in our case, the N- and COO–-containing ligand in compound III manifests the bridging properties and is coordinated to the silver ions through the N atom and COO– group. However, the N atom is in the composition of the aromatic ring. The Ag···Ag distances in compound III range from 2.97 to 3.29 Å, and the Ag–O(NO3) distance is 2.65 Å.
Interest in metal-organic framework compounds (MOFs) containing mixed bridging (anionic or neutral) ligands has increased in the recent years. It was proposed to call them mixed-component metal-organic framework compounds (MC-MOFs) [14]. In this work, we synthesized and studied the coordination polymer containing simultaneously two different anions. It is shown that the multifunctional organic ligands containing amino and carboxylate groups are good building blocks for the preparation of coordination and supramolecular ensembles with unusual structures.
REFERENCES
Che, C.-M., Tse, M.-C., Chan, M.C.W., et al., J. Am. Chem. Soc., 2000, vol. 122, p. 2464.
Wang, R., Hong, M., Luo, J., et al., Eur. J. Inorg. Chem., 2002, p. 2904.
Moulton, B. and Zaworotko, M.J., Chem. Rev., 2001, vol. 101, p. 1624.
Wang, R., Hong, M., Luo, J., et al., Inorg. Chim. Acta, 2004, vol. 357, p. 103.
Nomiya, K. and Yokoyama, H., Dalton Trans., 2002, p. 2483.
Ma, Y., Du, L., Wang, K., and Zhao, Q., Crystals, 2017, vol. 7, no. 1, p. 32.
Zheng, C., Shi, R., Jin, X., et al., Inorg. Chem. Commun., 2015, vol. 58, p. 74.
Kristiansson, O., Inorg. Chem., 2001, vol. 40, p. 5058.
Atria, A.M., Garland, M.T., and Baggio, R., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2014, vol. 70, p. 385.
Kokunov, Yu.V., Kovalev, V.V., Gorbunova, Yu.E., et al., Russ. J. Inorg. Chem., 2018, vol. 63, no. 3, р. 333. doi 10.1134/S0036023618030117
Sheldrick G.M., SHELXS-97. Program for the Solution of Crystal Structures, Göttingen: Univ. of Göttingen, 1997.
Sheldrick G.M., SHELXL-97. Program for the Refinement of Crystal Structures, Göttingen: Univ. of Göttingen, 1997.
Liu, Z., Liu, P., Chen, Y., et al., New J. Chem., 2005, vol. 29, p. 474.
Burrows, A.D., CrystEngComm, 2011, vol. 13, p. 3623.
ACKNOWLEDGMENTS
This work was supported by the Russian Science Foundation (project no. 14-23-00176). Some part of the work was carried out in terms of the state task of the Kurnakov Institute of General and Inorganic Chemistry (Russian Academy of Sciences) in the field of basic research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Kokunov, Y.V., Kovalev, V.V., Gorbunova, Y.E. et al. Coordination Polymeric Ensemble of Silver with Nitrate and 4-(Aminomethyl)benzoate: Synthesis, Crystal Structure, and Luminescence Properties. Russ J Coord Chem 44, 722–727 (2018). https://doi.org/10.1134/S1070328418120047
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328418120047