Abstract
In this study, a new Schiff base (3) was synthesised from the reaction of the 4-aminoantipyridine (1) with 2-pyridine carboxaldehyde (2). Then the amine ligand 4 was synthesised by reduction of 3 with NaBH4. Nickel and cobalt complexes (5–6) of 4 were prepared. The antioxidant activity of (III)–(VI) was investigated using DPPH• free radical scavenging, ABTS•+ radical cation scavenging, and reducing power assays. As a result of this work, it was seen that all compounds revealed good antioxidant activity. While metal complexes show better activity then ligands, the nickel complexe revealed more activity than that of the cobalt complex.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Schiff bases are known as the Azomethine group (‒HC = N–) [1] were first synthesized in 1864 by the famous German chemist Hugo Schiff and got its name from here. Since Schiff bases are structurally diverse, they are both easy to obtain and the ease of steric and electronic controls in their structure [2] have made them useful chelators. Schiff bases are equally widespread [3] in areas of use as they have potential regions such as nitrogen and other donors and are stable [4]. Since Schiff bases are special ligands, they can be synthesized easily and in a versatile way, as well as dissolving easily in co-solvents. In addition, the requirement of C=N bonds for biologic activation in Azomethine derivatives has further increased the importance of these ligands. Azomethine is involved in the synthesis of nitrogen atom components and can interfere with normal cell processes [5].
Schiff bases are ligands that have an important place in coordination chemistry. Schiff bases are formed by the condensation reaction of ketones and aldehydes with primary amines. These Schiff bases are represented by the general formula RCH=NR'. In this representation, R and R' are aryl or alkyl substituents. There are several synthesis methods of Schiff bases, which are widely used. The first of these is formed as a result of the reaction of primary amines with carbonyl compounds, which takes place in two stages. Firstly, the carbonyl amine intermediate is obtained by a condensation reaction of the carbonyl group with the primary amine. In the second stage, the Schiff base is obtained by amine dehydration. Also, Schiff bases are synthesized by condensation of aldehydes or ketones with amines. It has been stated that they are widely used in many coordination compounds due to their ability to donate electrons [6, 7].
Schiff bases are among the most widely used organic compounds whose electroluminescence effects [8], fluorescence properties [9], nonlinear optical properties reveal a wide range of applications [10]. In addition, many scientific studies conducted in recent years have proven that Schiff base and their metal complexes show many biological activations such as anticancer, antioxidant [11] antibacterial, antibiofilm, anti-inflammatory, hemocompatibility, cytotoxic [12] pesticide, nematicidal activities [13]. Due to their flexible and stereo-electronic structure, Schiff bases have mostly been used as highly attractive ligands that can form stable complexes with transition metals [14]. Schiff some complexes reveal important catalytic activities for many organic transformation [15].
One of the fastest and most important developing branches of Inorganic Chemistry is Coordination Chemistry. The branch of science that studies the properties of the complexes formed by coordination compounds metal ions with electron-pair donor molecules called ligands is called Coordination Chemistry. Coordination compounds play an important role in inorganic chemistry due to their colors, structures, large numbers, chemical reactions, and magnetic properties and have a wide range of research areas. Today, coordination compounds are widely used in dye and polymer technology, in the explanation of biological events in medicine, in the pharmaceutical industry, in the removal of water hardness, in the synthesis of disinfectants and antioxidants, in various chemical processes such as the preparation of rocket fuel, in the field of agriculture, in biological systems and industry [16].
Free radicals are molecules containing one or more unpaired electrons. Since these radicals have lost one electron from the outermost electron shell, they are willing to share the electrons of other atoms to compensate for this electron deficit [17]. There are many factors causing radicals such as radiation, X-rays, UV-rays, cosmic rays, air pollution, cigarette smoke, vehicle exhaust gases, industrial wastes, diseases, stress, toxic products of cell metabolism, chemicals, agriculture, and pesticides. The uncontrolled proliferation of radicals within the body can cause great danger for living things. Antioxidants are compounds that neutralize free radicals and stop and slow their oxidation. Antioxidants inhibit lipid oxidation by stopping the peroxidation chain reaction or accumulating reactive oxygen species [18]. Antioxidants have some important advantages. For example, since it can easily inhibit the formation of free radicals, it can delay lipid peroxidation that causes the spoilage of these products during the processing and storage of food and pharmaceutical products, and can protect the human body from ROS. Antioxidants are widely used in foods to prevent radical chain reactions that cause food spoilage [19–23].
In this work, a new Schiff base and a new amine compounds have been synthesized starting from 4‑aminoantipyridine, and cobalt and nickel complexes of amine compound have been prepared for the first time. The antioxidant activity of these new compounds and complexes has been investigated using DPPH• free radical scavenging, ABTS•+ radical cation scavenging, and reducing power assays.
RESULTS AND DISCUSSION
A Schiff base, 4-(2-pyridylmethylene) amino-1-phenyl-2,3-dimethyl-5-pyrazolone 3 (III) was synthesized by the reaction of 4-amino anti-pyrene (I) with pyridine carboxyl aldehyde (II) with 96.23% yield according to [27]. Compound (III) was reduced by NaBH4 to obtain a new amine compound, 1,5‑dimethyl-2-phenyl-4-(pyridine-2-yl-methylamino)-1H-pyrazole-3(2H) (IV), with 86.9% yield. Then a nickel complex (V) and a cobalt complex (VI) of IV were prepared in the MeOH and CHCl3 mixture at 60°C (Fig. 1). The structures of synthesized compounds were characterized by spectroscopic methods such as 1H NMR, 13C NMR, HRMS, and FTIR, elemental analysis (Figs. S1–S7, Supplementary Information).
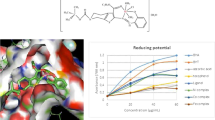
Antioxidant activity of compound (III)–(VI) was carried out using the BHT, BHA, and Trolox as the standards (Table 1). Compound (IV) showed a spectacular DPPH• free radical scavenging effect with the value of 5.91. Nickel complex displayed very good DPPH• free radical scavenging effect with the value of 7.78 (lC50, µg/mL) in comparison to the standards and cobalt complex (12.12, IC50, µg/mL). In concern to the ABTS•+ radical cation scavenging activity, the nickel complex (V) showed excellent activity with a value of 3.62 (IC50, µg/mL). Moreover, cobalt complex (VI) (4.32, IC50, µg/mL), revealed a higher effect significantly than that of the standard, BHA, BHT, and Trolox with the values of 7.14, 10.34, and 6.92 (IC50, µg/mL) respectively. The same trend was observed in the reducing power assay, in this essay, nickel complex activity was detected as 8.14 (µmol TE/mg sample) which was statistically higher than that of the cobalt complex and BHT with the values of 6.93, 5.29 (µmol TE/mg sample) respectively (Fig. 2). On the other hand, comparing compound (III) with its reduced form, compound (IV) it is seen that compound (IV) shows much higher DPPH• free radical scavenging effect, ABTS•+ radical cation scavenging activity and reducing power.
EXPERIMENTAL
Chemical and Reagents
NMR spectra of the synthesized new ligands were determined by a Bruker spectrometer by 1H NMR at 400 MHz and 13C NMR at 100 MHz. As an internal reference for 1H NMR chemical shifts were measured against CDCl3 (CDCl3: 8 7.26, DMSO-d6: 8 2.5). A thin layer chromatography was performed on alumina plates (60F254). A UV (UV-260 Shimadzu) spectrometer device was used to determine the antioxidant activities of the new metal complexes. Silica gel (Kiesegel 60, 0.063–0.200 mm, Merck) was used for column chromatography. A Bianchi B-540 apparatus was used for melting points determination and IR analyses were performed with a Jasco FT/IR47700 spectrometer. All chemicals and solvents used in the synthesis of reactions and also in antioxidant experiments were obtained from E. Merck (Darmstadt, Germany).
Synthesis of 4-(2-pyridylmethylene) amino-1-phenyl-2,3-dimethyl-5-pyrazol-one (III). 0.0187 moles (3.8 g) of 4-amino antipyrine (I) was dissolved in 10 mL of methanol in a 50 ml flask. The glass flask was rifled in an oil bath. 0.0187 mole (2 g) of 2-pyridinecarboxaldehyde was dissolved in 10 mL of methanol in the tube. The 2-pyridinecarboxyaldehyde-methanol mixture was slowly added dropwise within 120 min to the refluxed 4 amino antipyrine–methanol mixture with a micropipette. After 24 h, the reaction mixture was cooled to the room temperature. The precipitated solids were filtered through a flask with cold methanol and dried. 5.26 g of the pure product was obtained. Yield: 96.23%. Anal. calcd. (%) for C17H16N4O: C, 69.85, H, 5.52. Found: C, 70.02, H, 5.56. IR: ν/cm (FTIR-4700 type A) 3042, 2960, 2924, 1638, 1563, 1484, 1411, 1380, 1339, 1304, 1134, 1041, 1020, 989, 957, 763, 694. 1H NMR (400 MHz, CDCl3) δ 9.80 (s, 1H), 8.70 (ddd, J = 4.8, 1.6, 0.9 Hz, 1H), 8.02 (d, J = 7.9 Hz, 1H), 7.75 (td, J = 7.6, 1.6 Hz, 1H), 7.49 (m, 2H), 7.42 (m, 2H), 7.34 (m, 1H), 7.27 (ddd, J = 7.5, 4.9, 1.2 Hz, 1H), 3.19 (s, 3H), 2.53 (s, 3H). 13C NMR (100 MHz, CDCl3) δ: 160.4, 156.8, 156.2, 152.5, 149.8, 136.2, 134.7, 129.3, 127.1, 124.7, 123.9, 121.7, 118.3, 35.6, 10.3.
Synthesis of 5-dimethyl-2-phenily-4-(pyridine-2-dimethylamino)-1H-pyrazole-3(2H)-on (IV). 9.51 g of III was dissolved in a 250 mL flask with enough MeOH (45 mL). A magnet was thrown into the flask and placed on the heated stirrer in ice water and mixed at 500 rpm. 1.848 g of NaBH4 was added gradually into the flask and the mixture was stirred at room temperature overnight. Methanol was evaporated to concentrated. Some water (near 100 mL) was added to the mixture. Then the aqueous media was extracted with 100 × 3 mL of chloroform. The chloroform phase was dried with MgSO4, filtered, and evaporated. The crude product was purified by column chromatography with (EtOAc/H/TEA: 5 : 1 : 0.2). Yield: 8.32 g (89.6%). Anal. calcd. (%) for C17H18N4O: C, 69.37, H, 6.16. Found: C, 70.09, H, 6.26. IR: ν/cm 3316, 3060, 3009, 2980, 2921, 2803, 2232, 1644, 1590, 1492, 1455,1432, 1348, 1311, 1292, 1266, 1179, 1164, 1133, 1105, 1074, 1049, 992, 907, 752, 727, 693. 1H NMR (400 MHz, CDCl3) δ 8.54 (m, 1H), 7.62 (td, J = 7.7, 1.8 Hz, 1H), 7.46–7.36 (m, 5H), 7.21 (DDT, J = 8.7, 7.5, 1.5 Hz, 1H), 7.14 (m, 1H), 4.42 (s, 2H), 3.65 (s, 1H, NH), 2.80 (s, 3H), 2.09 (s, 3H). 13C NMR (100 MHz, CDCl3) δ: 162.4, 159.6, 149.2, 140.2, 136.6, 135.4, 129.0, 125.8, 122.8, 122.0, 121.8, 121.3, 52.6, 37.8, 10.7. Rf in TLC: 0.42 (see Fig. S8, Supplementary Information).
Synthesis of nickel metal complex synthesis (V). In a 100 mL flask, 1.7 mmol (0.5 g) IV was dissolved in 20 mL chloroform and refluxed at 60°C. 1.7 mmol (0.22 g) of NiCl2 was dissolved in 35 ml of methanol and added to the flask which was refluxed within 4 minutes. The solution colour changed from yellow to green (khaki). One day later, the reaction was terminated, and the solvent evaporated. Khaki (green) solid-viscose formed. It was washed 3× (5/5 chloroform hexane) and filtered. The solid part was dried and weighed. Yield: 0.6 g, mp 250°C (decomposition). Anal. calcd. (%) for C34H34N8NiO2: C, 63.28, H, 5.31. Found: C, 63.72, H, 5.42. Rf in TLC: 0.0 (see Fig. S8, Supplementary Information).
Synthesis of cobalt metal complex synthesis (VI). In a 100 ml flask, 1.7 mmol (0.5 g) compound IV was dissolved in 10 mL of chloroform and refluxed at 60°C. 1.7 mmol (0.495 g) Co (NO3)2⋅6H2O was dissolved in 10 mL of methanol and added to the flask refluxed within 4 min. The solution changed colour from yellow to brown. One day later, the reaction was terminated, and the solvent evaporated. Brown solid viscous formed. It was washed 3× (5/5 chloroform–hexane) and filtered. The solid part was dried and weighed. Weighing: 0.644 g. Anal. calcd (%) for C34H34CoN8O2: C, 63.25, H, 5.31. Found: C, 63.89, H, 5.49. Rf in TLC: 0.0 (see Fig. S8, Supplementary Information).
Antioxidant Assays
ABTS+• radical scavenging test. Free radical (ABTS•+) scavenging activity was performed according to the method suggested by [24]. For free radical (ABTS•+) scavenging activity, PO43– buffer with 0.1 M pH 7.4, 2 mM ABTS•+, and 2.45 mM K2S2O8 solution was prepared. ABTS•+ and K2S2O8 solutions (1 : 2) were mixed to ABTS•+–K2S2O8 and incubated in the dark for 6 h. Sample and standard solutions were taken at different concentrations (10–20–40 mL) and 1 mL of ABTS•+–K2S2O8 solution was added to a total volume of 4 mL. The mixture was vortexed vigorously and incubated for 30 min. Spectrophotometric measurement was done under room conditions at 734 nm. Measurements were done in triplicate and averaged. The % cation free radical scavenging activity of samples and standard was calculated according to the formula below.
Results, % activity versus concentration plotted. Using the slope equation, it was calculated as IC50.
DPPH• free radical scavenging assay. Free radical (DPPH) scavenging activity was performed according to the method outlined by Liyana [25]. DPPH (2,2‑diphenyl-1-picryl hydrazyl) sample solution at different concentrations (40–80–160 mg mL–1) was added onto 1 mL of 0.135 mM ethanol solution. The final volume was completed to 4 mL with ethanol. The mixture was vortexed vigorously and incubated at room temperature and in the dark. Spectrophotometric measurement was made at room conditions at 517 nm. The % free radical scavenging activity of the samples was calculated according to the formula below.
Results were calculated as % free radical removal ± standard deviation.
Reducing power. The reducing power of the molecules to be isolated and the crude extract was evaluated according to the Oyaizu method [26]. Phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%) were added to different concentrations of the standard and samples in ethanol (40, 80 and 120 mg/mL). This mixture was incubated at 50°C for 20 min. After incubation, TCA (2.5 mL, 10%) was added to this mixture and then centrifuged at 3000 rpm for 10 min. By taking 2.5 mL of the mixture, distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%) were added, and the absorbance of the final mixture was measured at 700 nm. High absorbance value will be considered as high reducing power [27].
Statistical analysis. Statistical analysis of antioxidant assays was carried out using the GraphPad prism (8.0.1), ANOVA with Tukey’s multiple comparison test. The results were indicated as mean values ± standard deviation (P ˂ 0.05)
CONCLUSIONS
In the work, a new Schiff bases was synthesised. By the reduction of the new Schiff bases with NaBH4, a new amine compound and its Ni and Co complexes were synthesised, and their structures were elucidated completely. Antioxidant activity of all compounds synthesised were investigated using the DPPH•, ABTS•+, and reducing power assays. Both complexes revealed excellent antioxidant activity, especially nickel complex displayed the best antioxidant activity. Also compound (IV) shows much higher DPPH• free radical scavenging effect, ABTS•+ radical cation scavenging activity and reducing power than compound (III). Indeed, these complexes have the potential to be used as an antioxidant reagent. Moreover, anticancer and other biological activity assays should be carried out.
REFERENCES
Anacona, J., Pineda, Y., Bravo, A., and Camus J., Med. Chem., 2016, vol. 6, pp. 467–473. https://doi.org/10.4172/2161-0444.1000385
Eman, T., Int. J. Curr. Res. Chem. Pharm. Sci., 2016, vol. 3, pp. 118–123. http://s-o-i.org/1.15/ijcrcps-2016-3-4-12.
Abu-Khadra, A.S., Farag, R.S., and Abdel-Hady, A.E.-D.M., Am. J. Anal. Chem., 2016, vol. 7, pp. 233–245. https://doi.org/10.4236/ajac.2016.73020
Rafi, U.M., Mahendiran D., Haleel, A.K., Nankar, R.P., and Doble, M., New J. Chem., 2016, vol. 40, pp. 2451–2465. https://doi.org/10.32571/ijct.474279
Shanty, A.A., Philip J.E., Sneha, E.J., Kurup, M.R.P., and Balachandran, S., Bioorg. Chem., 2017, vol. 70, pp. 67–73. https://doi.org/10.1016/j.bioorg.2016.11.009
Chakraborty, H., Paul, N., and Rahman, M.L., Transition Met. Chem., 1994, vol. 19, pp. 524–526. https://doi.org/10.1007/BF00136366
Reddy, P.R., Shilpa, A., Raju, N., and Raghavaiah, P., J. Inorg. Biochem., 2011, vol. 105, pp. 1603–1612. https://doi.org/10.1016/j.jinorgbio.2011.08.022
Burlov, A., Vlasenko, V., Koshchienko, Y.V., Makarova, N., and Zubenko, A., Polyhedron, 2018, vol. 154, pp. 65–76. https://doi.org/10.1016/j.poly.2018.07.034
Satapathy, A.K., Behera, S.K., Yadav, A., Mahour, L.N., and Yelamaggad, C., J. Lumin., 2019, vol. 210, pp. 371–375. https://doi.org/10.1016/j.jlumin.2019.02.056
Zhang, M., Gong, L., Sun, C., Li, W., and Chang, Z., Spectrochim. Acta, Part A, 2019, vol. 214, pp. 7–13. https://doi.org/10.1016/j.saa.2019.01.089
Pasa, S., Erdogan, O., and Yenisey, C., J. Mol. Struct., 2019, vol. 1186, pp. 458–467. https://doi.org/10.1016/j.molstruc.2019.03.061
Ali, S.S., Kenawy, E., Sonbol, FI., Sun, J.Z., and Al-Etewy, M., Pharm. Res., 2019, vol. 36, p. 18. https://doi.org/10.1007/s11095-018-2535-x
Masih, I., Fahmi, N., and Rajkumar, J. Enzyme. Inhib. Med. Chem., 2013, vol. 28, pp. 33–40. https://doi.org/10.3109/14756366.2011.625022
Clarke, R.M. and Storr, T., Dalton Trans., 2014, vol. 43, pp. 9380–9391. https://doi.org/10.1039/C4DT00591K
Das, P. and Linert, W., Coord. Chem. Rev., 2016, vol. 311, pp. 1–23. https://doi.org/10.1016/j.ccr.2015.11.010
Maldonado, N., and Amo-Ochoa, P., Dalton Trans., 2021, vol. 50, pp. 2310–2323. https://doi.org/10.1039/D0DT04066E
Halliwell, B., Lancet, 1994, vol. 344, pp. 721–724. https://doi.org/10.1016/s0140-6736(94)92211-x
Halliwell, B., Gutteridge, J.M., and Cross, C.E., J. Lab. Clin. Med., 1992, vol. 119, pp. 598–620. http://europepmc.org/article/med/1593209.
Erenler, R., Adak, T., Karan, T., Elmastas, M., and Yildiz, I., Eurasia Proc. Sci. Tech. Eng. Math., 2017, vol. 1, pp. 139–145. http://www.epstem.net/en/download/article-file/379950.
Erenler, R., Sen, O., Aksit, H., Demirtas, I., and Yaglioglu, A.S., J. Sci. Food Agric., 2016, vol. 96, pp. 822–836. https://doi.org/10.1002/jsfa.7155
Erenler, R., Meral, B., Sen, O., Elmastas, M., and Aydin, A., Pharm. Biol., 2017, vol. 55, pp. 1646–1653. https://doi.org/10.1080/13880209.2017.1310906
Guzel, A., Aksit, H., Elmastas, M., and Erenler, R., Pharmacogn. Mag., 2017, vol. 13, p. 316. https://doi.org/10.4103/0973-1296.204556
Elmastas, M., Celik, S.M., Genc, N., Aksit, H., and Erenler, R., Int. J. Food Prop., 2018, vol. 21, pp. 374–384. https://doi.org/10.1080/10942912.2017.1416399
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C., Free Radical Biol. Med., 1999, vol. 26, pp. 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Liyana-Pathirana, C.M. and Shahidi, F., J. Agric. Food Chem., 2005, vol. 53, pp. 2433–2440. https://doi.org/10.1021/jf049320i
Oyaizu, M., Jpn. J. Nutr., 1986, vol. 103, pp. 413–419.
Kızılkaya, H., Dağ, B., Aral, T., Genç, N., and Erenler, R., J. Chin. Chem. Soc., 2020, vol. 67, pp. 1696–1701.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Supplementary Information
Rights and permissions
About this article
Cite this article
Dag, B., Tenekecioğlu, Y., Aral, T. et al. Synthesis, Characterization, and Antioxidant Activity of a Novel Schiff Base, an Amine and Amine–Metal Complexes. Russ J Bioorg Chem 49, 861–866 (2023). https://doi.org/10.1134/S1068162023040106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162023040106