Abstract
A new series of 5,6-dichloro-benzimidazoles containing thiophene ring derivatives of thiosemicarbazides and triazoles were designed, synthesized and characterized by spectral methods like as IR, 1H-NMR, 13C-NMR and elemental analysis. The compounds antiurease activity studies have done according to VanSlyke method and IC values were calculated in μM unit. All newly synthesized compounds containing thiophene ring showed urease inhibitory activity with IC50 values between 1.52 and 0.07 µM. 5-{[5,6-Dichloro-1-(2-thienylmethyl)-1H-benzimidazol-2-yl]methyl}-4-methyl-4H-1,2,4-triazol-3-thiol (Va) proved to be the most potent enzyme inhibition activity with IC50 = 0.07 ± 0.008 μM. Especially compounds (Va–f) have best results with triazole ring. All synthesized compounds were docked at the active sites of the Jack bean urease enzyme to investigate the reason of the inhibitory activity and the possible binding interactions of enzyme-ligand complexes. 2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-(4-clorophenyl)hydrazine carbothioamide (IVe), which has the second highest in vitro urease inhibitory activity with an IC50 of 0.11 μM compared to other compounds, has the highest binding energy of –8.97 kcal/mol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Benzimidazole ring has a significant place in biochemistry as it possesses many pharmacological properties extensively explored with a potent inhibitor of various enzymes [1]. Benzimidazoles with thiophene ring gained popularity in the field of organic and medicinal chemistry because of having wide range of biological activities [2], such as antioxidant [3], antiurease [4], analgesic, anti-inflammatory [5, 6], anticonvulsant [7]. Benzimidazole nucleus is used as a structural motif in the development of a wide range of drugs in medicinal areas. The benzimidazole group is a big target for modifying its structure and converting into potential molecules for further exploration in different ailments [8, 9].
Urease enzyme catalyzes the fast conversion of urea into ammonia and carbamic acid, the carbamate rapidly and spontaneously decomposes to yield a second molecule of ammonia and one of carbon dioxide [10]. Uncontrolled production of ammonia by urease causes various pathological conditions and damages the cells of living system [11]. It is considered as a virulent factor in many diseases such as kidney stones, gastric cancer, gastric ulcer, duodenal ulcer, chronic gastritis, urolithiasis, pyelonephritis in humans and animals [10, 12, 14]. Many methods such as the use of urease inhibitors have been used to control the activity of urease. Studies on urease inhibition and the synthesis of new inhibitors are gaining importance at the present time for minimizing these damages in the cells and treating these diseases. The urease inhibitors can be extensively classified in some compounds such as Schiff bases, benzimidazoles, 1,4-benzoquinone, hydroxamic acid, phosphorodiamidates and humic acid [15–18].
Thiophene shows a wide range of therapeutic properties and molecules containing this structure have an important place in the synthesis of potential bioactive compounds. Some thiophene ring-containing compounds are also used as medicine such sertaconazole nitrate, tiquizium bromides, tioconazole, dorzolamide, citizolam, and benocyclidine [19]. Recently, thiophenes derivatives containing sulphonylacetamide reported as anti-urease with IC50 values 92.12 ± 0.21 and 94.66 ± 0.11 [20]. In 2010, Khan assessed the antiurease activity of 2-aminothiophenes derivatives with IC50 values ranging from 5.04 ± 0.0043 to 31.84 ± 0.0053 μM [21]. The triazole ring is one of the heterocyclic structures frequently used in the development of urease inhibitors. Khan and coworkers synthesized 4,5-disubstituted-2,4-dihydro-3H-1,2,4-triazole-3-thiones as potent urease inhibitors with their activities in the range of 45.60 ± 0.04 to 483.55 ± 1.99 mM [22]. Similarly, Özil et al. synthesized bis-triazole derivatives linking with triazole moiety and screened for their urease inhibitory potential [23]. Hameed et al. synthesized and screened antiurease activity of 1,2,4-triazole-3-thione derivatives [24]. Recently, some 5,6-dichloro-benzimidazole derivatives containing triazole ring have been reported as potent urease inhibitors [25–27].
In our current study, we synthesized new 5,6-dichloro-benzimidazoles containing thiophene ring derivatives of thiosemicarbazides and triazoles and then evaluated their urease inhibitory activities with docking studies.
RESULTS AND DISCUSSION
Chemisty
The synthetic pathway of the target compounds is outlined in Scheme 1. First the starting compound 5,6-dichloro-2-(2-thienylmethyl)-1H-benzimidazole (I) with only cas number in literature (cas no. 1176092-81-1) was synthesized with 4,5-dicloro-1,2 phenylenediamine and ethyl-2-(thiophen-2-yl)acetimidate hydrochloride in dry methanol at room temperature for 12 hours. The reaction of compound (I) with ethyl bromoacetate in presence of K2CO3 concluded ethyl[5,6-dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetate (II), it was converted by hydrazine hydrate in ethanol to afford desired 2-[5,6-dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]-acetohydrazide (III). Thiosemicarbazides (IVa–f) were synthesized by the nucleophilic addition of compound (III) with an appropriate isothiocyanate. Compounds of (Va–f) were obtained by intramolecular cyclization of compounds (IVa–f) in the presence of NaOH. The structures of all new synthesized compounds were confirmed by 1H-NMR, 13C-NMR spectroscopy and elemental analysis. Spectroscopic results of all new synthesized molecules matched the proposed structure.

Scheme 1 . The synthetic pathway of the target compounds derivatives of benzimidazole.
Biological Activity
All newly synthesized benzimidazole compounds showed effective urease inhibitory activity in the range of IC50 = 0.07 ± 0. 008 μM and 1.52 ± 0.042 μM (Table 1). 5-{[5,6-Dichloro-1-(2-thienylmethyl)-1H-benzimidazol-2-yl]methyl}-4-methyl-4H-1,2,4-triazol-3-thiol (Va) has the best enzyme inhibition activity with an IC50 value of 0.07 ± 0.008 μM. The antiurease activities of compounds (II), (III), (IVe), (IVf), (Va) and (Vd) are more potent than the standard compound thiourea (IC50: 0.267 ± 0.012 μM). If we compare the compound (I) with previously reported compounds having aryl or benzyl group on at the position-2 of benzimidazole as a potent inhibitor against urease, it is clear that thiophene ring at the position-2 on the benzimidazole has a positive effect on the inhibitory activity (Scheme 2) [26, 27].

Scheme 2 . Comparison of structure activity relationship between compounds (I), and previously reported analogs.
Molecular Docking Study
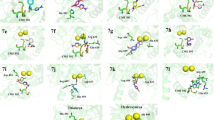
To explore the urease inhibitor potential of the synthesized compounds in terms of enzyme-ligand interaction, molecular docking studies were performed using Maestro Molecular Modeling platform (V10.5) by Schrödinger, LLC [28]. The crystal structure of JBU was obtained from the Protein Data Bank using pdb code 3LA4 [29]. The docking procedures were performed as described in our previous studies [30]. Most of the synthesized compounds appear to interact perfectly at the active site of the urease enzyme. Interaction type and interacting residues with docking score were tabulated in Table 2. The mercaptotriazole ring of the (Va–e) series and thiosemicarbazide moiety of the (IVa–e) series of synthesized compounds extends towards both the Ni2+ cations in the catalytic region of JBU and forms a salt bridge or metallic interactions over the S atom (Fig. 1).
The compound (Va) has fitted well in the binding site making hydrogen bonding and halogen bonding with ARG439 and salt bridge with ASP633, Ni841, Ni842 residues which might be responsible for making it competitive inhibitor. The chlorine portion of the p-chloro-benzyl moiety extends towards the solvent side, contributing to the repulsion of the compound towards the nickel-containing active site.
2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimi-dazol-1-yl]acetyl}-N-(4-clorophenyl) hydrazine carbothioamide (IVe), which has the second highest in vitro urease inhibitory activity with an IC50 of 0.11 µM compared to other compounds, has the highest binding energy of –8.97 kcal/mol. Compound (IVe) forms four hydrogen bonds with ARG609, HIS492, ALA440 residues through N and S atoms of thiosemicarbazide moiety and forms π–π interactions over the chloro-benzyl rings with HIS593, ARG609.The strong hydrogen bonding and salt bridge between (IVe) and Ni2+ centers are responsible for the higher activity of this compound.
EXPERIMENTAL
Chemistry. General
The chemicals were supplied from Merck and isolab. Melting points were uncorrected and determined in open capillary tubes on a Büchioil-heated melting point apparatus. Reactions were monitored by thin-layer chromatography (TLC) using using silica gel plates visualized by UV light. 1H-NMR and 13C-NMR spectra were recorded by a VarianMercury 400 (1H, 400 MHz; 13C, 100 MHz) spectrometer using DMSO‑d6 as solvent. Elemental analyses were performed on a Carla Erba 1106 CHN analyzer (Heraeus, Hanau, Germany); the experimental values were in agreement (±0.4%) with calculated ones.
General Procedure for the Synthesis of Compound (I)
A mixture of 4,5-dichloro-1,2-phenylenediamine (0.010 mol) and 2-thiopheneacetonitrile in dry methanol (15 mL) was stirred for 12 h at room temperature. The precipitated product was filtered, washed and recrystallized from ethanol–water (1 : 3). Yield 80%, mp: 204–205°C, IR: 3280 (NH), 1510 (C=N), 1H-NMR (DMSO-d6, 400 MHz): 4.39 (s, 2H, CH2), 6.95–7.75 (m, 5H, ArH) and 12.62 (s, 1H, NH). 13C-NMR (DMSO-d6, 100 MHz): 29.57 (CH2), 124.36, 124.44, 125.64, 126.62, 126.70, 127.21, 127.43, 139.04 (ArC) and 155.13 (C=N). Anal. calculated for C12H8Cl2N2S: C, 50.90; H, 2.85; N, 9.89; S, 11.32; found: C: 51.03, H: 2.94, N: 9.99, S: 11.39.
General Procedure for the Synthesis of Compound (II)
In a balloon a solution of compound (I) (0.010 mol) in 10 mL of acetone and K2CO3 (0.025 mol) was mixed. Then the mixture was stirred at room temperature for 1 h. Ethyl bromoacetate (0.011 mol) was added to the mixture and stirred at room temperature for 8 h. The reaction mixture was poured into water the white product was filtered off, washed with water and recrystallized. Yield 92%, mp: 135–136°C, IR: 1737 (C=O), 1518 (C=N). 1H-NMR (DMSO-d6, 400 MHz): 1.12 (t, 3H, J = 7.2 Hz, CH3), 4.02 (m, 2H, OCH2), 4.48 (s, 2H, CH2), 5.22 (s, 2H, NCH2), 6.92 (m, 2H, ArH), 7.37 (m, 1H, ArH), 7.91 (s, 1H, ArH), 8.21 (d, J = 7.2 Hz, 1H, ArH). 13C-NMR (DMSO-d6, 100 MHz): 14.35 (CH3), 27.97 (CH2), 45.24 (NCH2), 61.73 (OCH2), 112.76, 120.38, 124.77, 125.17, 126.01, 127.10, 127.14, 135.77, 138.00, 141.94 (ArC), 156.17 (C=N) and 167.78 (C=O). Anal. calculated for C16H14Cl2N2O2S: C, 52.04; H, 3.82; N, 7.59; S, 8.68; found: C: 52.17, H: 3.91, N: 7.67, S: 8.80.
General Procedure for the Synthesis of Compound (III)
To a solution of compound (II) (0.010 mol) in 10 mL of ethanol, hydrazine monohydrate (0.035 mol) was added and the mixture was stirred for 4 h at room temprature. The reaction was scanned by TLC (ethanol : ethyl acetate, 3 : 1). When the reaction was completed the mixture was filtered off and dried. Yield 85%, mp: 223–225°C. IR: 3310–3175 (NH–NH2), 1659 (C=O), 1509 (C=N). 1H-NMR (DMSO-d6, 400 MHz): 9.47 (s, 1H, NH), 7.85 (m, 2H, ArH), 7.39 (d, 2H, J = 7.6 Hz, ArH), 6.98 (s, 1H, ArH), 6.95 (t, J = 7.6 Hz, 2H, ArH), 4.86 (s, 2H, NCH2), 4.32 (s, 2H, NH2), 4.02 (s, 2H, CH2). 13C-NMR (DMSO-d6, 100 MHz): 28.09 (CH2), 45.19 (NCH2), 112.55, 120.34, 124.50, 124.95, 125.86, 127.09, 127.25, 135.75, 138.13, 142.02 (ArC), 156.45 (C=N), 165.80 (C=O). Anal. calculated for C14H12Cl2N4OS: C, 47.34; H, 3.41; N, 15.77; S, 9.02; found: C: 47.45, H: 3.54, N: 15.89, S: 9.17.
General Procedure for the Synthesis of Compound (IVa–f)
A mixture of compound (III) (0.010 mol) in ethanol (15 mL) and an appropriate isothiocyanate (0.011) was refluxed for 4 h. The solid products were filtrated and recrystallized from ethanol to obtain the pure (IVa–f).
2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-methylhydrazine carbothioamide (IVa). Yield 82%, mp: 220–222°C. IR: 3263 (NH), 1682 (C=O), 1560 (CN), 1232 (C=S). 1H-NMR (400 MHz, DMSO-d6): 10.23 (s,1H, NH), 9.41 (s, 1H, NH), 8.63 (s, 1H, NH), 8.0–7.74 (m, 3H, ArH), 7.02 (s, 1H, ArH), 6.98 (s, 2H, ArH), 4.98 (s, 2H, NCH2), 4.46 (s, 2H, CH2), 2.89 (s, 3H, NCH3). 13C‑NMR (100 MHz, DMSO-d6): 28.05 (CH2), 31.38 (NCH3), 45.20 (NCH2), 112.57, 120.34, 124.68, 125.01, 125.86, 127.04, 127.31, 135.70, 138.09, 142.42 (ArC), 156.46 (C=N), 166.52 (C=O), 188.09 (C=S). Anal. calculated for C16H15Cl2N5OS2: C, 44.86; H, 3.53; N, 16.35; S, 14.97; found: C: 44.97, H: 3.61, N: 16.47, S: 15.10.
2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-ethylhydrazine carbothioamide (IVb). Yield 85%, mp: 230–232°C. IR: 3359 (NH), 1689 (C=O), 1541 (CN), 1233 (C=S). 1H-NMR (400 MHz, DMSO-d6): 10.22 (s,1H, NH), 9.70 (s, 1H, NH), 9.21 (s, 1H, NH), 8.06 (s, 1H, ArH), 7.85 (s, 2H, ArH), 7.73 (s, 1H, ArH), 7.38–7.40 (m, 1H, ArH), 6.93–6.98 (m, 2H, ArH), 4.99 (s, 2H, NCH2), 4.46 (s, 2H, NCH2), 3.46 (s, 2H, CH2), 1.09 (s, 3H, NCH3). 13C-NMR (100 MHz, DMSO-d6): 14.87 (CH3), 28.05 (CH2), 38.72 (CH2), 45.19 (CH2), 112.53, 112.77, 120.34, 124.67, 125.01, 125.64, 127.01, 127.31, 135.73, 138.11, 142.03 (ArC), 156.48 (C=N), 166.38 (C=O), 188.10 (C=S). Anal. calculated for C17H17Cl2N5OS2: C, 46.16; H, 3.87; N, 15.83; S, 14.49; found: C: 46.29, H: 3.98, N: 15.99, S: 14.63.
2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-phenylhydrazinecarbo thioamide (IVc). Yield 80%, mp: 200–202°C. IR: 3326 (NH), 1691 (C=O), 1523 (CN), 1212 (C=S). 1H-NMR (400 MHz, DMSO-d6): 10.48 (s,1H, NH), 9.68 (s, 1H, NH), 8.90 (s, 1H, NH), 7.90 (s, 1H, ArH), 7.53 (d, J = 8 Hz, 1H, ArH), 7.32–7.53 (m, 5H, ArH), 6.95-6.99 (m, 3H, ArH), 5.06 (s, 2H, NCH2), 4.50 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO-d6): 28.04 (CH2), 45.28 (CH2), 112.76, 120.35, 124.54, 124.68, 124.90, 125.04, 125.86, 127.07, 127.30, 128.72, 135.75, 138.12, 138.24, 139.33, 142.05 (ArC), 156.51 (C=N), 160.38 (C=O), 188.11 (C=S). Anal. calculated for C21H17Cl2N5OS2: C, 51.43; H, 3.49; N, 14.28; S, 13.07; found: C: 51.65, H: 3.60, N: 14.41, S: 13.30.
2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-(4-nitrophenyl) hydrazinecarbothioamide (IVd). Yield 78%, mp: 168–170°C. IR: 3308 (NH), 1672 (C=O), 1506 (CN), 1203 (C=S). 1H-NMR (400 MHz, DMSO-d6): 11.59 (s,1H, NH), 10.55 (s, 1H, NH), 9.78 (s, 1H, NH), 8.22 (d, J = 8.0 Hz, 1H, ArH), 7.87–765 (m, 2H, ArH), 7.39–7.53 (m, 4H, ArH), 6.95–6.99 (m, 1H, ArH), 6.56 (s, 1H, ArH), 5.08 (s, 2H, NCH2), 4.53 (s, 2H, CH2). 13C‑NMR (100 MHz, DMSO-d6): 27.04 (CH2), 45.26 (NCH2), 112.71, 112.91, 120.38, 121.51, 121.67, 124.54, 124.68, 125.08, 125.86, 126.60, 127.31, 128.72, 135.74, 138.12, 138.24, 142.04 (ArC), 156.49 (C=N), 165.38 (C=O) and 188.10 (C=S). Anal. calculated for C21H16Cl2N6O3S2: C, 47.11; H, 3.01; N, 15.70; S, 11.98; found: C: 47.34, H: 3.13, N: 15.88, S: 12.13.
2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-(4-clorophenyl) hydrazinecarbothioamide (IVe). Yield 82%, mp: 198–200°C. IR: 3333 (NH), 1660 (C=O), 1459 (CN), 1247 (C=S). 1H-NMR (400 MHz, DMSO-d6): 10.24 (s, 1H, NH), 9.74 (s, 1H, NH), 8.98 (s, 1H, NH), 8.31 (s,1H, ArH), 7.77–7.92 (m, 5H, ArH), 7.30–7.57 (m, 4H, ArH), 6.99 (d, J = 7.2 Hz, 1H, ArH), 5.22 (s, 2H, NCH2), 4.46 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO-d6): 28.09 (CH2), 45.26 (NCH2), 112.75, 112.81, 120.35, 121.62, 121.77, 123.54, 124.68, 125.08, 125.86, 126.60, 127.31, 135.75, 138.09, 138.12, 142.04 (ArC), 156.49 (C=N), 164.54 (C=O) and 188.11 (C=S). Anal. calculated for C21H16Cl3N5OS2: C, 48.06; H, 3.07; N, 13.34; S, 12.22; found: C: 48.29, H: 3.15, N: 13.47, S: 12.41.
2-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-(4-methylphenyl) hydrazinecarbothioamide (IVf). Yield 74%, mp: 120–122°C. IR: 3207 (NH), 1716 (C=O), 1512 (CN). 1H-NMR (400 MHz, DMSO-d6): 10.94 (s, 1H, NH), 10.47 (s, 1H, NH), 9.69 (s, 1H, NH), 8.31 (s,1H, ArH), 7.91 (s, 1H, ArH), 7.28–7.47 (m, 4H, ArH), 7.15 (d, J = 7.2 Hz, 2H, ArH), 6.92–7.00 (m, 3H, ArH), 5.00 (s, 2H, NCH2), 4.50 (s, 2H, CH2), 2.29 (s, 3H, CH3). 13C‑NMR (100 MHz, DMSO-d6): 14.30 (CH3), 28.09 (CH2), 45.26 (NCH2), 112.69, 112.77, 120.30, 121.52, 121.61, 123.54, 124.68, 125.00, 126.60, 127.10, 127.31, 135.74, 138.08, 142.04 (ArC), 156.46 (C=N), 165.54 (C=O), 188.11 (C=S), Anal. calculated for C22H19Cl2N5OS2: C, 52.38; H, 3.80; N, 13.88; S, 12.71; found: C: 52.53, H: 3.89, N: 13.97, S: 12.95.
General Procedure for the Synthesis of Compounds (Va–f)
In a reflux balloon 2 N NaOH (10 mL) was added to a solution of thiosemicarbazides (IVa–f) (0.01 mol) in ethanol (5 mL). Then the mixture was refluxed for 12 h. Then the balloon was cooled to room temperature and the mixture was acidified to pH 5 or 6 with dripping HCl (37%). The precipitated product was filtrated off, washed with water and recrystallized from ethanol.
5-{[5,6-Dichloro-1-(2-thienylmethyl)-1H-benzimidazol-2-yl]methyl}-4-methyl-4H-1,2,4-triazol-3-thiol (Va). Yield 88%, mp: 210–212°C. IR: 3142 (NH), 1620, 1572 (C=N), 1325 (C=S). 1H-NMR (400 MHz, DMSO-d6): 13.44 (s, 1H, NH), 7.70-8.07 (m, 3H, ArH), 7.39 (s, 1H, ArH), 6.98 (s, 1H, ArH), 4.98 (s, 2H, NCH2), 4.46 (s, 2H, CH2), 1.04 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6): 28.06 (CH3), 31.40 (CH2), 45.20 (NCH2), 112.71, 120.35, 124.60, 125.01, 127.05, 127.32, 135.71, 138.10, 142.06 (ArC), 160.46 (C=N), 166.52 (C=N), 170.27 (C=S). Anal. calculated for C16H13Cl2N5S2: C, 46.83; H, 3.19; N, 17.07; S, 15.63; found: C: 47.03, H: 3.25, N: 17.26, S: 15.78.
5-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]methyl}-4-ethyl-4H-1,2,4-triazol-3-thiol (Vb). Yield 85%, mp: 280°C (decomp.). IR: 2635 (SH), 1620, 1575 (C=N). 1H-NMR (400 MHz, DMSO-d6): 13.45 (s,1H, NH), 7.98 (s, 1H, ArH), 7.89 (s, 1H, ArH), 7.34 (d, J = 8Hz, 1H, ArH), 6.87–6.93 (m, 2H, ArH), 5.71 (s, 2H, NCH2), 4.52 (s, 2H, CH2), 4.00 (q, J = 8Hz, 2H, CH2), 1.18 (t, J = 8.0 Hz, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6): 13.50 (CH3), 27.94 (CH2), 39.05 (NCH2), 112.87, 124.93, 125.30, 125.87, 127.05, 135.62, 137.96, 142.02, 147.75 (ArC), 156.35 (C=N), 167.47 (C=N). Anal. calculated for C17H15Cl2N5S2: C, 48.12; H, 3.56; N, 16.50; S, 15.11; found: C: 48.33, H: 3.75, N: 16.87, S: 15.37.
5-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]methyl}-4-phenyl-4H-1,2,4-triazole-3-thiol (Vc). Yield 75%, mp: 250°C (decomp.). IR: 2923 (SH), 1620, 1596, 1497 (C=N). 1H-NMR (400 MHz, DMSO-d6): 13.83 (s, 1H, NH), 7.81 (s, 1H, ArH), 7.70 (s, 1H, ArH), 7.26–7.51 (m, 5H, ArH), 6.86 (t, J = 8.0 Hz, 1H, ArH), 6.82 (d, J = 8.0 Hz, 2H, ArH), 5.38 (s, 2H, NCH2), 4.32 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO-d6): 27.91 (CH2), 39.32–40.58 (DMSO-d6 + NCH2), 112.83, 120.22, 124.76, 125.12, 125.95, 126.97, 127.11, 128.57, 129.85, 130.16, 133.30, 135.43, 137.85, 141.72, 147.58 (ArC), 155.89 (C=N), 169.07 (C=N). Anal. calculated for C21H15Cl2N5S2: C, 53.39; H, 3.20; N, 14.83; S, 13.57; found: C: 53.54, H: 3.30, N: 15.03, S: 13.80.
5-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]methyl}-4-(4-nitrophenyl-4H-1,2,4-triazole-3-thiol (Vd). Yield 78%, mp: 125°C (decomp.). IR: 3284 (NH), 1620, 1596 (C=N) 1306 (C=S). 1H‑NMR (400 MHz, DMSO-d6): 13.95 (s, 1H, NH), 8.33 (s, 1H, ArH), 8.21 (d, J = 8.0 Hz, 1H, ArH), 8.17–7.36 (m, 4H, ArH), 6.97 (s, 1H, ArH), 6.87–6.77 (m, 2H, ArH), 5.46 (s, 2H, NCH2), 4.53 (s, 2H, CH2). 13C-NMR (100 MHz, DMSO-d6): 27.96 (CH2), 48.72 (NCH2), 112.63, 120.25, 121.55, 124.79, 124.98, 125.12, 125.82, 125.94, 127.11, 130.39, 135.39, 137.78, 137.87, 138.08, 141.65 (ArC), 156.09 (C=N), 168.92 (C=S). Anal. calculated for C21H14Cl2N6O2S2 C, 48.75; H, 2.73; N, 16.24; S, 12.39; found: C: 49.04, H: 2.85, N: 16.44, S: 12.60.
5-{[5,6-Dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]methyl}-4-(4-methylphenyl)-4H-1,2,4-triazole-3-thiol (Vf). Yield 88%, mp: 150°C (decomp.). IR: 3036 (NH), 1514 (C=N), 1317 (C=S). 1H-NMR (400 MHz, DMSO-d6): 10.94 (s, 1H, NH), 7.91 (s, 1H, ArH), 7.87 (s, 1H, ArH), 7.76 (d, J = 8.0 Hz, 2H, ArH), 7.40 (s, 1H, ArH), 7.32 (s, 1H, ArH), 7.15–6.95 (m, 3H, ArH), 5.06 (s, 2H, NCH2), 4.50 (s, 2H, CH2) 1.20 (s, 3H, CH3). 13C-NMR (100 MHz, DMSO-d6): 21.00 (CH3), 28.00 (CH2), 45.36 (NCH2), 112.76, 120.34, 124.56, 124.79, 124.70, 125.05, 125.84, 127.07, 127.32, 129.15, 135.75, 136.74, 137.78, 138.12, 142.05 (ArC), 156.51 (C=N), 166.43 (C=S). Anal. calculated for C22H17Cl2N5S2: C, 54.32; H, 3.52; N, 14.40; S, 13.18; found: C: 54.50, H: 3.60, N: 14.70, S: 13.34.
In vitro Urease Inhibition Assay
The urease inhibition assay was determined spectrophotometrically according to the method of Van Slyke and Archibald [31]. Benzimidazole derivatives and standard thiourea were dissolved in DMSO at different concentrations (1.0 × 10–5–1.0 × 10–2 µm). Enzyme solution was prepared in pH: 6.8 phosphate buffer at 100 Mm last concentration with Jack bean urease. 500 µL of Jack bean urease solution was added to 0.5 mL of the test compounds and the standard. The mixture was incubated for 15 min at room temperature. After incubation, 400 µL phenol red solution which was prepared in urea-phosphate buffer (pH 6.8) was transferred to the mixture. The absorbance was read at 570 nm using UV spectrophotometer. The assays were done in triplicate, and the IC values were calculated in μM.
CONCLUSIONS
A novel series of 5,6-dichloro-2-(2-thienylmethyl)-1H-benzimidazole derivatives containing triazole ring was synthesized and then screened for urease inhibitory activities. In vitro antiurease activity evaluation of title compounds revealed that all the synthesized derivatives exhibited excellent inhibitory potency compared with thiourea (IC50 = 0.267 ± 0.022 µM). Compound 5-{[5,6-dichloro-1-(2-thienylmethyl)-1H-benzimidazol-2-yl]methyl}-4-methyl-4H-1,2,4-triazol-3-thiol (Va) derivatives of triazole has the best inhibition activity with IC50 = 0.07 μM. The mercaptotriazole ring of the (Va–e) series and thiosemicarbazide moiety of the (IVa–e) series of synthesized compounds extends towards both the Ni2+ cations in the catalytic region of JBU and forms a salt bridge or metallic interactions over the S atom. Docking studies showed that 2-{[5,6-dichloro-2-(2-thienylmethyl)-1H-benzimidazol-1-yl]acetyl}-N-(4-clorophenyl) hydrazinecarbothioamide (IVe), which has the second highest in vitro urease inhibitory activity with an IC50 of 0.11 µM compared to other compounds, has the highest binding energy of -8.97 kcal/mol. Thiophene ring at the position-2 on the benzimidazole has a positive effect on the inhibitory activity of main structure. The results obtained here will contribute to the synthesis of more effective urease inhibiting agents.
REFERENCES
Palit, R., Kumar, R., Saraswat, N., Wal, A., and Upadhyaya, P.K., Res. Ayurveda Phar., 2017, vol. 7, pp. 68–73.
Mabkhot, N.Y., Showiman, S.S., Soliman, G.H.A., and Aldamen, M.A., Chem. Cent. J., 2017, vol. 11. https://doi.org/10.1186/s13065-017-0280-6
Yılmaz, F., Menteşe, E., and Baltaş, N., Lett. Drug. Dis. Dis., 2017, vol. 14, pp. 201–208. https://doi.org/10.2174/1570180813666160609082633
Menteşe, E., Emirik, M., and Sökmen, B.B., Bioorg. Chem., 2019, vol. 86, pp. 151–158. https://doi.org/10.1016/j.bioorg.2019.01.061
Ragavendran, J., Sriram, D., Patil, S., Reddy, I.V., Bharathwajan, N., Stables, J., and Yogeeswari, P., Eur. J. Med. Chem., 2007, vol. 42, pp. 146–151. https://doi.org/10.1016/j.ejmech.2006.08.010
Polivka, Z., Holubek, J., Svatek, E., Metys, J., and Protiva, M., Collect. Czech. Chem. Commun., 1984, vol. 49, pp. 122–123. https://doi.org/10.1002/CHIN.198427237
Yogeeswari, P., Thirumurugan, R., Kavya, R., Samuel, J.S., Stables, J., and Siram, D., Eur. J. Med. Chem., 2004, vol. 39, pp. 729–734. https://doi.org/10.1016/j.ejmech.2004.03.008
Paramashivappa, R., Phani Kumar, P., Subba Rao, P.V., and Srinivasa Rao, A., Bioorg. Med. Chem. Lett., 2003, vol. 13, pp. 657–660. https://doi.org/10.1016/s0960-894x(02)01006-5
Keter, F. and Darkwa, J., Biometals, 2012, vol. 25, pp. 9–21. https://doi.org/10.1007/s10534-011-9496-4
Mobley, H.L.T., Island, M.D., and Hausinger, R.P., Microbiol. Rev., 1995, vol. 59, pp. 451–480. https://doi.org/10.1128/mr.59.3.451-480.1995
Maroney, M.J. and Ciurli, S., Chem. Rev., 2014, vol. 114, pp. 4206–4228. https://doi.org/10.1021/cr4004488
Mobley, H. and Hausinger, R., Microbiol. Mol. Biol. Rev., 1989, vol. 53, pp. 85–108.
Karplus, P.A., Pearson, M.A., and Hausinger, R.P., Acc. Chem. Res., 1997, vol. 30, pp. 330–337. https://doi.org/10.1021/ar960022j
Lodhi, M.A., Ajmal, M., Choudhary, M.I., Ahmed, V.U., Nat. Prod. Res., 2007, vol. 21, pp. 721–725. https://doi.org/10.1080/14786410600906913
Velík, J., Baliharová, V., Fink-Gremmels, J., Bull, S., Lamka, J.L., and Skálová, Res. Vet. Sci., 2004, vol. 76, pp. 95–108. https://doi.org/10.1016/j.rvsc.2003.08.005
Amtul, Z., Rahman, A., Siddiqui, R., and Choudhary, M.I., Curr. Med. Chem., 2002, vol. 9, pp. 1323–1348. https://doi.org/10.2174/0929867023369853
Saeed, A., Imran, A., Channar, P.A., Shahid, M., Mahmood, W., and Iqbal, J., Chem. Biol. Drug. Des., 2015, vol. 85, pp. 225–230. https://doi.org/10.1111/cbdd.12379
Akyüz, G., Cumhuriyet Sci. J., 2021, vol. 42, pp. 649–651. https://doi.org/10.17776/csj.867783
Shah, R. and Verma, P.K., Chem. Cent. J., 2018, vol. 12, p. 137. https://doi.org/10.1186/s13065-018-0511-5
Noreen, M., Rasool, N., Gull, Y., Zubair, M., Mahmood, T., Ayub, K., Nasim, F.U., Yaqoob, A., Zia-Ul-Haq, M., and de Feo, V., Molecules, 2015, vol. 20, pp. 19914–19928. https://doi.org/10.3390/molecules201119661
Khan, K.M., Wadood, A., Ali, M., Ul-Haq, Z., Lodhi, M.A., Khan, M., Perveen, S., and Choudhary, I., J. Mol. Graph. Mod., 2010, vol. 28, pp. 792–798. https://doi.org/10.1016/j.jmgm.2010.02.004
Khan, I., Ali, S., Hameed, S., Rama, N.H., Hussain, M.T., and Wadood, A., Eur. J. Med. Chem., 2010, vol. 45, pp. 5200–5207. https://doi.org/10.1016/j.ejmech.2010.08.034
Özil, M., Bodur, O., Ülker, S., and Kahveci, B., Chem. Heterocycl. Comp., 2015, vol. 51, pp. 88–96. https://doi.org/10.1007/s10593-015-1664-y
Akhtar, T., Hameed, S., Khan, K.M., Khan, A.L., and Choudhary, M., J. Enzym. Inh. Med. Chem., 2010, vol. 25, p. 572. https://doi.org/10.3109/14756360903389864
Menteşe, E., Emirik, M., and Sökmen, B.B., Bioorg. Chem., 2019, vol. 86, pp. 151–158.
Arshad, T., Khan, K.M., Rasool, N., Salar, U., Hussain, S., Asghar, H., Ashraf, M., Wadood, A., Riaz, M., Perveen, S., Taha, M., and Ismail, N.H., Bioorg. Chem., 2017, vol. 72, pp. 21–31. https://doi.org/10.1016/j.bioorg.2017.03.007
Menteşe, E., Bektaş, H., Emirik, M., Sökmen, B.B., and Kahveci, B., Bioorg. Med. Chem. Lett., 2017, vol. 27, pp. 3014–3018. https://doi.org/10.1016/j.bmcl.2017.05.019
Schrödinger, Suite 2009 Induced Fit Docking Protocol; Glide version 5.5, S., LLC, New York, NY, 2009; Prime version 2.1, Schrödinger, LLC, New York, NY, 2009.
Balasubramanian, A. and Ponnuraj, K., J. Mol. Biol., 2010, vol. 400, pp. 274–283. https://doi.org/10.1016/j.jmb.2010.05.009
Akyüz, G., Menteşe, E., Emirik, M., and Baltaş, N., Bioorg. Chem., 2018, vol. 80, pp. 121–128.
Van Slyke, D.D. and Archibald, R.M., J. Biol. Chem., 1944, vol. 154, pp. 623–628.
Funding
This work was supported by Recep Tayyip Erdogan University Scientific Research Project Unit (BAP) under the project number of FBA-2020-1128.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Akyüz, G., Emirik, M., Sökmen, B.B. et al. Synthesis and Docking Studies of Novel Benzimidazole Derivatives Containing Thiophene and Triazole Rings as Potential Urease Inhibitors. Russ J Bioorg Chem 48 (Suppl 1), S87–S95 (2022). https://doi.org/10.1134/S106816202301003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202301003X