Abstract—
We report a novel derivative of the conformationally locked derivative of the green fluorescent protein chromophore, (Z)-5-((2-(difluoroboryl)-4-(dimethylamino)phenyl)(4-(dimethylamino)phenyl)methylene)-2-propyl-3,5-dihydro-4H-imidazol-4-on. The presence of an additional aryl substituent in the structure causes a decrease in the fluorescence intensity and does not lead to absorption and emission spectra shifts. A promising direction of research is the replacement of this substituent with the electron-withdrawing groups, such as the nitrile or CF3 group, capable of more efficient conjugation with the π-system of the molecule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Studies in recent decades show that fluorescent labels are of high practical importance for visualization and study of biological processes [1]. Synthetic fluorophores include various benzylidene imidazolones, derivatives of the chromophore of the green fluorescent protein GFP [2]. This class of substances is represented by a large number of compounds that have different chemical structures and, as a result, are characterized by different properties. A number of well-developed methods for the chemical synthesis of these compounds are described in the literature [3].
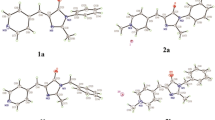
Important properties of fluorophores include the ability to absorb and emit light in the far-red region of the spectrum and a high quantum yield of fluorescence. It is well known that the GFP chromophore and other benzylidene imidazolones weakly fluoresce due to the mobility of the benzylidene fragment of the molecule [4]. However, it was shown that this fragment can be rigidly fixed, for example, using a difluoroboryl bridge (for example, the compound (Ia) in Fig. 1) or other fixing methods. Such modifications made it possible to multiply the fluorescence quantum yield [5–7]. “Red” benzylidene imidazolones can be obtained by increasing the conjugated π-system (introducing styrene or arylacetylene substituents) [8–11] or by expanding the aromatic system by annulating the benzylidene moiety [12–14].
Previously, we synthesized a pair of annulated derivatives (Fig. 1, compounds (Ib) and (Ic)) [12, 15]. It was found that an increase in the aromatic system led to a bathochromic shift of the absorption maxima by 54–65 nm and emission by 50–77 nm relative to the unmodified derivative (Ia). However, it also turned out that fluorescence is retained only for the compound (Ic), while the fluorescence quantum yield of the derivative (Ib) does not exceed 0.2% (Fig. 1). We found that this behavior is caused by the presence of a covalent bond between two phenyl substituents in the derivative (Ib), due to which an additional free molecular orbital arises, fluorescence from which is impossible. It is likely that the removal of this bond can solve this problem and lead to an increase in fluorescence.
The purpose of this work is the synthesis the corresponding diarylmethylene-imidazolone, in which the aryl substituents are not linked to each other by a covalent bond, and the study of the optical properties of this compound.
RESULTS AND DISCUSSION
As a model, we chose the compound (II) (Fig. 1) [16]. This compound and its closest analogs act as substrates for lipocalin-based fluorogen-activating proteins and can be used in genetically encoded labeling [17, 18].
Benzylidene-imidazolone (V) was synthesized using the ketone condensation reaction (III) and saturated imidazolone (IV) in pyridine (Scheme 1). The conformationally fixed derivative (VI) was obtained by the action of boron tribromide on benzylidene-imidazolone (V) in dichloroethane in the presence of molecular sieves (Scheme 1).
The study of optical properties showed that the new derivative (VI), as well as the model connection (II), absorbs light in the region of 450–480 nm, and fluoresces in the region of 530–555 nm (Fig. 2, Table 1). It is likely that the absence of a significant difference is due to incomplete conjugation of the second aryl substituent with the π-system of the molecule.

Scheme 1 . Scheme for the synthesis of compounds (V) and (VI).
The mobility of the second aryl substituent in the structure of compound (VI) also had a negative effect on the fluorescence intensity. It was found that the fluorescence quantum yield of this substance in acetonitrile does not exceed 0.5%, while the quantum yield of compound (II) is 48% (Fig. 1).
The results obtained indicate that a promising direction for further research may be the introduction other groups more capable of effective conjugation with the π-system of the molecule, for example, the introduction of not the second aryl substituent, as in the case of the compound (VI), but with various electron-acceptor groups, such as a nitrile or CF3 group.
EXPERIMENTAL
Equipment. NMR spectra (δ, ppm; J, Hz) were recorded on an Avance spectrometer III NMR (700 MHz; Bruker, United States) at 303 K in DMSO-d6 (internal standard Me4Si), absorption spectra on a Cary 100 Bio spectrophotometer (Varian, United States), and fluorescence spectra on a Cary Eclipse spectrofluorimeter (Varian, United States). Melting points were determined on an SMP 30 instrument (Stuart Scientific, UK) and were not corrected. High-resolution mass spectra were recorded on a micrOTOF II instrument (Bruker, Germany), electrospray ionization.
Synthesis of 5-(bis(4-(dimethylamino)phenyl)methylene)-2-propyl-3,5-dihydro-4H-imidazol-4-one (V). 4,4'-Bis(dimethylamino)benzophenone (2.5 g, 9 mmol), 2-propyl-3,5-dihydro-4H-imidazol-4-one (2.3 g, 18 mmol) and molecular sieves (1.2 g, 3 Å). The flask was evacuated, filled with argon, and 10 mL of pyridine was added. The resulting mixture was stirred at 120°C for 50 h. Then, the reaction mixture was cooled to room temperature and evaporated. The resulting product was purified using column chromatography (eluent, chloroform and ethanol, 50 : 1).
Orange powder (409 mg, 15%); m.p. 206–209°C. 1H-NMR: 10.85 (s, 1H), 7.31 (d, J2 9.0, 2H), 7.03 (d, J2 8.8, 2H), 6.68 – 6.63 (m, 4H), 2.97 (s, 6H), 2.96 (s, 6H), 2.37 (t, J2 7.5, 2H), 1.64 (sext, J2 7.4, 2H), 0.94 (t, J2 7.4, 3H). 13C-NMR: 169.8, 159.3, 150.5, 150.4, 145.1, 134.0, 133.5, 132.4, 127.2, 125.2, 110.8, 110.6, 39.8, 39.7, 31.2, 19.2, 13.6. HRMS (ESI) m/z: found M 391.2466; calculated for C24H31N4O+, [M + H]+ 391.2492.
Synthesis of (Z)-5-((2-(difluoroboryl)-4-(dimethylamino)phenyl)(4-(dimethylamino)phenyl)methylene)-2-propyl-3,5-dihydro-4H-imidazol-4-one (VI). The product of the first stage (V) (0.2 g, 0.5 mmol) was mixed with molecular sieves (2 g, 3 Å and 2 g, 4 Å) and boron tribromide (5 mmol) in 50 mL of dichloroethane. The resulting mixture was refluxed in an argon atmosphere for 6 h. The mixture was then cooled and filtered. The molecular sieves were washed with cold ethanol (2 × 10 mL) and chloroform (50 mL). HF solution (40%, 5 mL) was added to the resulting solution and stirred for 30 min. The mixture was diluted with EtOAc (100 mL), washed with saturated K2CO3 (2 × 50 mL), water (2 × 50 mL) and saturated NaCl solution (2 × 50 mL). Organic extracts were dried over anhydrous Na2SO4 and evaporated. The resulting product was purified by flash chromatography (eluent, chloroform and ethanol, 20 : 1).
Red powder (22 mg, 10%); m.p. ~250°C with decomposition. 1H-NMR: 12.58 (s, 1H), 7.09 (d, J2 8.6, 2H), 6.94–6.90 (m, 2H), 6.78 (d, J2 8.6, 2H), 6.59 (dd, J2 9.1, 2.9, 1H), 3.03 (s, 6H), 3.00–2.93 (m, 8H), 1.78 (sext, J2 7.5, 2H), 0.98 (t, J2 7.3, 3H). 13C-NMR: 163.0, 162.7, 151.7, 150.2, 145.6, 132.2, 130.3, 123.7, 120.6, 119.0, 113.7, 111.3, 110.8, 110.8, 39.9, 39.8, 28.5, 19.9, 13.5. HRMS (ESI), m/z: found M 439.2480; calculated for C24H30BF2N4O+, [M + H]+ 439.2475.
CONCLUSIONS
In this work, a new derivative of the chromophore of the GFP protein, (Z)-5-((2-(difluoroboryl)-4-(dimethylamino)phenyl)(4-(dimethylamino)phenyl)methylene)-3-methyl-2-propyl-3,5-dihydro-4H-imidzol-4-one was synthesized. The optical properties of this compound have been studied and it was found that in the absence of rigid fixation of the second aryl substituent and the resulting incomplete conjugation with the π-system of the molecule leads to a sharp decrease in the intensity fluorescence of the new compound. It is likely that an effective modification aimed at increasing the fluorescence quantum yield of such compounds will be the replacement of an unfixed aryl substituent with various electron-acceptor groups, such as a nitrile or CF3 group, capable of more efficient conjugation with the π-system of the molecule.
REFERENCES
Sahoo, H., RSC Adv., 2012, vol. 2, pp. 7017–7029. https://doi.org/10.1039/C2RA20389H
Walker, C.L., Lukyanov, K.A., Yampolsky, I.V., Mishin, A.S., Bommarius, A.S., Duraj-Thatte, A.M., Azizi, B., Tolbert, L.M., and Solntsev, K.M., Curr. Opin. Chem. Biol., 2015, vol. 27, pp. 64–74. https://doi.org/10.1016/j.cbpa.2015.06.002
Baleeva, N.S. and Baranov, M.S., Chem. Heterocycl. Compd., 2016, vol. 52, pp. 444–446. https://doi.org/10.1007/s10593-016-1909-4
Svendsen, A., Kiefer, H.V., Pedersen, H.B., Bochenkova, A.V., and Andersen, L.H., J. Am. Chem. Soc., 2017, vol. 139, pp. 8766–8771. https://doi.org/10.1021/jacs.7b04987
Baranov, M.S., Lukyanov, K.A., Borissova, A.O., Shamir, J., Kosenkov, D., Slipchenko, L.V., Tolbert, L.M., Yampolsky, I.V., and Solntsev, K.M., J. Am. Chem. Soc., 2012, vol. 134, pp. 6025–6032. https://doi.org/10.1021/ja3010144
Zaitseva, S.O., Farkhutdinova, D.A., Baleeva, N.S., Smirnov, A.Y., Zagudaylova, M.B., Shakhov, A.M., Astafiev, A.A., Baranov, M.S., and Bochenkova, A.V., RSC Adv., 2019, vol. 9, pp. 38730–38734. https://doi.org/10.1039/c9ra08808c
Baldridge, A., Solntsev, K.M., Song, C., Tanioka, N., Kowalik, J., Hardcastle, K., and Tolbert, L.M., Chem. Commun., 2010, vol. 46, pp. 5686–5688. https://doi.org/10.1039/B927313A
Shen, B. and Qian, Yi., Dyes Pigm., 2019, vol. 166, pp. 350–356. https://doi.org/10.1016/j.dyepig.2019.03.034
Baleeva, N.S., Myannik, K.A., Yampolsky, I.V., and Baranov, M.S., Eur. J. Org. Chem., 2015, vol. 26, pp. 5716–5721. https://doi.org/10.1002/ejoc.201500721
Feng, G., Luo, C., Yi, H., Yuan, L., Lin, B., Luo, X., Hu, X., Wang, H., Lei, C., Nie, Z., and Yao, S., Nucleic Acids Res., 2017, vol. 45, pp. 10380–10392. https://doi.org/10.1093/nar/gkx803
Baleeva, N.S. and Baranov, M.S., Russ. J. Bioorg. Chem., 2017, vol. 43, pp. 612–615. https://doi.org/10.1134/S106816201705003X
Baleeva, N.S., Khavroshechkina, A.V., Zaitseva, E.R., Myasnyanko, I.N., Zagudaylova, M.B., and Baranov, M.S., Tetrahedron Lett., 2019, vol. 60, p. 150963. https://doi.org/10.1016/j.tetlet.2019.150963
Huang, G., Zhang, G., Wu, Y., Liao, Q., Fu, H., and Zhang, D., Asian J. Org. Chem., 2012, vol. 1, pp. 352–358. https://doi.org/10.1002/ajoc.201200086
Muselli, M., Baudequin, C., Perrio, C., Hoarau, C., and Bischoff, L., Chemistry, 2016, vol. 22, pp. 5520–5524. https://doi.org/10.1002/chem.201600602
Sinenko, G.D., Farkhutdinova, D.A., Myasnyanko, I.N., Baleeva, N.S., Baranov, M.S., and Bochenkova, A.V., Int. J. Mol. Sci., 2021, vol. 22, p. 13645. https://doi.org/10.3390/ijms222413645
Baranov, M.S., Solntsev, K.M., Baleeva, N.S., Mishin, A.S., Lukyanov, S.A., Lukyanov, K.A., and Yampolsky, I.V., Chemistry, 2014, vol. 20, pp. 13234–13241. https://doi.org/10.1002/chem.201403678
Bozhanova, N.G., Baranov, M.S., Baleeva, N.S., Gavrikov, A.S., and Mishin, A.S., Int. J. Mol. Sci., 2018, vol. 19, p. 3778. https://doi.org/10.3390/ijms19123778
Muslinkina, L., Gavrikov, A.S., Bozhanova, N.G., Mishin, A.S., Baranov, M.S., Meiler, J., Pletneva, N.V., Pletnev, V.Z., and Pletnev, S., ACS Chem. Biol., 2020, vol. 15, pp. 2456–2465. https://doi.org/10.1021/acschembio.0c00440
Funding
The study was financed by the Russian Science Foundation (project no. 18-73-10105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any research involving humans and animals as research objects.
Conflict of Interests
The authors declare they have no conflicts of interest.
Additional information
Abbreviation: GFP, green fluorescent protein.
Rights and permissions
About this article
Cite this article
Baleeva, N.S., Smirnov, A.Y. & Baranov, M.S. Synthesis and Optical Properties of the Conformationally Locked Diarylmethene Derivative of the GFP Chromophore. Russ J Bioorg Chem 48, 1101–1104 (2022). https://doi.org/10.1134/S1068162022050077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022050077