Abstract
The main classes of antitumor drugs (antimetabolites, anthracyclines, taxanes, and alkylating agents) act via DNA damage, inhibit DNA synthesis, and/or represent antimicrotubule agents. The phosphorus-containing alkyl glycerolipids induce death of tumor cells of various tissue origin, whereas nonmalignant counterparts are less damaged. One serious drawback of these compounds is their hemolytic activity, that is, disruption of red blood cells. Moreover, intracellular phospholipases can hydrolyze the phosphorus-containing glycerolipids and reduce their antitumor potency. This justifies the search for new antitumor, nonhemolytic phosphorus-free lipids. Modifications of the hydrophobic and polar groups yielded new lipid-based agents. In particular, the replacement of the hydrophilic phosphate group with a carbohydrate residue yielded a new chemotype of glycosylated glycerolipids. Further developments resulted in a series of non-phosphorus cationic, polycationic, and neutral glycoglycerolipids. New non-phosphorus lipids retained the antiproliferative and cytotoxic properties similarly to edelfosine and other alkyl phosphoglycerolipids. Importantly, the hemolytic activity was negligible or absent. This review analyzes the structure–activity relationship within the class of non-phosphorus glycerolipids as the original antitumor drug candidates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cancer is a major cause of death in Russia and around the world. The efficacy of antitumor therapy still remains not so high [1] due to lack of selectivity for tumor tissues, induction of drug resistance [2, 3], high hydrophobicity [4, 5] and low efficacy of drug delivery to the tumor [6, 7], serious side effects, etc. [8, 9]. Hence, the most urgent task is to optimize the structure of chemical compounds, which represent candidates for antitumor drugs. This review analyzes the chemical classes of synthetic lipids that hold promise as antitumor compounds but missed the attention of researchers.
ANTITUMOR PROPERTIES OF LIPIDS
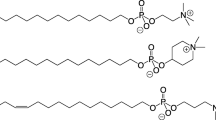
The investigations into the antitumor properties of lipids were started in the second half of the twentieth century, when it was revealed that phagocytosis in macrophages was significantly increased by small amounts of the natural phospholipid 2-lysophosphatidylcholine (compound (I), Fig. 1) [10]. This indicated that compound (I) could play an active role in the body’s defense. Nonetheless, phospholipid (I) is unstable and is rapidly metabolized into phosphatidylcholine (II) or glycerophosphocholine (III) by acetyltransferase or lysophospholipase, which made it impossible to use compound (I) as a drug [11].
Determination of the structure of the platelet-activating factor (PAF, compound (IV), Fig. 2) was a major step in the search for new antitumor compounds. In endothelial cells, compound (IV) stimulates cell motility, expression of adhesion molecules, promotes destruction of the extracellular matrix, cell migration, and neoangiogenesis [12]. Upregulation of PAF receptors induces antiapoptotic factors, contributing to the survival of tumor cells [13, 14]. It is suggested that the combination of PAF-receptor antagonists with chemotherapy may be a promising strategy for the treatment of tumors [15]. Many compounds of a lipid nature have structural similarity with compound (IV), which explains the possibility of developing antitumor agents based on certain lipids.
The investigations of the biological activity of synthetic and natural PAF analogs have shown that various modifications of the bond type at the C1 position, as well as substituents at the C2 positions of glycerol, lead to the appearance of antagonistic properties in the lipid analogs of PAF. Since compound (IV) causes significant platelet aggregation, it is difficult to use it for tumor treatment [16]. A large number of PAF structural analogs have been synthesized, which exhibit antitumor properties due to their various effects on the inhibition of cellular enzymes, induction of necrosis, activation of immune effectors, and metastasis restriction [17, 18].
The biological effect of PAF-like glycerolipids with a simple ether linkage depends on the length of hydrocarbon substituents that form their hydrophobic domain. The replacement of the acetyl group at the C2-glycerol atom with a short-chain alkyl group leads to the appearance of antitumor properties [19]. Zwitterionic phospholipids of the alkyl type (structural analogs of PAF) do not provoke platelet aggregation, but they inhibit the proliferation of tumor cells and cause their death. These lipids exhibit antitumor, antiviral and antibacterial effects and, in contrast to many antitumor drugs, they do not cause serious side effects, except for hemolysis [20–22].
Further developments led to the synthesis of 1-O-octadecyl-2-O-methyl-3-glycerophosphocholine (ET-18-OCH3, edelfosine, compound (V), Fig. 2), which showed higher antitumor activity than compound (I) [23]. Subsequent studies confirmed that edelfosine and related alkyl-lysophospholipids have potential antitumor activity [17, 24]. Edelfosine was the first successful glycerophospholipid after PAF (IV) that was active in micromolar concentrations, entered phase II clinical trials, and became the gold standard among antitumor lipids. It is noted that the nature of edelfosine action was not initially determined and due to its similarity to compound (IV), PAF receptors were considered the most likely targets. It was later shown that the addition of edelfosine to HL60 cells with low PAF receptor expression caused apoptosis [25], while incubation of edelfosine-sensitive cells with compound (IV) did not lead to apoptosis [26].
Edelfosine is the first antitumor agent that interacts with lipid rafts on the cell periphery [27, 28]. It accumulates in the endoplasmic reticulum, which elicits endoplasmic reticulum stress response and apoptosis, and also inhibits the synthesis of phosphatidylcholine by inactivating phosphocholine cytidylyltransferase [29, 30]. It exhibits some selectivity of action and is less toxic to nontumor cells [17, 31, 32]. The selective cytotoxic action of edelfosine is associated with an increased rate of endocytosis in transformed cells [33, 34].
The replacement of the methyl group to an ethyl group at C2-position of the glycerol backbone in edelfosine significantly reduces the cytotoxic effect (Table 1, [19]; compound (VI), Fig. 2).
Edelfosine has a high hemolytic activity [19]. It has a relatively low bioavailability (<10% upon a single administration) and a low rate of excretion from the body [35]. These factors led to the search for more effective structural analogs of edelfosine [36, 37]. The search for less hemotoxic, more stable and no less active analogs of edelfosine is still ongoing. Cationic and neutral lipids have been synthesized, including glycerolipids, glycoglycerolipids, and polycationic glycerolipids (glyceropolyamines) (Scheme 1).

Scheme 1 . Classification of antitumor lipids.
The side effects of edelfosine and its analogs necessitate the development of new approaches to deliver compounds in order to improve bioavailability. Parenteral administration of phosphorus-containing glycerolipids permits a rapid distribution in the organs [38], but the doses need to be limited because of their hematotoxicity. Oral administration of compound (V) provides the necessary concentration in blood plasma only upon repeated use [35]. Subcutaneous administration and topical application of glycerolipids neither provide an effective concentration in plasma, but in some cases they are used to treat skin metastases and minimize systemic side effects [40]. Modern approaches used to improve bioavailability and delivery of glycerolipids are based on the use of lipid nanoparticles [41–43]. In addition to improved bioavailability due to encapsulation of compound (V) into lipid nanoparticles, the researchers succeeded in overcoming the resistance of leukemia cells to edelfosine [39].
This review presents a classification of phosphorus-containing and non-phosphorus lipids with antitumor activity (scheme 1), justifies the structural modifications of various lipids, and analyzes how they work.
The primary analysis of the activity was performed using the MTT test to evaluate the cytotoxicity of a compound on tumor and nontumor cells. Glycerolipids were added to the cells at final concentrations of 0.1–50.0 μM. After a 72-h incubation, viable cells reduced the tetrazolium dye MTT to form an insoluble formazan, the amount of which was detected in a colorimetric assay (absorbance at 575 nm). The cytotoxicity of the compounds was calculated as 50% cell growth inhibitory concentration (IC50) by comparing the degree of light absorbance of the formazan product in cells treated with the compounds and in intact (control) cells. In our studies, compounds with IC50 > 20–50 μM were considered to be inactive against tumor lines. Cytotoxicity of IC50 ≤ 20 μM was regarded as a threshold for further testing.
EDELFOSINE ANALOGS
Ilmofosine (compound (VII, Fig. 3), a thioether variant of edelfosine, demonstrated cytotoxicity in vitro and in vivo [44]. In clinical trials, ilmofosine did not show any activity in patients and caused side effects similar to edelfosine [45]. A similar situation was observed for miltefosine (hexadecylphosphocholine, compound (VIII)), whereas in preclinical trials it showed higher bioavailability and accumulation in blood plasma compared to compound (V) [46]. Miltefosine is primarily metabolized by phospholipases and is toxic upon oral and intravenous administration [47]. However, compound (VIII), in contrast to edelfosine (V), is used for the treatment of several tumors (skin lymphoma, superficial metastases from breast cancer) and leishmaniasis [48]. Replacement of the choline part in miltefosine with heterocyclic piperidine (perifosine, compound (IX)) expanded the range of susceptible tumor cells and contributed to favorable pharmacokinetics: perifosine (IX) is absorbed from the gastrointestinal tract within 24 h and does not undergo metabolic transformations. The maximum accumulation in the tumor is reached in 48 h [49, 50]. Hence, perifosine (IX) can be administered orally, similar to miltefosine (VIII). It is known that perifosine (IX), similar to edelfosine (V), causes apoptosis and is selective for myeloma [31]. Erucylphosphocholine (X) and its homolog erufosine (XI) are active against brain tumors [51, 52].
The substantial limitations of edelfosine and its phosphorus-containing analogs, i.e., hydrolysis under the action of phospholipases and a significant hemolytic effect, launched the beginning of structural and functional investigations into non-phosphorus lipids.
NON-PHOSPHORUS POSITIVELY CHARGED LIPIDS
The search for candidate phosphorus-free analogs of edelfosine involved the variation in the hydrophobic and hydrophilic structural domains. All the phosphorus-free lipids discussed below can be divided into three main types (Figure 4): (1) positively charged glycerolipids (Fig. 4a); (2) positively charged glycoglycerolipids (Fig. 4b); (3) polycationic glycerolipids (Fig. 4c).
Structural and functional studies were conducted for each group, which investigated the contribution of individual domains to cytotoxicity. The analysis of the dependence between activity and structure is largely complicated by the fact that tumor cells of different origins differ significantly in their sensitivity to lipidotherapy. In addition, an increase in toxicity caused by a change in the lipid structure sometimes reduces the selectivity of its action.
Positively Charged Glycerolipids
The long-term structural and functional investigations contributed to the elaboration of alkyl cationic glycerolipids, a class of non-phosphorus analogs of edelfosine [53]. These compounds are diverse in the structure; the common feature is the presence of a positively charged head and hydrophobic substituents placed on the glycerol matrix (Fig. 4a). There are glycerolipids with a cationic head attached to the glycerol fragment directly or via a spacer group.
The analysis of the structure–activity relationship showed that the length of the substituent chain at the C1 and C2 glycerol atoms, the type of cationic head and the presence of a spacer group, which separates the head from the glycerol fragment, have a significant effect on the activity of the lipid [19, 54]. The following fragments in the structure of alkyl cationic glycerolipids are essential for the cytotoxic activity [19]: (1) a long-chain alkyl substituent in the C1 position of the glycerol backbone (14–19 carbon atoms); (2) a short-chain alkyl substituent at the C2 position (1–2 carbon atoms); (3) a cationic head represented by heterocyclic and aliphatic amines with a range of different functional groups and attached directly to the C3 atom of the glycerol backbone or via an acyl-type spacer group.
The action of cationic alkyl glycerolipids is not fully understood, but it is assumed that it involves endocytosis and glycerolipid entry into early endosomes [55]. It has been established that antitumor glycerolipids are able to integrate into the membranes of tumor cells and cause biophysical (changes in the fluidity of the plasma membrane) and biochemical changes, leading to cell lysis [56].
The main reason for a special attention to phosphorus-free alkyl type cationic glycerolipids is that they have only a minor negative effect on nontumor cells (including blood cells), which makes these compounds safe [57].
New cationic glycerolipids with various aliphatic or heterocyclic amines were obtained and studied. The data presented in Table 1 for phosphorus-free glycerolipids (Fig. 4a) indicate that colon adenocarcinoma cell line НСТ116 is sensitive to all lipids (XIIa–XIIg) [36, 53, 57]. Compounds with methylimidazolium (XIIe) and ethylimidazolium (XIIf) cationic heads showed the highest activity. The IC50 values for the rest of the studied compounds did not exceed 20 µM.
The ovarian adenocarcinoma cell line SKOV3 was found to be less susceptible to compounds (XIIa–XIIc). Acceptable results were shown for lipids with pyridinium (XIId), methylimidazolium (XIIe), ethylimidazolium (XIIf), and dimethyl-2-hydroxyethylammonium (XIIg) fragments (IC50 does not exceed 13 µM). The IC50 values for the remaining compounds were more than 35 µM, which indicates an insignificant cytotoxic effect of these compounds against SKOV3 line.
Lipids with pyridinium (XIId) and dimethyl-2-hydroxyethylammonium (XIIg) polar heads displayed a significant cytotoxic effect against breast cancer cell line MCF-7, the most resistant cell to the tested lipids. The rest phosphorus-free compounds had IC50 values higher than 20 µM. For MCF-7, lipids with the imidazolium group in the polar domain (XIIe, XIIf) were found to be low-toxic, and lipids with the methylpiperidinium (XIIa), methyl-4–hydroxypiperidinium (XIIb) and methylmorpholinium (XIIc) groups did not kill 50% of cells in the range of the concentrations used (0.1–50.0 µM).
In terms of B16 cells (mouse melanoma), lipids with heterocyclic pyridinium (XIId), methylimidazolium (XIIe), ethylimidazolium (XIIf), and dimethyl-2-hydroxyethylamonium (XIIg) heads were found to be more toxic than compounds with methylpiperidinium (XIIa), methyl-4-hydroxypiperidinium (XIIb), and methylmorpholinium (XIIc) cationic heads.
In contrast to the listed adhesion cultures, all the studied lipids, except for compounds with methylpiperidinium (XIIa), methyl-4-hydroxypiperidinium (XIIb) and especially with methylmorpholinium (XIIc) groups, had a pronounced cytotoxic effect on suspension (leukemia) cells (HL60 line), which insignificantly exceeded the cytotoxicity of edelfosine (V). A compound with a methylimidazolium (XIIe) heterocyclic head was the most active compound against the HL60 line. It was found that the quaternary ammonium group is necessary for the manifestation of activity, whereas the hydroxyl group in the cationic head reduces the cytotoxicity of the compounds. The action of this group of compounds on the K562 line has not been investigated in any studies.
The study of the effect on non-tumor cells (fibroblasts) showed that 50% cell death occurs in response to the action of lipids (XIId–XIIg) in concentrations exceeding 25 µM [53]. A weak effect on fibroblasts may indicate the selectivity of non-phosphorus alkyl glycerolipids to tumor cells. This important property distinguishes this chemotype from edelfosine (V) [53].
Positively Charged Glycoglycerolipids
Glycosylated antitumor ether lipids (GAELs) are a class of synthetic antitumor lipids in which the sugar moiety replaces the phosphocholine fragment of edelfosine (V). GAELs are glycolipids with a significant cytotoxicity for a range of tumor models [58, 59]. These compounds induce cancer cell death in an apoptosis-independent manner and eliminate tumor stem cells. The cytotoxicity of some GAELs exceeds that of edelfosine (V), i.e., the most studied representative of nonglycosylated alkyl lipids [60].
The molecular actions of GAELs have not been elucidated comprehensively. GAELs have been shown to cause nonapoptotic death in stem tumor cells. Hence, GAELs hold promise in overcoming drug resistance [58, 59, 61, 62]. Glycosylated lipids enter breast cancer cells by endocytosis and are incorporated into early endosomes [55, 61]. GAELs prevent the maturation of endocytic vesicles, which leads to the formation of large acidic vacuoles (possibly via paraptosis). Apparently, the release of cathepsins from vacuoles into the cytosol induces cell death in a way that differs from classic apoptosis [61].
One of the reasons for studying GAELs was the observation that certain plant species (for example, oats) are able to partially replace phosphoglycerolipids with glycoglycerolipids (lipids based on mono- or oligosaccharides) as a response to phosphate starvation [63]. This observation suggests that a glycerolipid containing a carbohydrate unit can mimic phosphocholine-glycerolipids due to the polarity of the carbohydrate group [63].
One of the directions in the search for new antitumor glycerolipids is associated with a change in the positions of a cationic domain, glycerol backbone, and a carbohydrate fragment (Fig. 4b). The carbohydrate unit may be located in the center of the molecule (XIII–XV) or occupy a terminal position (XVI). The carbohydrate fragment consists of glucose, galactose, mannose, and glucosamine residues. The cationic head is represented by heterocyclic bases, similar to cationic glycerolipids (Fig. 4b) [64].
Table 1 shows that glycolipids that include mannose with a pyridinium cationic head (XIIIa) showed the greatest activity on K562 and HCT116 cells [64]. Mannose glycerolipid with a methylimidazolium cationic head (XIIIb), galactoglycerolipids (XIVa, XIVb), and glucosamine glycerolipids (XVa, XVb) (our unpublished data) demonstrated good results on K562, HL60, HCT116 and B16 cell lines. These compounds showed significantly better results compared to compounds with a terminal location of carbohydrate residue (XVIa–XVIc, Fig. 4b) [64, 65]. Therefore, subsequent development of mannose-containing GAELs appears reasonable.
Polycationic Glycerolipids
It has been proposed to use polyamines with an open or substituted terminal group as the cationic domains of glycerolipids. This choice is explained by the structure and biological properties of polyamines. This class includes natural compounds putrescine, spermine, and spermidine. The positive charge of the four amino groups allows spermine and spermidine to interact with negatively charged molecules: nucleic acids, ATP, phospholipids, and proteins [66, 67]. Most of the polyamines exist in the bound state with RNA [68]. Spermine and spermidine are able to remodel chromatin and thus affect gene expression [69, 70]. The inhibition of enzymes involved in the biosynthesis and metabolism of polyamines using antimetabolites-mimetics slows down cell proliferation. Thus, the inhibition of ornithine decarboxylase by the addition of α-difluoromethylornithine decreased the level of intracellular spermine to a half of control cells and reduced the rate of cell proliferation [71].
Therefore, the use of polyamines and their derivatives as positively charged substituents in glycerolipids appears advantageous in terms of the search for new antitumor compounds [72]. Modified polyamines are investigated as inhibitors of polyamine transport systems [73] and enzymes of their biosynthesis [74–76]. In this review, we consider alkyl polycationic glycerolipids (Fig. 4c), in which the cationic domain is represented by a residue of unprotected or terminally alkylated tetraamine.
Glyceropolyamines (XVIIa–XVIIi) have a strong cytotoxic effect (IC50 < 6 µM) on tumor cells of various tissue origins, including chronic myeloid leukemia K562/4 subline resistant to doxorubicin and other drugs transported by P-glycoprotein (Table 1), as well as on nontumor human embryonic fibroblasts [77].
Based on an analysis of the influence of the alkyl substituent length in the first position of glycerol in glyceropolyamines (Table 1), it is noted that for the K562, НСТ116, SKOV3, and MCF-7 cell lines, the chain lengthening from 10 (XVIIc) to 16 (XVIIf) carbon atoms has an insignificant effect on cytotoxicity (IC50 = 1.82–4.88 µM). Further lengthening of the chain to 18 carbon atoms (XVIIg) reduces cytotoxicity (no data available for the SKOV3 line). The presence of an ethyl group at the terminal nitrogen atom of polyamine slightly reduces the value of IC50, i.e., increases the efficacy of compounds (XVIIc–XVIIg, XVIIi). This effect may be due to the fact that the terminal ethyl group prevents potential acylation and further oxidation of the compound, which increases its stability in cells [78].
NON-PHOSPHORUS NEUTRAL GLYCOGLYCEROLIPIDS
Neutral glycoglycerolipids do not contain a cationic head, but remain amphiphilic due to the hydrophilic carbohydrate unit. This chemotype represents interest for targeted drug delivery. For example, D-galactose and D-mannose serve as carbohydrate ligands recognized by the liver and dendritic cell receptors [79–81].
Neutral glycoglycerolipids contain both alkyl and acyl residues, which have different localizations in the glycerol backbone and carbohydrate fragment. The structural domains are represented by the glycosyl unit and long-chain alkyl and acyl residues located on the glycerol skeleton or on the carbohydrate unit. The classification of these lipids is based on the nature of long-chain substituents, their localization on the glycosylglycerol matrix, and the distance of the carbohydrate unit to the glycerol backbone. Therefore, synthetic glycoglycerolipids are divided into:
(1) Alkyl glycoglycerolipids:
(a) Alkyl glycoglycerolipids with a carbohydrate fragment directly attached to the glycerol backbone.
(b) Alkyl glycoglycerolipids with a carbohydrate fragment attached via a spacer group.
(2) Acyl glycoglycerolipids.
(3) Combined glycoglycerolipids (contain alkyl and acyl residues).
Alkyl Glycoglycerolipids
Neutral glycoglycerolipids (GAELs) are the closest analogs of natural glycoglycerolipids with high bioavailability, which increases their clinical significance. It was shown [55] that cell death under the action of GAELs does not occur via classical apoptosis, which distinguishes GAELs from non-phosphorus alkyl glycerolipids. In addition, an important advantage of neutral GAELs is related to the almost complete lack of hemolytic activity [82].
Alkyl glycoglycerolipids with a carbohydrate fragment directly attached to the glycerol backbone. The carbohydrate fragment of compounds (XVIIIa–XVIIIf) includes D-mannose, D-galactose, D-glucosamine, N-acetyl-D-glucosamine, D-galactosamine, and N-acetyl-D-galactosamine residues attached to the C3 atom of glycerol (Fig. 5a). Compounds (XVIIIа, XVIIIb) demonstrated similar cytotoxicity and selectivity to the K562 leukemia cell line (Table 2 [82]): The IC50 values of the two compounds are lower in comparison with the values obtained for other cell lines and lower than the corresponding values for edelfosine (V) (Table 1).
Compounds (XVIIIc–XVIIIf) are alkyl aminoglycosides containing residues of D-glucosamine and D-galactosamine (XVIIIc, XVIIIe) or N-acetyl-D-glucosamine and N-acetyl-D-galactosamine (XVIIId, XVIIIf) (our unpublished data are provided for compounds (XVIIId, XVIIIf).
Glycerolipids (XVIIIc–XVIIIf) have a similar cytotoxic effect on the K562 cell line (IC50 values are in the range of 4–8 µM). The most active compound is (XVIIIe) with the D-galactosamine residue, while its acetylated derivative (XVIIIf) shows lower activity. Acetylation of the amino group for both glucose (XVIIId) and galactose derivatives (XVIIIf) decreases toxicity of the compounds. Compounds (XVIIIс) and (XVIIIe) were more active on MCF-7 cells than the other representatives of this group. Glycerolipids (XVIIIc–XVIIIf) were less active on the HCT116 and SKOV3 cell lines, but also showed similar results (IC50 were in the range of 9–16 µM).
Alkyl glycoglycerolipids with the carbohydrate fragment attached via a spacer group. In the compounds (XIXa–XIXf) (Fig. 5a), the carbohydrate residue is attached to the glycerol unit by spacer groups of different lengths, which makes it possible to analyze the effect of the distance of the carbohydrate fragment to the diglyceride backbone on the cytotoxic properties of neutral alkyl glycoglycerolipids.
In comparison with compounds (XVIIIa, XVIIIb) [82], glycoglycerolipids containing a spacer group were less active for the K562 line and did not show selectivity. Compound (XIXa) with a D-mannose residue attached via a dimethylene spacer showed higher toxicity to nontumor cells (IC50 = 12.5 ± 5.2 µM on fibroblasts) than its analog with a tetramethylene spacer (XIXe) (IC50 > 50 µM).
The best results were shown for compound (XIXс) with a D-mannose residue attached via a trimethylene spacer on the HL60, HCT116, and B16 cell lines. Among the compounds containing the D-galactose residue (XIXb, XIXd, XIXf), glycoglycerolipids linked to galactose via a di- or trimethylene spacer (XIXb, XIXd) were the most active against HCT116, B16 and K562 cells (only for compound (XIXd)). Glycoglycerolipids with tetramethylene spacer (XIXe, XIXf) showed the worst results on the studied cell lines, regardless of the type of carbohydrate residue.
While summarizing the data on neutral GAELs, it is concluded that GAELs in which the D-mannose residue is attached either directly to the glycerol skeleton (XVIIIa) (for K562 cells) or via a trimethylene spacer group (XIXc) (Table 2) demonstrate high activity. Compounds with dimethylene spacer (XIXa, XIXb) exhibit moderate activity on the studied cell lines. The mean IC50 values for the group of compounds (XVIII) were lower than for the group of compounds (XIX), which indicates that the spacer has a negative effect on the cytotoxic properties of the considered glycoglycerolipids.
Acyl Glycoglycerolipids
Compounds (XXa) and (XXb) are neutral glycosylated glycerolipids containing a D-galactose residue as a carbohydrate fragment and differ by the unsaturation degree of acyl substituents at the C1 and C2 positions of glycerol (linoleic and oleic acid residues) (Fig. 5b). We present our data regarding acyl glycoglycerolipids.
The cytotoxicity of glycosylated acyl glycerolipid with linoleic acid residue (XXb) on the K562 line was insignificantly higher than that of edelfosine (V) (Table 1). The compound with oleic acid residue (XXa) showed marked selectivity for the ovarian cancer line SKOV3 and IC50 similar to that of edelfosine (V). Both compounds were less active on HCT116 cells, but on the p53 gene knockout subline, compound (XXb) with two unsaturated double bonds was significantly more active (IC50 = 13.6 ± 0.21) than compound (XXa) containing one unsaturated double bond (IC50 > 50 µM). The p53 protein plays a major role in apoptosis [83]. Hence, it is assumed that the mechanism of action associated with the polyunsaturated acyl residue of compound (XXb) involves apoptosis, which distinguishes this compound from other GAELs that cause non-apoptotic cell death.
Combined Glycoglycerolipids Containing Alkyl and Acyl Residues
Combined medications (prodrugs) are compounds that have low pharmacological activity or lack any, but they are metabolized in vivo into a pharmacologically active drug [84]. Using the physicochemical properties of liposomes, which incorporate prodrugs, and the pathophysiological characteristics of tumors, it is possible to trigger the release and activation of ether lipids (AELs) in a tumor. This is achieved with the help of polymer-coated liposomes, which contain ether pro-lipids (proAELs) [85]. Pro-lipids are metabolized into active drugs upon hydrolysis by phospholipase A2 (sPLA2) in a tumor [86–88]. Phospholipids are hydrolyzed by sPLA2 to form lysophospholipids and free fatty acids [86]. One of the most attractive substances is lysophosphatidic acid (LPA, (XXIII), Fig. 6), a natural phospholipid that functions as a bioactive lipid mediator and secondary messenger. Compound (XXIII) regulates the proliferation, migration, and survival of tumor cells.
The ability of tumor cells to acquire resistance to clinical drugs reduces the efficacy of antitumor therapy [2]. Such resistance commonly develops to multiple drugs and causes insensitivity to many anticancer drugs [3]. Creation of antitumor agents whose components induce various mechanisms of cell death may help overcome drug resistance [50, 89].
As mentioned above, GAELs activate tumor cell death via the non-apoptotic pathway, and the presence of a glycosyl residue in the structure of glycerolipids may affect amphiphilic property of the molecules, which is necessary to improve the biological activity and pharmacokinetics.
A combination of antitumor actions may elicit an additive or synergistic effect [89]. Modification of antitumor agents by conjugation with a fatty acid can increase selectivity and yield new hybrid structures with optimized biological activity. Modified fatty acids may improve efficacy of chemotherapy and make it less toxic [90]. In this regard, it appears attractive to create alkyl–acyl glycoglycerolipids containing alkyl and acyl residues, which differ by saturation and length in the diglyceride or by carbohydrate domains.
Compounds (XXIa–XXIc) are alkyl glycoglycerolipids linked to fatty acid residues, which are localized at the C6 position of the carbohydrate domain (Fig. 5c). The concept of such molecular engineering is based on that the ester bond is expected to be degraded by cellular esterases to yield two antitumor agents that trigger different ways of cell death.
The cytotoxicity of glycoglycerolipids (XXIa–XXIc) on the K562, HCT116, MCF-7, and SKOV3 lines was unsatisfactory (IC50 > 50 µM) (Table 2). This indicates that this combination of structural elements is significantly inferior to compounds (XVIIIa, XVIIIb) without an acyl residue at the C6 position.
ω3-Polyunsaturated fatty acids are also used in chemotherapy [91]. The key mechanism proposed for overcoming multidrug resistance is based on the effect of ω3-acids on the structure of the plasma membrane and transmembrane transport [93] and on the presence of tissue specificity depending on the structure [90, 92, 93]. In addition, they may slow down the growth of tumors, cause apoptosis or inhibit angiogenesis [91, 94].
Combined antitumor lipids upon intracellular cleavage may exhibit additive effects due to a combination of the following properties: 1) lysolipids are cytotoxic in micromolar concentrations; 2) fatty acids may inhibit the proliferation of tumor cells and increase their sensitivity to antitumor drugs; 3) fatty acids and lysolipids may reduce the permeability barrier of lipid membranes [88].
Lysoglycerolipid (XXIIa) and its derivatives with mono- (XXIIb) and diunsaturated (XXIIc) fatty acids at the C2 position of glycerol were synthesized, and their cytotoxicity was studied (Fig. 5c, Table 2). The compounds demonstrated the greatest effect on the K562 and HL60 leukemia cell lines (our unpublished data). In the A375, MCF-7, HBL100, MDA-MB-231, and HCT116 tumor cell lines, as well as in nontumor fibroblasts, the IC50 values exceeded 50 µM. The presence and unsaturation degree of fatty acids at the C2 position of glycerol affect cytotoxicity, but both modifications are important for selectivity to leukemia cells. Replacement of the monounsaturated oleic acid residue with diunsaturated linoleic acid enhances the cytotoxic effect on the K562 cells, and lack of a substituent at C2 or the presence of a more saturated substituent improve cytotoxic efficacy for the HL60 line.
CONCLUSIONS
At the initial stage of the study of antitumor lipids, the main investigated compounds were phosphorus-containing glycerolipids. Edelfosine remains the gold standard because of its high activity. However, the consideration of edelfosine as the gold standard among antitumor lipids requires revision, since its toxicity to normal cells and low selectivity do not allow one to obtain an acceptable therapeutic window for this substance, i.e., the relation between the amount of edelfosine that causes the therapeutic effect and the amount that causes toxicity. In addition, it has low bioavailability, instability, slow excretion from the body, and a high hemolytic effect.
Among non-phosphorus lipids, there are individual chemotypes (for example, positively charged glycerolipids), which, along with antineoplastic activity comparable to edelfosine, cause minimal damage to non-tumor cells. Some compounds (cationic glycolipids, neutral glycoglycerolipids, etc.) demonstrated specificity for leukemia, which may open up new prospects for the development of drugs for the treatment of this disease. Despite the relatively low antitumor effect of phosphorus-free lipids, their advantage is low toxicity to non-tumor cells. In addition, individual compounds (cationic glycerolipids) are more active than edelfosine and cause less damage to red blood cells.
The elaboration of non-phosphorus analogs of edelfosine and their further modifications is a promising direction in the search for new candidate antitumor agents among representatives of this class of chemical compounds.
REFERENCES
Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A., and Bray, F., CA Cancer J. Clin., 2021, pp. 1–41. https://doi.org/10.3322/caac.21660
Mansoori, B., Mohammadi, A., Davudian, S., Shirjang, S., and Baradaran, B., Adv. Pharm. Bull., 2017, vol. 7, pp. 339–348. https://doi.org/10.15171/apb.2017.041
Tan, B.L. and Norhaizan, M.E., Molecules, 2019, vol. 24, p. 2527. https://doi.org/10.3390/molecules24142527
Gulyakin, I.D., Oborotova, N.A., and Pechennikov, V.M., Pharm. Chem. J., 2014, vol. 48, pp. 209–213. https://doi.org/10.1007/s11094-014-1078-7
Burton, P.S., J. Pharm. Exp. Ther., 2002, vol. 303, pp. 889–895. https://doi.org/10.1124/jpet.102.035006
Utreja, P., Jain, S., and Tiwary, A., Curr. Drug Delivery, 2010, vol. 7, pp. 152–161. https://doi.org/10.2174/156720110791011783
Piao, X., Yin, H., Guo, S., Wang, H., and Guo, P., Adv. Sci., 2019, vol. 6, p. 1900951. https://doi.org/10.1002/advs.201900951
Tewari, D., Rawat, P., and Singh, P.K., Food Chem. Toxicol., 2019, vol. 123, pp. 522–535. https://doi.org/10.1016/j.fct.2018.11.041
Oun, R., Moussa, Y.E., and Wheate, N.J., Dalton Transaction, 2018, vol. 7, pp. 6645–6653. https://doi.org/10.1039/c8dt00838h
Munder, P.G., Ferber, E., Modolell, M., and Fischer, H., Int. Arch. Allergy Appl. Immunol., 1969, vol. 36, pp. 117–128. https://doi.org/10.1159/000230731
Mulder, E. and van Deenen, L.L., Biochim. Biophys. Acta, 1965, vol. 106, pp. 348–356. https://doi.org/10.1016/0005-2760(65)90043-3
Iatrou, C., Frangia, C., and Demopoulos, C.A., Infect. Disord. Drug Targ., 2009, vol. 9, pp. 390–399. https://doi.org/10.2174/187152609788922555
Jancar, S. and Chammas, R., Curr. Drug Targ., 2014, vol. 15, pp. 982–987. https://doi.org/10.2174/1389450115666140903111812
Onuchic, A.C., Machado, C.M.L., Saito, R.F., Rios, F.J., Jancar, S., and Chammas, R., Med. Inflam., 2012, pp. 1–6. https://doi.org/10.1155/2012/175408
Silva, JuniorI., Andrade, L.N., Jancar, S., and Chammas, R., Clinics, 2018, vol. 73. https://doi.org/10.6061/clinics/2018/e792s
Kozlovskii, V.I., Kovtun, O.M., Seroukhova, O.P., Detkovskaya, I.N., and Kozlovskii, I.V., Vestn. Viteb. Gos. Med. Univ., 2013, vol. 12, no. 4, pp. 79–91.
Mollinedo, F., de la Iglesia-Vicente, J., Gajate, C., de Mendoza, A.E.H., Villa-Pulgarin, J.A., Frias, M., Roué, G., Gil, J., Colomer, D., Campanero, M.A., and Blanco-Prieto, M.J., Clin. Cancer Res., 2010, vol. 16, pp. 2046–2054. https://doi.org/10.1158/1078-0432.ccr-09-2456
Rahmani, M., Reese, E., Dai, Y., Bauer, C., Payne, S.G., Dent, P., Spiegel, S., and Grant, S., Cancer Res., 2005, vol. 65, pp. 2422–2432. https://doi.org/10.1158/0008-5472.can-04-2440
Markova, A.A., Plyavnik, N.V., Pletneva, M.V., Serebryannikova, G.A., and Shtil’, A.A., Klin. Onkogematol., 2012, vol. 5, no. 2, pp. 141–143.
Berdel, W.E., Fink, U., and Rastetter, J., Lipids, 1987, vol. 22, pp. 967–969. https://doi.org/10.1007/bf02535566
Drings, P., Gunther, I., Gatzemeier, U., Ulbrich, F., Khanavkar, B., Schreml, W., Lorenz, J., Brugger, W., Schick, H., Pawel, J., and Nordstrom, R., Oncol. Res. Treat., 1992, vol. 15, pp. 375–382. https://doi.org/10.1159/000217391
Chee, K.G., Longmate, J., Quinn, D.I., Chatta, G., Pinski, J., Twardowski, P., Pan, C.X., Cambio, A., Evans, C.P., Gandara, D.R., and Lara, P.N., Clin. Genit. Cancer, 2007, vol. 5, pp. 433–437. https://doi.org/10.3816/CGC.2007.n.031
Munder, P.G., Modolell, M., Andreesen, R., Weltzien, H.U., and Westphal, O., in Immunostimulation, Chedid, L., Miescher, P.A., and Mueller-Eberhard, H.J, Eds., Berlin: Springer, 1980m pp. 177–193. https://doi.org/10.1007/978-3-642-67809-7_12
Tarnowski, G.S., Mountain, I.M., Stock, C.C., Munder, P.G., Weltzien, H.U., and Westphal, O., Cancer Res., 1978, vol. 38, pp. 339–344.
Müller, E., Dupuis, G., Turcotte, S., and Rola-Pleszczynski, M., Biochem. Biophys. Res. Commun., 1991, vol. 181, pp. 1580–1586. https://doi.org/10.1016/0006-291x(91)92119-5
Mollinedo, F., Fernandez-Luna, J.L., Gajate, C., and Martin-Martin, B., Cancer Res., 1997, vol. 57, pp. 1320–1328.
Ausili, A., Torrecillas, A., Aranda, F.J., Mollinedo, F., Gajate, C., Corbalán-García, S., Godos, A., and Gómez-Fernández, J.C., J. Phys. Chem. B, vol. 112, pp. 11643–11654. https://doi.org/10.1021/jp802165n
Kostadinova, A., Topouzova-Hristova, T., Momchilova, A., Tzoneva, R., and Berger, M.R., Adv. Protein Chem. Struct. Biol., 2015, vol. 101, pp. 27–66. https://doi.org/10.1016/bs.apcsb.2015.08.001
Van der Luit, A.H., Budde, M., Ruurs, P., Verheij, M., and Blitterswijk, W.J., J. Biol. Chem., 2002, vol. 277, pp. 39541–39547. https://doi.org/10.1074/jbc.m203176200
Gajate, C. and Mollinedo, F., Anticancer Agents Med. Chem., 2014, vol. 14, pp. 509–527. https://doi.org/10.2174/1871520614666140309222259
Gajate, C. and Mollinedo, F., Blood, 2007, vol. 109, pp. 711–719. https://doi.org/10.1182/blood-2006-04-016824
Hąc-Wydro, K. and Dynarowicz-Łątka, P., Colloids Surf., 2010, vol. 76, pp. 366–369. https://doi.org/10.1016/j.colsurfb.2009.10.012
Farooqui, A.A., Farooqui, T., and Horrocks, L.A., in Metabolism and Functions of Bioactive Ether Lipids in the Brain, New York, NY: Springer, 2008, pp. 219–235. https://doi.org/10.1007/978-0-387-77401-5_11
Bazill, G.W. and Dexter, T.M., Cancer Res., 1990, vol. 50, pp. 7505–7512.
de Mendoza, A.E.H., Campanero, M.A., de la Iglesia-Vicente, J., Gajate, C., Mollinedo, F., and Blanco-Prieto, M.J., Clin. Cancer Res., 2009, vol. 15, pp. 858–864. https://doi.org/10.1158/1078-0432.ccr-08-1654
Plyavnik, N., Shtil, A., and Serebrennikova, G., Min. Rev. Med. Chem., 2006, vol. 6, pp. 533–542. https://doi.org/10.2174/138955706776876221
Brachwitz, H. and Vollgraf, C., Pharm. Ther., 1995, vol. 66, pp. 39–82. https://doi.org/10.1016/0163-7258(95)00001-w
Arnold, B., Reuther, R., and Weltzien, H.U., Biochim. Biophys. Acta. Lipids Lipid Metab., 1978, vol. 530, pp. 47–55. https://doi.org/10.1016/0005-2760(78)90125-x
Lasa-Saracíbar, B., Estella-Hermoso de Mendoza, A., Mollinedo, F., Odero, M.D., and Blanco-Príeto, M.J., Cancer Lett., 2013, vol. 334, pp. 302–310. https://doi.org/10.1016/j.canlet.2013.01.018
Smorenburg, C.H., Seynaeve, C., Bontenbal, M., Planting, A.S., and Verweij, J., Anticancer Drugs, 2000, vol. 11, pp. 825–828. https://doi.org/10.1097/00001813-200011000-00006
de Mendoza, A.E.H., Préat, V., Mollinedo, F., and Blanco-Prieto, M.J., J. Controlled Release, 2011, vol. 156, pp. 421–426. https://doi.org/10.1016/j.jconrel.2011.07.030
Lasa-Saracibar, B., Aznar, M.Á., Lana, H., Aizpún, I., Gilb, A.G., and Blanco-Prietoa, M.J., Int. J. Pharmacol., 2014, vol. 474, pp. 1–5. https://doi.org/10.1016/j.ijpharm.2014.07.053
Shim, G., Yu, Y.H., Lee, S., Kim, J., and Oh, Y.K., Asian J. Pharm. Sci., 2016, vol. 11, pp. 596–602. https://doi.org/10.1016/j.ajps.2016.05.003
Herrmann, D.B.J., Pahlke, W., Opitz, H.G., and Bicker, U., Cancer Treat. Rev., 1990, vol. 17, pp. 247–252. https://doi.org/10.1016/0305-7372(90)90055-k
Giantonio, B.J., Derry, C., McAleer, C., McPhillips, J.J., and O’Dwyer, P.J., Clin. Cancer Res., 2004, vol. 10, pp. 1282–1288. https://doi.org/10.1158/1078-0432.ccr-0837-02
Yanapirut, P., Berger, M.R., Reinhardt, M., and Schmahl, D., Arzneimittelforschung, 1991, vol. 41, pp. 652–655.
Kötting, J., Marschner, N.W., Neumuller, W., Unger, C., and Eibl, H., in Alkylphosphocholines: New Drugs in Cancer Therapy, Eibl, H., Hilgard, C., and Unger, C., Eds., Prog. Tumor Res., Basel: Karger, 1992, vol. 34, pp. 131–142.
Escobar, P., Matu, S., Marques, C., and Croft, S.L., Acta Trop., 2002, vol. 81, pp. 151–157. https://doi.org/10.1016/S0001-706X(01)00197-8
Vink, S.R., Schellens, J.H.M., van Blitterswijk, W.J., and Verheij, M., Inv. New Drugs, 2005, vol. 23, pp. 279–286. https://doi.org/10.1007/s10637-005-1436-0
Gills, J.J. and Dennis, P.A., Curr. Oncol. Rep., vol. 11, pp. 102–110. https://doi.org/10.1007/s11912-009-0016-4
Rübel, A., Handrick, R., Lindner, L.H., Steiger, M., Eibl, H., Budach, W., Belka, C., and Jendrossek, V., Radiat. Oncol., 2006, vol. 1, p. 6. https://doi.org/10.1186/1748-717x-1-6
Erdlenbruch, B., Jendrossek, V., Marx, M., Hunold, A., Eibl, H., and Lakomek, M., Anticancer Res., 1998, vol. 18, pp. 2551–2557.
Markova, A.A., Plyavnik, N.V., Morozova, N.G., Maslov, M.A., and Shtil, A.A., Russ. Chem. Bull., 2014, vol. 63, pp. 1081–1087. https://doi.org/10.1007/s11172-014-0552-4
Romanova, S.G., Romanov, V.G., Serebrennikova, G.A., and Shtil’, A.A., Biomed. Khim., 2010, vol. 56, no. 4, pp. 457–470.
Gilbert, A. and Bittman, R., Anticancer Agents Med. Chem., 2014, vol. 14, pp. 592–606. https://doi.org/10.2174/1871520614666140309231144
Jaffrès, P.A., Gajate, C., Bouchet, A.M., Couthon-Gourvès, H., Chantôme, A., Potier-Cartereau, M., Besson, P., Bougnoux, P., Mollinedo, F., and Vandier, C., Pharmacol. Ther., 2016, vol. 165, pp. 114–131. https://doi.org/10.1016/j.pharmthera.2016.06.003
Markova, A.A., Pliavnik, N.V., Tatarskii, V.V., Shtil’, A.A., and Serebrennikova, G.A., Russ. J. Bioorg. Chem., 2010, vol. 36, pp. 532–534. https://doi.org/10.1134/S106816201004014X
Ogunsina, M., Samadder, P., Idowu, T., Arthur, G., and Schweizer, F., J. Med. Chem., 2017, vol. 60, pp. 2142–2147. https://doi.org/10.1021/acs.jmedchem.6b01773
Idowu, T., Samadder, P., Gilbert, A., and Schweizer, F., J. Med. Chem., 2017, vol. 60, pp. 9724–9738. https://doi.org/10.1021/acs.jmedchem.7b01198
Marino-Albernas, J.R., Bittman, R., Peters, A., and Mayhew, E., J. Med. Chem., 1996, vol. 39, pp. 3241–3247. https://doi.org/10.1021/jm960164j
Samadder, P., Bittman, R., Byun, H.-S., and Arthur, G., Biochem. Cell Biol., 2009, vol. 87, pp. 401–414. https://doi.org/10.1139/o08-147
Ogunsina, M., Samadder, P., Idowu, T., Nachtigal, M., Schweizer, F., and Gilbert, A., Molecules, 2020, vol. 25, p. 566. https://doi.org/10.3390/molecules25030566
Andersson, M.X., Larsson, K.E., Tjellstrom, H., Liljenberg, C., and Sandelius, A.S., J. Biol. Chem., 2005, vol. 280, pp. 27578–27586. https://doi.org/10.1074/jbc.M503273200
Morozova, N.G., Shmendel, E.V., Timofeev, G.A., Ivanov, I.V., Kubasova, T.S., Plyavnik, N.V., Markova, A.A., Maslov, M.A., and Shtil, A.A., Mend. Commun., 2019, vol. 29, pp. 166–168. https://doi.org/10.1016/j.mencom.2019.03.016
Shmendel’, E.V., Perevoshchikova, K.A., Shishova, D.K., Kubasova, T.S., Tyutyunnik, L.L., Maslov, M.A., Morozova, N.G., and Shtil’, A.A., Izv. Akad. Nauk, Ser. Khim., 2015, no. 7, p. 1648.
Ramani, D., De Bandt, J.P., and Cynober, L., Clin. Nutr., 2014, vol. 33, pp. 14–22. https://doi.org/10.1016/j.clnu.2013.09.019
Moinard, C., Cynober, L., and Debandt, J., Clin. Nutr., 2005, vol. 24, pp. 184–197. https://doi.org/10.1016/j.clnu.2004.11.001
Igarashi, K. and Kashiwagi, K., Int. J. Biochem. Cell Biol., 2019, vol. 107, pp. 104–115. https://doi.org/10.1016/j.biocel.2018.12.012
Wang, J.Y., Inflammopharmacology, 2005, vol. 13, pp. 91–101. https://doi.org/10.1163/156856005774423890
Pasini, A., Caldarera, C.M., and Giordano, E., Amino Acids, 2013, vol. 46, pp. 595–603. https://doi.org/10.1007/s00726-013-1550-9
Terui, Y., Sakamoto, A., Yoshida, T., Kasahara, T., Tomitori, H., Higashi, K., Igarashi, K., and Kashiwagi, K., Amino Acids, 2015, vol. 47, pp. 345–356. https://doi.org/10.1007/s00726-014-1867-z
Casero, R.A., Murray Stewart, T., and Pegg, A.E., Nat. Rev. Cancer, 2018, vol. 18, pp. 681–695. https://doi.org/10.1038/s41568-018-0050-3
Palmer, A.J. and Wallace, H.M., Amino Acids, 2009, vol. 38, pp. 415–422. https://doi.org/10.1007/s00726-009-0400-2
Casero, R.A. and Woster, P.M., J. Med. Chem., 2009, vol. 52, pp. 4551–4573. https://doi.org/10.1021/jm900187v
Goyal, L., Supko, J.G., Berlin, J., Blaszkowsky, L.S., Carpenter, A., Heuman, D.M., Hilderbrand, S.L., Stuart, K.E., Cotler, S., Senzer, N.N., Chan, E., Berg, C.L., Clark, J.W., Hezel, A.F., Ryan, D.P., and Zhu, A.X., Cancer Chem. Pharm., 2013, vol. 72, pp. 1305–1314. https://doi.org/10.1007/s00280-013-2293-8
Xie, Y., Murray-Stewart, T., Wang, Y., Yu, F., Laurence, J.L., Robert, J.M., Casero, A., and Oupickýa, D., J. Controlled Release, 2017, vol. 246, pp. 110–119. https://doi.org/10.1016/j.jconrel.2016.12.017
Perevoshchikova, K.A., Nichugovskiy, A.I., Isagulieva, A.K., Morozova, N.G., Ivanov, I.V., Maslov, M.A., and Shtil, A.A., Mend. Commun., 2019, vol. 29, pp. 616–618. https://doi.org/10.1016/j.mencom.2019.11.003
Weisell, J., Hyvonen, M., Alhonen, L., Vepsalainen, J., Keinanen, T.A., and Khomutov, A.R., Curr. Pharm. Des., 2014, vol. 20, pp. 262–277. https://doi.org/10.2174/13816128113199990037
Huang, K.W., Lai, Y.T., Chern, G.J., Huang, S.F., Tsai, C.L., Sung, Y.C., Chiang, C.C., Hwang, P.B., Ho, T.L., Huang, R.L., Shiue, T.Y., Chen, Y., and Wang, S.K., Biomacromolecules, 2018, vol. 19, pp. 2330–2339. https://doi.org/10.1021/acs.biomac.8b00358
Liu, L., Zong, Z.M., Liu, Q., Jiang, S.S., Zhang, Q., Cen, L.Q., Gao, J., Gao, X.G., Huang, J.D., Liu, Y., and Yao, H., Biomaterials, 2018, vol. 184, pp. 20–30. https://doi.org/10.1016/j.biomaterials.2018.08.064
DeRossi, C., Bambino, K., Morrison, J., Sakarin, I., Villacorta-Martin, C., Zhang, C., Ellis, J.L., Fiel, M.I., Ybanez, M., Lee, Y.A., Huang, K., Yin, C., Sakaguchi, T.F., Friedman, S.L., Villanueva, A., and Chu, J., Hepatology, 2019, vol. 70, pp. 2107–2122. https://doi.org/10.1002/hep.30677
Morozova, N.G., Timofeev, G.A., Timakova, A.A., Shmendel, E.V., Kubasova, T.S., Tyutyunnik, L.L., Markova, A.A., Maslov, M.A., and Shtil, A.A., Mend. Commun., 2015, vol. 25, pp. 248–249. https://doi.org/10.1016/j.mencom.2015.07.003
Vogelstein, B., Lane, D., and Levine, A.J., Nature, 2000, vol. 408, pp. 307–310. https://doi.org/10.1038/35042675
Rautio, J., Meanwell, N.A., Di, L., and Hageman, M.J., Nat. Rev. Drug Discov., 2018, vol. 17, pp. 559–587. https://doi.org/10.1038/nrd.2018.46
Abe, T., Sakamoto, K., Kamohara, H., Hirano, Y., Kuwahara, N., and Ogawa, M., Int. J. Cancer, 1997, vol. 74, pp. 245–250. https://doi.org/10.1002/(sici)1097-0215(19970620)74:3<245::aid-ijc2>3.0.co;2-z
Andresen, T.L., Davidsen, J., Begtrup, M., Mouritsen, O.G., and Jorgensen, K., J. Med. Chem., 2004, vol. 47, pp. 1694–1703. https://doi.org/10.1021/jm031029r
Arouri, A. and Mouritsen, O.G., Eur. J. Pharm. Sci., 2012, vol. 45, pp. 408–420. https://doi.org/10.1016/j.ejps.2011.09.013
Kaleağasıoğlu, F., Zaharieva, M.M., Konstantinov, S.M., and Berger, M.R., Anti-Cancer Agents Med. Chem., 2019, vol. 19, pp. 66–91. https://doi.org/10.2174/1871520618666181012093056
Saputra, E.C., Huang, L., Chen, Y., and Tucker-Kellogg, L., Cancer Res., 2018, vol. 78, pp. 2419–2431. https://doi.org/10.1158/0008-5472.can-17-1201
Jóźwiak, M., Filipowska, A., Fiorino, F., and Struga, M., Eur. J. Pharmacol., 2020, vol. 871, p. 172937. https://doi.org/10.1016/j.ejphar.2020.172937
Zhu, S., Lin, G., Song, C., Wu, Y., Feng, N., Chen, W., He, Z., and Chen, Y.Q., Oncotarget, 2017, vol. 8, pp. 109135–109150. https://doi.org/10.18632/oncotarget.22629
Abel, S., Riedel, S., and Gelderblom, W., Proc. Nutr. Soc., 2014, vol. 73, pp. 361–367. https://doi.org/10.1017/S0029665114000585
Gu, Z., Shan, K., Chen, H., and Chen, Y.Q., Curr. Pharm. Rep., vol. 1, pp. 283–294. https://doi.org/10.1007/s40495-015-0043-9
Li, Z., Li, Q., Wang, G., Huang, Y., Mao, X., Zhang, Y., and Wang, X., Int. J. Clin. Exp. Med., 2019, vol. 12, pp. 403–412.
ACKNOWLEDGMENTS:
The authors thank Professor G.A. Serebrennikova, the founder of the Antitumor Lipid Group in Russia.
We thank Cand. Sci. (Biol.) A.A. Markova, V.V. Tatarskii and the team of the laboratory of Tumor Cell Death Mechanisms, N.N. Blokhin National Medical Research Center of Oncology for the critical discussion of this work.
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation (project no. 0706-2020-0019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Translated by M. Novikova
The article is published based on the materials of the report presented at the conference “Lipids 2021” (Moscow, October 11–13, 2021). Abbreviations: GAELs, glycosylated antitumor ether lipids; IC50, 50% growth inhibitory concentration; PAF, platelet-activating factor.
Rights and permissions
About this article
Cite this article
Varlamova, E.A., Isagulieva, A.K., Morozova, N.G. et al. Non-Phosphorus Lipids As New Antitumor Drug Prototypes. Russ J Bioorg Chem 47, 965–979 (2021). https://doi.org/10.1134/S1068162021050356
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021050356