Abstract
This work provides the first example of incorporating the thiazole moiety into the aminobenzylnaphthol i.e. Betti base. A series of novel synthesized Betti bases via a one-pot three-component reaction of 2-amino-5-methyl thiazole, 2-naphthol, and substituted aldehydes are reported. The formation of desired products was confirmed using various spectroscopic techniques. The derivatives were screened in vitro for antibacterial activities. Molecular docking was also performed to predict the possible mode of action of these derivatives. The docking analysis ascertained that these derivatives regulate the antimicrobial potential via inhibition of DNA gyrase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Betti reaction is a special case of the Mannich reaction that produces aminobenzylnaphthol, known as Betti base [1]. The Betti bases serve as an important class of organic scaffolds. The reaction successfully procures the evolution of a new carbon–carbon bond along with one chiral carbon under mild experimental conditions. These optically active Betti bases have developed their importance as a ligand in asymmetric synthesis [2, 3]. Recent investigations have revealed their significance in pharmaceutical chemistry [4]. Betti bases have shown bioactivities including antibacterial [5], antitumor [6], bradycardiac [7], anti-inflammatory, and anthelmintic activities [8].
Multicomponent reactions (MCRs) represent an extraordinary tool for the construction of diverse and complex organic molecules. MCRs have their own advantages of saving time, energy, and raw materials [9]. Originally Betti bases were prepared in two steps, by the reaction of aldehyde, ammonia, and 2-naphthol in an ethanolic solution of KOH [10]. In recent years, one-pot synthesis is implemented for these reactions, with some catalysts thus making its synthetic pathway easy and rapid.
A critical analysis of the aforementioned reaction has prompted us to motif a novel Betti bases with 2-amino-5-methylthiazole, 2-naphthol, and substituted aldehydes. Present work deals with the synthesis, characterization, and molecular docking of a series of novel Betti bases. These compounds were then tested for in vitro antibacterial activities.
EXPERIMENTAL
Chemicals and reagents were purchased from S.D. Fine (Mumbai, India) and Sigma-Aldrich (United States) chemical companies. All the reactions were monitored by using thin-layer chromatography using Merck silica gel 60 F254 plates (TLC). Melting points were recorded on an Analab melting point apparatus. NMR (1H and 13C) spectra were recorded in a DMSO-d6 solvent using Bruker Avance II (300 MHz) NMR spectrometer and TMS was taken as an internal standard. IR spectra were done by using Perkin Elmer, Frontier equipment with a diamond tip. Elemental analysis was performed on model EA300, Euro Vector.
General Procedure for the Synthesis of Betti Bases (IVa–j)
Equimolar concentrations of 5-methyl-thiazol-2-ylamine (I), substituted aromatic aldehyde (II), and 2-napthol (III) were taken in a round bottom flask and sequentially dissolved in glycerol. A catalytic amount of silica sulfuric acid (SSA) was added and the mixture was kept on a water bath at 70–80°C for 30 min. The progress of the reaction was monitored on the TLC plate. After completion, the reaction mixture was quenched in water which results in the formation of a sticky mass. This sticky mass was freed using a pet ether and recrystallized using methanol.
1-[(2-Chloro-phenyl)-(5-methyl-thiazol-2-ylamino)-methyl]-naphthalen-2-ol (IVa). mp 168°C yield = 89.58% color = light yellow. IR(νmax, cm–1): 3438 (‒OH), 3368 (–NH), 3070 (Ar–H), 1542 (C=N), 742 (C–Cl). 1H NMR (300 MHz, DMSO): δ 10.09 (s, 1H, –OH), 8.11 (d, J = 8.6 Hz, 1H, Ar–H), 7.82 (s, 1H, Ar–H), 7.76–7.63 (m, 3H, Ar–H), 7.39 (t, J = 7.7 Hz, 1H, Ar–H), 7.31–7.21 (m, 1H, Ar–H), 7.18 (d, J = 8.9 Hz, 2H, Ar–H), 7.10 (t, J = 6.9 Hz, 2H, Ar–H), 6.88 (s, 1H,–NH), 6.63 (s, 1H, –CH), 2.21 (s, 3H, ‒CH3). 13C NMR (75 MHz, DMSO): δ 167.12 (‒C=N), 158.41, 153.27, 134.83, 132.13, 129.27, 129.15, 128.46, 128.38, 128.25, 126.17, 123.44, 122.85, 122.39, 120.33, 118.85, 117.95, 115.04, 114.75, 49.83 (benzyl-C), 11.71 (–CH3). Elemental analysis for C21H17ClN2OS: C, 66.22; H, 4.50; N, 7.35; found C, 66.36; H, 4.42; N, 7.46.
1-[(3-Chloro-phenyl)-(5-methyl-thiazol-2-ylamino)-methyl]-naphthalen-2-ol (IVb). mp 162°C yield = 84.34% color = light yellow. IR (νmax, cm–1): 3445 (‒OH), 3364 (–NH), 3059 (Ar–H), 1544 (C=N), 730 (C–Cl). 1H NMR (300 MHz, DMSO): δ 10.21 (s, 1H, –OH), 7.91 (d, J = 11.9 Hz, 2H, Ar–H), 7.72 (dd, J = 16.0, 8.4 Hz, 2H, Ar–H), 7.42-7.11 (m, 7H, Ar–H), 6.97 (s, 1H, –NH), 6.65 (s, 1H, –CH), 2.22 (s, 3H, ‒CH3). 13C NMR (75 MHz, DMSO): δ 167.68 (‒C=N), 153.31, 145.17, 134.68, 133.32, 132.10, 129.47, 129.37, 128.72, 128.50, 126.35, 126.12, 124.77, 123.18, 122.54, 120.40, 119.05, 119.00, 53.55 (benzyl-C), 11.74 (–CH3). Elemental analysis for C21H17ClN2OS: C, 66.22; H, 4.50; N, 7.35; found C, 66.16; H, 4.24; N, 7.44.
1-[(4-Chloro-phenyl)-(5-methyl-thiazol-2-ylamino)-methyl]-naphthalen-2-ol (IVc). mp 145°C yield = 90.28% color = light yellow. IR (νmax, cm–1): 3423 (‒OH), 3321 (–NH), 3056 (Ar–H), 1579 (C=N), 717 (C–Cl). 1H NMR (300 MHz, DMSO): δ 10.10 (s, 1H, –OH), 7.97–7.83 (m, 4H, Ar–H), 7.71–7.23 (m, 7H, Ar–H), 6.89 (s, 1H, –NH), 6.65 (s, 1H, –CH), 2.21 (s, 3H, –CH3). 13C NMR (75 MHz, DMSO): δ 167.80 (–C=N), 153.36, 141.91, 134.69, 132.14, 130.75, 129.43, 128.50, 128.33, 126.31, 122.53, 120.41, 119.59, 119.14, 119.11, 53.66 (benzyl-C), 11.73 (–CH3). Elemental analysis for C21H17ClN2OS: C, 66.22; H, 4.50; N, 7.35; found C, 66.30; H, 4.64; N, 7.48.
1-[(5-Methyl-thiazol-2-ylamino)-(2-nitro-phenyl)-methyl]-naphthalen-2-ol (IVd). mp 190°C yield = 86.90% color = yellow. IR (νmax, cm–1): 3430 (–OH), 3347 (–NH), 2980 (Ar–H), 1587 (C=N), 1538 (‒NO2). 1H NMR (300 MHz, DMSO): δ 9.87 (s, 1H, –OH), 7.91 (dd, J = 18.7, 9.9 Hz, 2H, Ar–H), 7.81–7.04 (m, 10H, Ar–H and –NH), 6.65 (s, 1H, –CH), 2.22 (s, 3H, –CH3). 13C NMR (75 MHz, DMSO): δ 167.43 (–C=N), 164.19, 153.91, 149.28, 136.19, 134.98, 133.06, 131.72, 129.95, 129.49, 128.40, 127.60, 126.64, 123.92, 122.71, 122.52, 121.06, 119.01, 116.46, 52.08 (benzyl-C), 11.81 (–CH3). Elemental analysis for C21H17N3O3S: C, 64.43; H, 4.38; N, 10.73; found C, 64.58; H, 4.26; N, 10.80.
1-[(5-Methyl-thiazol-2-ylamino)-(3-nitro-phenyl)-methyl]-naphthalen-2-ol (IVe). mp 168°C yield = 91.58% color = yellow. IR (νmax, cm–1): 3441 (–OH), 3346 (–NH), 3019 (Ar–H), 1599 (C=N), 1548 (‒NO2). 1H NMR (300 MHz, DMSO): δ 10.30 (s, 1H, –OH), 8.26 (s, 1H, Ar–H), 8.01 (d, J = 8.0 Hz, 1H, Ar–H), 7.91 (s, 1H, Ar–H), 7.74–7.26 (m, 8H, Ar–H), 7.05 (s, 1H, –NH), 6.69 (s, 1H, –CH), 2.25 (s, 3H, –CH3). 13C NMR (75 MHz, DMSO): δ 167.76 (–C=N), 153.50, 148.03, 145.21, 134.69, 132.91, 132.02, 129.90, 128.95, 128.88, 128.67, 126.64, 122.90, 122.75, 121.27, 121.09, 120.98, 119.13, 118.65, 53.80 (benzyl-C), 11.83 (–CH3). Elemental analysis for C21H17N3O3S: C, 64.43; H, 4.38; N, 10.73; found C, 64.50; H, 4.44; N, 10.84.
1-[(5-Methyl-thiazol-2-ylamino)-(4-nitro-phenyl)-methyl]-naphthalen-2-ol (IVf). mp 180°C yield = 94.39% color = yellow. IR (νmax, cm–1): 3445 (–OH), 3355 (–NH), 3066 (Ar–H), 1587 (C=N), 1540 (‒NO2). 1H NMR (300 MHz, DMSO): δ 10.21 (s, 1H, –OH), 8.06 (d, J = 8.5 Hz, 2H, Ar–H), 7.84 (d, J = 8.8 Hz, 2H, Ar–H), 7.74–7.17 (m, 7H, Ar–H), 7.06 (s, 1H, –NH), 6.68 (s, 1H, –CH), 2.24 (s, 3H, ‒CH3). 13C NMR (75 MHz, DMSO): δ 167.66 (‒C=N), 153.45, 146.18, 134.69, 132.03, 129.83, 128.82, 128.60, 127.25, 126.54, 122.99, 122.69, 120.89, 119.03, 118.66, 53.87 (benzyl-C), 11.79 (–CH3). Elemental analysis for C21H17N3O3S: C, 64.43; H, 4.38; N, 10.73; found C, 64.54; H, 4.36; N, 10.77.
1-[(3-Methoxy-phenyl)-(5-methyl-thiazol-2-ylamino)-methyl]-naphthalen-2-ol (IVg). mp 165°C yield = 87.65% color = brown. IR (νmax, cm–1): 3434 (–OH), 3368 (–NH), 3062 (Ar–H), 1580 (C=N), 1238 (‒OCH3). 1H NMR (300 MHz, DMSO): δ 10.27 (s, 1H, –OH), 7.90 (d, J = 5.2 Hz, 2Ar–H), 7.70 (dd, J = 16.9, 8.1 Hz, 2H, Ar–H), 7.32–6.94 (m, 7H, Ar–H), 6.73 (m, 1H, –NH), 6.64 (s, 1H, –CH), 3.68 (s, 3H, –OCH3), 2.21 (s, 3H, –CH3). 13C NMR (75 MHz, DMSO): δ 168.01 (–C=N), 159.23, 153.34, 143.97, 134.56, 132.21, 129.21, 128.92, 128.79, 128.42, 126.12, 123.40, 122.46, 120.18, 119.59, 119.20, 118.70, 112.64, 110.89, 101.57, 54.82 (–OCH3), 54.20 (benzyl-C), 11.75 (–CH3). Elemental analysis for C22H20N2O2S: C, 70.19; H, 5.35; N, 7.44; found C, 70.25; H, 5.42; N, 7.56.
1-[(5-Methyl-thiazol-2-ylamino)-p-tolyl-methyl]-naphthalen-2-ol (IVh). mp 156°C yield = 92.88% color = buff. IR (νmax, cm–1): 3442 (–OH), 3350 (–NH), 3060 (Ar–H), 1581 (C=N). 1H NMR (300 MHz, DMSO): δ 10.29 (s, 1H, –OH), 7.88 (d, J = 14.6 Hz, 2H, Ar–H), 7.70 (dd, J = 18.1, 8.4 Hz, 2H, Ar–H), 7.41–7.02 (m, 7H, Ar–H), 6.84 (s, 1H, –NH), 6.65 (s, 1H, –CH), 2.26 (s, 3H, –CH3), 2.22 (s, 3H, CH3). 13C NMR (75 MHz, DMSO): δ 168.15 (C=N), 153.38, 139.06, 135.51, 132.22, 129.16, 128.86, 128.59, 128.44, 126.19, 126.10, 125.48, 122.47, 120.19, 119.73, 119.34, 54.41 (benzyl-C), 20.77 (–CH3), 11.78 (–CH3). Elemental analysis for C22H20N2OS: C, 73.30; H, 5.59; N, 7.77; found C, 73.38; H, 5.72; N, 7.83.
1-(((4-Methylthiazol-2-yl)amino)(thiophen-2-yl)-methyl)naphthalen-2-ol (IVi). mp 166°C yield = 88.64% color = brown. IR (νmax, cm–1): 3435 (–OH), 3355 (–NH), 3068 (Ar–H), 1598 (C=N). 1H NMR (300 MHz, DMSO): δ 10.45 (s, 1H, –OH), 8.00 (d, J = 8.5 Hz, 1H, Ar–H), 7.81 (s, 1H), 7.72 (dd, J = 17.0, 8.4 Hz, 2H, Ar–H), 7.39 (t, J = 7.5 Hz, 1H, Ar–H), 7.31–7.19 (m, 2H, Ar–H), 7.15 (d, J = 4.9 Hz, 1H, Ar–H), 7.03 (s, 1H, Ar–H), 6.87–6.82 (m, 1H), 6.79 (s, 1H, –NH), 6.67 (s, 1H, –CH), 2.22 (s, 3H, ‒CH3). 13C NMR (75 MHz, DMSO): δ 172.51 (‒C=N), 158.31, 151.65, 139.60, 137.01, 134.52, 133.63, 133.44, 131.40, 131.32, 129.10, 128.96, 127.76, 127.58, 125.74, 124.14, 123.87, 56.97 (benzyl-C), 16.72 (–CH3). Elemental analysis for C19H16N2OS2: C, 64.74; H, 4.58; N, 7.95; found C, 64.79; H, 4.65; N, 7.92.
1-(Benzo[D] [1, 3]dioxol-5-yl((4-methylthiazol-2-yl)amino)methyl)naphthalen-2-ol (IVj). mp 174°C yield = 84.45.19% color = brown. IR (νmax, cm–1): 3422 (–OH), 3349 (–NH), 3063 (Ar–H), 1576 (C=N). 1H NMR (300 MHz, DMSO): δ 10.33 (s, 1H, –OH), 7.90 (d, J = 8.6 Hz, 1H, Ar–H), 7.78–7.65 (m, 3H, Ar–H), 7.41–7.33 (m, 1H, Ar–H), 7.24 (dd, J = 18.3, 8.1 Hz, 2H, Ar–H), 6.81 (d, J = 5.0 Hz, 2H, Ar–H), 6.68 (s, 1H, –NH), 6.67 (s, 1H, –CH), 2.23 (s, 3H, ‒CH3). Elemental analysis for C22H18N2O3S: C, 67.67; H, 4.65; N, 7.17; found C, 67.78; H, 4.60; N, 7.25.
Biological Evaluation
Antibacterial activity. Antimicrobial activity of the synthesized derivatives was determined using disk diffusion methods on four nonpathogenic organisms S. aureus, E. coli, P. aeruginosa, and B. substilus. The selected bacterial suspension was inoculated on agar plates, and the plates were then allowed to dry for 5 minutes. The sterile filter paper disks (Whatman no. 1, diameter = 6 mm) were soaked in each sample solution. Ciprofloxacin disk was used as a positive control. The Petri plates were incubated for 18 h at 35 ± 2°C. After incubation, the MIC of compounds was determined [15].
Molecular docking. Molecular docking was carried out using the structure of the S. aureus DNA gyrase (PDB ID 6QX1) which was downloaded from the free protein-free protein database (http://www.rcsb.org). Biopredicta module of Vlife MDS v. 4.6 was utilized for the docking analysis [16, 17].
RESULTS AND DISCUSSIONS
Chemist ry
Synthesis of aminobenzylnaphthol (Betti base) was carried out using a general pathway outlined in Scheme 1. The one-pot reaction was incorporated for the synthesis of Betti bases using silica sulfuric acid (SSA) as a catalyst. 2-Amino-5-methylthiazole, 2‑naphthol, and substituted aldehydes were initially dissolved in glycerol. To this small portion of SSA was added and the mixture was allowed to heat at 70–80°C. The products obtained were characterized using IR, 1HNMR, 13C NMR, and elemental analysis. The characterization data of synthesized novel compounds are presented in the experimental section.
IR data for Betti bases reveals a characteristic absorption band in the range of 3420–3430 cm–1 for phenolic –OH stretching while a band at 3350–3360 cm–1 was observed for –NH stretching. 1H NMR spectra depict the presence of singlet for phenolic proton, –NH and chiral –CH at δ ~10.20 ppm, δ ~7.00 ppm, and δ ~6.60 ppm respectively. A singlet that corresponds to three protons of methyl in thiazole moiety was observed at δ ~2.20 ppm. In 13C NMR spectra, a peak around δ ~167 ppm indicates the presence of the C=N group of thiazole. The two characteristic peaks for chiral benzyl carbon and methyl carbon were evidenced at δ ~53 ppm and δ ~11.7 ppm respectively [11]. As further, elemental analysis of C, N, and H was obtained for synthesized compounds and the experimental composition was in good agreement with the theoretical values.

Scheme 1 . Schematic representation for the route of synthesis of Schiff’s base.
Antibacterial Activity
Antibacterial activity of the synthesized derivatives was performed via the disc diffusion method on four different organisms S. aureus, B. substilus, E. coli, and P. aeruginosa, using ciprofloxacin as a standard. Table 1 shows the minimum inhibitory concentration (MIC) in μg/mL for all the compounds and standards. The result reveals that the bacterial activity depends not only on nature but also on the position of the substituents on the benzene ring of Betti bases. Although the results were not so great, among all the synthesized compounds (IVa–f) has depicted satisfactory results against all microbial cultures which emphasize the importance of the electron-withdrawing group. It was also noted that the withdrawing group at the ortho position raises the bacterial activity as compared to the meta and para position. This is due to the meta and para substitutions gives conformation restriction to fit in the receptor pocket. On the other hand, compounds (IVg) and (IVh) have exhibited less activity against all microbial cultures as they have electron-donating substituents on benzene i.e. methoxy and methyl respectively. The comparative bar graph of the antibacterial study is shown in Fig. 1.
Antimicrobial Docking
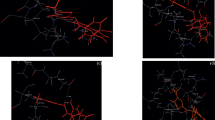
Docking analysis was performed to ascertain the mechanism of action of the synthesized derivatives. The crystal structure of DNA gyrase was utilized for the docking simulations. All the molecules were found to have a good binding affinity towards the DNA gyrase. Interactions of all synthesized derivatives are shown in Table S1 (Supplementary Information). Some of the most promising derivatives (IVa, IVc, and IVd) and their interactions with protein are elaborated below.
A molecule (IVa) shows hydrogen bond interaction with ARG630 and hydrophobic interactions with GLU634, ILE336, ARG342, LYS344, LEU345 (Fig. 2).
A molecule (IVc) shows hydrogen bond interaction with PRO343 and hydrophobic interactions with GLU634, ASP635, ALA637, VAL638, ARG342 (Fig. 3).
A molecule (IVd) shows hydrogen bond interaction with ARG630 hydrophobic interaction with ILE633, GLU634, ALA637, ILE30, MET179 (Fig. 4).
Insilico ADMET Prediction
All the synthesized derivatives were scrutinized for in silico ADMET prediction using admetSAR server (http://lmmd.ecust.edu.cn/admetsar2/about/) and the results are listed in Table 2. All the derivatives were marked under category III (500 mg kg–1 < LD50 ≤ 5000 mg kg–1) for acute oral toxicity which indicates that these derivatives are nontoxic [12]. The nitro, methoxy, and methyl substituted derivatives (IVd–h) were indicated for possible carcinogenicity (trinary). No Lipinski rule violation was observed for any molecule, thus making it suitable for oral consumption [13, 14].
CONCLUSION
In conclusion, the novel derivatives of Betti bases (IVa–j) were synthesized using SSA as an acid catalyst and characterized using FTIR, NMR, and Elemental analysis. These aminobenzylnaphthol derivatives i.e. Betti bases were screened for in vitro antibacterial activity which affirms that the electron-withdrawing groups are more potent than electron-donating groups as well as a substitution at ortho position shows most effectual activity than others. Molecular docking simulations were performed to ascertain the mechanism of action of these derivatives. The docking analysis brings to light that, these molecules are exhibiting action via inhibition of DNA gyrase for antibacterial potential. In silico ADMET prediction reveals that no violation of the Lipinski rule was observed as well the acute oral toxicity was marked under category III for all molecules.
REFERENCES
Gao, H., Sun, J., and Yan, C.G., Chin. Chem. Lett., 2015, vol. 26, pp. 353–356. https://doi.org/10.1016/j.cclet.2014.11.009
Olyaei, A. and Sadeghpour, M., RSC Adv., 2019, vol. 9, pp. 18467–1897. https://doi.org/10.1039/c9ra02813g
Cardellicchio, C., Ciccarella, G., Naso, F., Perna, F., and Tortorella, P., Tetrahedron., 1999, vol. 55, pp. 14685–14692. https://doi.org/10.1016/S0040-4020(99)00914-X
Georgieva, N.V., Yaneva, Z.L., Simova, S.D., and Nikolova, G.D., Bulg. Chem. Commun., 2017, vol. 49, pp. 201–208.
Chopde, H.N., Meshram, J.S., Pagadala, R., and Mungole, A.J., Int. J. ChemTech Res., 2010, vol. 2, pp. 1823–1830.
Adrián, P., Alexis, R.G., Roderick, A., Kaylie, D., Miguel, X.F., and Giovanna, B., J. Mol. Clin. Med., 2019, vol. 2, p. 35. https://doi.org/10.31083/j.jmcm.2019.02.7181
Shen, A.Y., Tsai, C.T., and Chen, C.L., Eur. J. Med. Chem., 1999, vol. 34, pp. 877–882. https://doi.org/10.1016/S0223-5234(99)00204-4
Mohanram, I. and Meshram, J., ISRN Org. Chem., 2014, vol. 2014, pp. 1–7. https://doi.org/10.1155/2014/639392
Cimarelli, C., Molecules, 2019, vol. 24, pp. 4–5. https://doi.org/10.3390/molecules24132372
Cardellicchio, C., Capozzi, M.A.M., and Naso, F., Tetrahedron Asymmetry, 2010, vol. 21, pp. 507–517. https://doi.org/10.1016/j.tetasy.2010.03.020
Olyaei, A., Abforushha, E.S., and Khoeiniha, R., Lett. Org. Chem., 2017, vol. 14, pp. 103–108. https://doi.org/10.2174/15701786146661702011714
Guan, L., Yang, H., Cai, Y., Sun, L., Di, P., and Li, W., Med. Chem. Commum., 2019, vol. 10, pp. 148–157. https://doi.org/10.1039/C8MD00472B
Cheng, F., Li, W., Zhou, Y., Shen, J., Wu, Z., Liu, G., J. Chem. Inf. Model., 2012, vol. 52, pp. 3099–3105. https://doi.org/10.1021/ci300367a
Yang, H., Lou, C., Sun, L., Li, J., Cai, Y., Wang, Z. Bioinformatics, 2019, vol. 35, pp. 1067–1069. https://doi.org/10.1093/bioinformatics/bty707
Balouiri, M., Sadiki, M., and Ibnsouda, S.K., J. Pharm. Anal., 2016, vol. 6, pp. 71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Chen, P., Lee, N.V., Hu, W., Xu, M., Ferre, R.A., and Lam, H., Mol. Cancer Ther., 2016, vol. 15, pp. 2273–2281. https://doi.org/10.1158/1535-7163.MCT-16-0300
Thalji, R.K., Raha, K., Andreotti, D., Checchia, A., Cui, H., Meneghelli, G. Bioorg. Med. Chem. Lett., 2019, vol. 29, pp. 1407–1412. https://doi.org/10.1016/j.bmcl.2019.03.029
ACKNOWLEDGMENTS
The authors are grateful to the Department of Chemistry, University of Mumbai, Santacruz (E.) for spectral analysis, Bharati Vidyapeeth College of Pharmacy, Kolhapur for computational studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1134/S1068162021040075.
Rights and permissions
About this article
Cite this article
Dandekar, S.N., Lotlikar, O.A., Ramana, M.M. et al. Synthesis, Molecular Docking, and In Vitro Antibacterial Activities of Some Novel Aminobenzylnaphthol Derivatives via One-Pot Three-Component Reaction. Russ J Bioorg Chem 47, 874–881 (2021). https://doi.org/10.1134/S1068162021040075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021040075