Abstract
Discovery towards the potent antimicrobial agents is indispensable for the treatment of infections caused by resistant microbes. Thus, we prepared a novel series of 3-substituted 5-phenylindeno-thiazolopyrimidinone derivatives following conventional method. All the molecules were investigated for their in vitro antimicrobial potency against different bacteria and fungi, and the results were compared with streptomycin and clotrimazole standard drugs, respectively. Among the twelve analogs, 4-methoxyphenyl-5-phenylindeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-one showed equipotent activity against a bacterium, Staphylococcus aureus (minimum inhibitory concentration 25 µg/mL and zone of inhibition 22 mm), and a fungus, Aspergillus niger (zone of inhibition 20 mm). The rest of the thiazolo[3,2-a]pyrimidin-6(5H)-one derivatives exhibited week to reasonable activities against the tested bacterial and fungal strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Antibacterial resistance is growing tremendously throughout the world due to the misuse and overuse of the antibiotics. Thus, it is a serious threat for the successful treatment of the infectious diseases and becomes a foremost clinical and public health problem. According to the World Health Organization (WHO), approximately 700 000 deaths occur annually due to drug-resistant pathogens, and this number is likely to reach 10 million by 2050 if the existing trend continues [1–3]. Hence, it is essential to develop new classes of highly effective antimicrobial agents with no side effects to fight against pathogenic microorganisms that developed resistance to the antibiotics used in the current regimen [4–6].
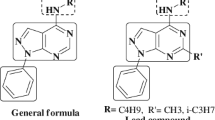
Thiazolopyrimidine derivatives have received a significant interest from the medicinal and synthetic chemists because of their extensive pharmacological applications, such as antimicrobial [7–9], antibiofilm [10, 11], anticancer [12, 13], antiviral [14], anti-inflammatory [15–18], antitubercular [8], anti-tumoural [19], antimalarial [20], anti-HIV [20, 21], antioxidant [7, 22], and calcium channel blocking [23] activities. They are also reported as inhibitors of acetylcholinesterase (AchE) [24, 25], CDC25B phosphatase [26], and xanthine oxidase [27] enzymes, and Bcl-2 family proteins [28]. These are also considered as potential purine antagonists [29, 30]. The structures of some of the thiazolopyrimidine derivatives and their biological properties are specified in Fig. 1 [31].
In view of the remarkable pharmacological properties of thiazolo[3,2-a]pyrimidines, numerous methods have been accounted for their synthesis. One of the simplest method is the cyclization of dihydropyrimidine-thiones (Biginelli product) with halogen- (bromo- or chloro-) containing electrophiles like 2‑bromo-ketones [32], chloroacetyl chloride [26], chloroacetic acid [12–14], 1,2-dichloroethane [33], and methyl chloroacetate [34] to yield various C2–N3 linked various thiazolo[3,2-a]pyrimidines. Motivated by the aforementioned findings, and in continuation of our investigation towards the development of highly potent antimicrobial agents [35–37], herein we report the synthesis, characterization, and in vitro antimicrobial investigation of fused thiazolopyrimidine derivatives.
RESULTS AND DISCUSSION
In our previous report [7], we synthesized several fused thiazolo[3,2-a]pyrimidines starting from 1‑tetralone and investigated their in vitro antibacterial potency against different bacteria. We observed that the analogs bearing aryl substitutions on the thiazole ring had nearly 1.3 fold potency against a gram-negative bacteria Pseudomonas aeruginosa when compared with an antibiotic penicillin. Keeping this in mind and to examine the effect of fused ring on antimicrobial activity, we designed to synthesize a small library of thiazolo[3,2-a]pyrimidines starting from 1,3-indandione. Biginelli product, 4-phenyl-2-thioxo-3,4-dihydro-1H-indeno[1,2-d]pyrimidin-5(2H)-one (III) was prepared via a three-component condensation reaction of 1,3-indandione, benzaldehyde, and thiourea utilizing a catalyst, poly(4-vinylpyridinium)hydrogen sulfate [P(4-VPH)HSO4]. The reaction mixture was subjected to heat at 120°C under neat conditions. The intermediate (III) was obtained in 94% yield in short reaction time (15 min). The expected products (IVa–l), were obtained in 80–90% yields via the cyclization of an intermediate (III) with a variety of bromine derived electrophiles, such as phenacyl bromides and 3-(bromoacetyl)coumarins (IIa–e) under conventional refluxing in acetic acid (Scheme 2). 3-(Bromoacetyl)coumarin derivatives (IIa–e) were prepared following the earlier literature procedure (Scheme 1) [38].

Scheme 1. Synthesis of 3-(2-bromoacetyl)-2H-chromen-2-ones and 2-(2-bromoacetyl)- 3H-benzo[f]chromen-3-one (IIa–e).

Scheme 2. Synthesis of 5-phenylindeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-one derivatives.
Formation of the target compounds (IVa–l) from the intermediate dihydropyrimidine-thione (III) was confirmed by FT-IR, 1H, 13C NMR, mass spectrometry and elemental analyses data. The disappearance of –NH bands at 3406 and 3280 cm–1 of the dihydropyrimidin-thione (III) and the existence of imine ‒C=N– band at 1604–1647 cm–1 in the FT-IR spectra confirm the product formation. In case of compounds (IVh–l), a strong band at 1732–1738 cm–1 signifies the existence of pyranone (–O–C=O) group. The disappearance of singlets at 9.78 and 11.68 ppm from the 1H NMR spectra and a signal at 174.32 ppm from 13C NMR spectra of the starting material, as well as the presence of a peak –of –C=N (imine carbon) at 157.47–158.64 ppm indicate the construction of the product. Elemental analyses and mass spectral data further strengthened the formation of products.
Antibacterial activity of all the synthesized molecules (IVa–l) was measured against gram-positive Streptococcus pyogene and Staphylococcus aureus and gram-negative Klebsiella pneumonia and Pseudomonas aeruginosa bacterial strains. Streptomycin was used as a standard antibacterial drug. Almost all the compounds (except for compound (IVa)) showed moderate activity against a gram-negative bacterium P. aeruginosa in terms of ZOI (14–18 mm) and MIC (50–100 µg/mL) values comparing with a standard antibiotic drug streptomycin. Compound (IVa) showed weak activity against P. aeruginosa. But the compound derived from 4-methoxyphenacyl bromide (IVf) showed equipotent activity against S. aureus (gram-positive) in terms of ZOI (22 mm) MIC (25 µg/mL) values (Table 1, Fig. 2). Replacing the 4-methoxyphenyl group of thiazole ring with 4-chlorophenyl (IVb), 4-bromophenyl (IVc), 4-nitrophenyl (IVd), 4-methylphenyl (IVe), and biphenyl (IVg) groups did not improve the antibacterial activity against S. aureus. Introducing coumarinyl groups (IVh–l) into thiazole ring also did not enhance the activity. Weak activity was observed for all the synthesized molecules against S. pyogene and K. pneumonia bacterial strains comparing with the reference drug.
Antifungal activity was assayed against Candida glabrata, Candida albicans, Aspergillus parasiticus, and Aspergillus niger fungal strains. Clotrimazole was used as a reference drug. Activity results reveal that the maximum ZOI (20 mm) has observed in the case of compound (IVf) against A. niger. Compounds (IVa), (IVc), (IVf), and (IVk) showed good activity (ZOI 11–20 mm) against all the tested fungal strains (Table 2, Fig. 2). Moderate-to-weak activity was observed in the case of the rest of the molecules.
CONCLUSION
3-Aryl/Heteryl substituted 5-phenylindeno-thiazolopyrimidinone derivatives (IVa–l) were prepared with promising yields under conventional method. All the synthesized molecules (IVa–l) were investigated for their antimicrobial potency against different microbes. The activity data revealed that the compound containing the 4-methoxyphenyl group on the thiazole ring (IVf) has broad-spectrum antibacterial and antifungal activities against all the microbes used for the investigation. Compounds (IVa), (IVc), and (IVk) also showed good antifungal activity. Taking compound (IVf) as a lead, further modifications by substituents on the pyrimidine ring are under investigation in our laboratory.
EXPERIMENTAL
All of the solvents and reagents used were obtained commercially from Aldrich, SRL, and Qualigens chemicals, and are used as is unless otherwise mentioned. Thin layer chromatography (silica gel 60F254 pre-coated aluminum sheets obtained from Aldrich) was used to examine the development of the reaction. Column chromatography (silica-gel, 230–400 mesh procured from Aldrich) with 80% hexanes and ethyl acetate as eluent was used for the purification of the final molecules. Buchi (R-100) rotary evaporator was used to remove the solvents. Stuart SMP30 melting point apparatus was used to find out the melting point of the compounds. Perkin-Elmer 100S IR spectrophotometer was used to record the IR spectra (νmax, cm–1) in KBr. Bruker 400MHz spectrometer was used to record the NMR spectra in DMSO-d6 at 400 (1H) and 100 (13C) MHz; chemical shifts values are given in ppm (parts per million). Jeol JMSD-300 spectrometer and Carlo Erba EA1108 analytical unit were used to record mass spectra and elemental analysis data.
4-Phenyl-2-thioxo-3,4-dihydro-1H-indeno[1,2-d]pyrimidin-5(2H)-one (III). To a mixture of 1,3-indandione (10 mmol, 1.461 g), benzaldehyde (10 mmol, 1.02 mL), and thiourea (12 mmol, 0.913 g), 15 mg of P(4-VPH)HSO4 was added. The mixture was heated at 120°C for 15 min under neat conditions. Dichloromethane (50 mL) was added to the reaction mixture and stirred at ambient temperature for additional 5 min, and the catalyst was filtered off. The crude product was purified by crystallization using ethanol to obtain pure intermediate (III) as a red solid (2.748 g, 94%). mp 224–226°C; 1H NMR: 5.36 (s, 1H, H4pyrimidine), 7.25–7.39 (m, 8H, Ar-H), 7.86–7.91 (m, 1H, Ar-H), 9.77 (bs, 1H, NH), 11.68 (bs, 1H, NH); MS (ESI, m/z): 293 [M + H]+; Anal. calcd. for C17H12N2OS: C, 69.84; H, 4.14; N, 9.58. Found: C, 69.67; H, 4.31; N, 9.41.
3,5-Diphenylindeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-one (IVa). To the stirred intermediate compound (III) (1 mmol, 292.35 mg) in glacial AcOH (4 mL) phenacyl bromide (1 mmol, 199.05 mg) was added. The temperature of the reaction mixture was raised to 120°C and the mixture was stirred at that same temperature for 3 h, then kept a side for 12 h. Precipitate separated out was filtered and washed with small amount of cold acetic acid. The crude material obtained was subjected to column chromatography using 20% ethyl acetate in hexanes to obtain the pure product (IVa) as an orange solid (83%, 325.73 mg). mp 261–263°C; IR: 1707 (C=O), 1600 (C=N), 1562 (C=C). 1H NMR: 6.35 (s, 1H, H4pyrimidine), 6.63–6.65 (m, 2H, Ar–H and H2thiazole), 7.10 (t, J = 7.2 Hz, 4H, Ar–H), 7.22–7.24 (m, 3H, Ar–H), 7.32 (d, J = 7.2 Hz, 1H, Ar–H), 7.36–7.46 (m, 4H, Ar–H), 7.55 (d, 3H, Ar–H, J = 7.6 Hz); MS (ESI, m/z): 393 [M + H]+; Anal. calcd. for C25H16N2OS: C, 76.51; H, 4.11; N, 7.14. Found: C, 76.40; H, 4.25; N, 7.33.
Compounds (IVb–l) were synthesized similarly as compound (IVa).
3-(4-Chlorophenyl)-5-phenylindeno[1,2-d]thiazolo-[3,2-a]pyrimidin-6(5H)-one (IVb). 4-Chlorophenacyl bromide (1 mmol, 233.49 mg) was used as reagent. Orange solid (90%, 384.22 mg); mp 254–256°C; IR: 1717 (C=O), 1605 (C=N), 1575 (C=C), 749 (C–Cl). 1H NMR: 6.34 (s, 1H, H4pyrimidine), 6.68–6.71 (m, 2H, Ar–H and H2thiazole), 7.11–7.15 (m, 7H, Ar–H), 7.26 (t, 3H, J = 8.4 Hz, Ar–H), 7.43 (t, 2H, J = 8.4 Hz, Ar–H); MS (ESI, m/z): 427 [M + H]+; Anal. calcd. for C25H15ClN2OS: C, 70.33; H, 3.54; N, 6.56; Found: C, 70.15; H, 3.67; N, 6.39.
3-(4-Bromophenyl)-5-phenylindeno[1,2-d]thiazolo-[3,2-a]pyrimidin-6(5H)-one (IVc). 4-Bromophenacyl bromide (1 mmol, 277.94 mg) was used as reagent. Orange solid (88%, 414.8 mg); mp 264–266°C; IR: 1714 (C=O), 1604 (C=N), 1576 (C=C), 616 (C–Br). 1H NMR: 6.34 (s, 1H, H4pyrimidine), 6.69–6.71 (m, 2H, Ar–H and H2thiazole), 7.11–7.23 (m, 7H, Ar–H), 7.31 (d, 1H, J = 7.2 Hz, Ar–H), 7.40 (t, 2H, J = 7.6 Hz, Ar–H), 7.58 (d, 2H, J = 8.8 Hz, Ar–H); MS (ESI, m/z): 472 [M + H]+; Anal. calcd. for C25H15BrN2OS: C, 63.70; H, 3.21; N, 5.94. Found: C, 63.57; H, 3.39; N, 5.80.
3-(4-Nitrophenyl)-5-phenylindeno[1,2-d]thiazolo-[3,2-a]pyrimidin-6(5H)-one (IVd). 4-Nitrophenacyl bromide (1 mmol, 244.04 mg) was used as reagent. Yellow solid (81%, 354.35 mg); mp 271–273°C; IR: 1715 (C=O), 1604 (C=N), 1574 (C=C), 1548, 1384 (NO2). 1H NMR: 6.39 (s, 1H, H4pyrimidine), 6.69–6.71 (m, 2H, Ar–H and H2thiazole), 7.07–7.11 (m, 3H, Ar–H), 7.22–7.34 (m, 3H, Ar–H), 7.40 (t, 2H, J = 6.8 Hz, Ar–H), 7.56 (d, 2H, J = 8.8 Hz, Ar–H), 8.21 (d, 2H, J = 8.8 Hz, Ar–H); MS (ESI, m/z): 438 [M + H]+; Anal. calcd. for C25H15N3O3S: C, 68.64; H, 3.46; N, 9.61. Found: C, 68.47; H, 3.31; N, 9.47.
5-Phenyl-3-(p-tolyl)indeno[1,2-d]thiazolo[3,2-a]-pyrimidin-6(5H)-one (IVe). 4-Methylphenacyl bromide (1 mmol, 213.07 mg) was used as reagent. Orange solid (82%, 333.33 mg); mp 267–269°C; IR: 1714 (C=O), 1647 (C=N), 1578 (C=C). 1H NMR: 2.34 (s, 3H, CH3), 6.36 (s, 1H, H4pyrimidine), 6.67 (s, 1H, H2thiazole), 7.13 (t, 6H, J = 8.0 Hz, Ar–H), 7.19 (d, 3H, J = 7.6 Hz, Ar–H), 7.33 (t, 2H, J = 7.2 Hz, Ar–H), 7.42 (t, 2H, J = 7.2 Hz, Ar–H); 13C NMR: 188.85, 157.47, 141.47, 139.62, 132.19, 130.51, 129.25, 128.92, 128.39, 128.19, 125.88, 125.37, 120.89, 119.46, 105.42, 58.89, 20.88; MS (ESI, m/z): 407 [M + H]+; Anal. calcd. for C26H18N2OS: C, 76.82; H, 4.46; N, 6.89; Found: C, 76.68; H, 4.29; N, 6.73.
3-(4-Methoxyphenyl)-5-phenylindeno[1,2-d]thiazolo-[3,2-a]pyrimidin-6(5H)-one (IVf). 4-Methoxyphenacyl bromide (1 mmol, 229.07 mg) was used as reagent. Orange solid (85%, 359.12 mg); mp 248–250°C; IR: 1715 (C=O), 1647 (C=N), 1578 (C=C), 1190 (C–O–C). 1H NMR: 3.79 (s, 3H, OCH3), 6.36 (s, 1H, H4pyrimidine), 6.69 (s, 1H, H2thiazole), 6.93 (d, 3H, J = 8.4 Hz, Ar–H), 7.12–7.17 (m, 6H, Ar–H), 7.25–7.46 (m, 4H, Ar–H); MS (ESI, m/z): 423 [M + H]+; Anal. calcd. for C26H18N2O2S: C, 73.91; H, 4.29; N, 6.63. Found: C, 73.79; H, 4.13; N, 6.51.
3-([1,1'-Biphenyl]-4-yl)-5-phenylindeno[1,2-d]-thiazolo[3,2-a]pyrimidin-6(5H)-one (IVg). 4-Phenylphenacyl bromide (1 mmol, 275.14 mg) was used as reagent. Yellow solid (80%, 374.85 mg); mp 276–278°C; IR: 1711 (C=O), 1645 (C=N), 1571 (C=C). 1H NMR: 6.45 (s, 1H, H4pyrimidine), 6.72–6.74 (m, 2H, Ar–H and H2thiazole), 7.11 (t, 3H, J = 7.2 Hz, Ar–H), 7.31 (t, 5H, J = 8.4 Hz, Ar–H), 7.42–7.51 (m, 5H, Ar–H), 7.67–7.73 (m, 4H, Ar–H); 13C NMR: 188.98, 157.84, 141.35, 141.12, 140.47, 139.01, 138.34, 133.98, 132.11, 130.41, 129.93, 129.05, 128.40, 128.18, 128.04, 127.49, 126.79, 126.54, 125.96, 120.71, 119.35, 108.94, 105.32, 58.99; MS (ESI, m/z): 469 [M + H]+; Anal. calcd. for C31H20N2OS: C, 79.46; H, 4.30; N, 5.98. Found: C, 79.31; H, 4.47; N, 5.81.
3-(2-Oxo-2H-chromen-3-yl)-5-phenylindeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-one (IVh). 3-(2-Bromoacetyl)-2H-chromen-2-one (1 mmol, 267.07 mg) was used as reagent. Yellow solid (84%, 386.82 mg); mp 238–240°C; IR: 1737 (C=Olactone) 1702 (C=Oindanone), 1646 (C=N), 1581 (C=C); 1H NMR: 5.76 (s, 1H, H4pyrimidine), 6.82 (s, 1H), 6.91 (t, 3H, J = 8.0 Hz), 7.13 (d, 2H, J = 8.0 Hz), 7.25 (d, 1H, J = 6.8 Hz), 7.29 (s, 1H, H2thiazole), 7.36 (t, 2H, J = 6.4 Hz, Ar–H), 7.51 (d, 2H, J = 8.4 Hz, Ar–H), 7.61 (d, 1H, J = 7.6 Hz, Ar–H), 7.82 (d, 1H, J = 7.6 Hz, Ar–H), 8.34 (s, 1H, H4coumarin); 13C NMR: 189.19, 170.86, 157.64, 153.33, 142.26, 138.95, 137.06, 132.83, 132.78, 130.71, 129.53, 128.42, 128.33, 127.86, 127.75, 127.60, 127.11, 125.29, 124.53, 121.43, 120.00, 118.16, 115.49, 109.08, 55.77; MS (ESI, m/z): 461 [M + H]+; Anal. calcd. for C28H16N2O3S: C, 73.03; H, 3.50; N, 6.08. Found: C, 73.23; H, 3.31; N, 6.27.
3-(8-Methoxy-2-oxo-2H-chromen-3-yl)-5-phenyl-indeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-one (IVi). 3-(2-Bromoacetyl)-8-methoxy-2H-chromen-2-one (1 mmol, 297.1 mg) was used as reagent. Orange solid (81%, 397.33 mg); mp 242–244°C; IR: 1736 (C=Olactone), 1703 (C=Oindanone), 1644 (C=N), 1584 (C=C), 1243 (C–O–C); 1H NMR: 3.94 (s, 3H, OCH3), 6.34 (s, 1H, H4pyrimidine), 6.79 (s, 1H, H2thiazole), 6.86 (d, 1H, J = 7.2 Hz, Ar–H), 6.97 (t, 2H, J = 7.2 Hz, Ar–H), 7.14 (t, 3H, J = 7.6 Hz, Ar–H), 7.29–7.40 (m, 5H, Ar–H), 7.88 (s, 1H, Ar–H), 8.29 (s, 1H, H4coumarin); 13C NMR: 189.25, 170.83, 157.27, 146.31, 145.99, 142.89, 142.24, 132.99, 132.56, 130.49, 128.23, 127.48, 127.00, 125.59, 124.57, 124.38, 121.13, 120.57, 119.69, 118.78, 115.11, 108.84, 56.23, 55.62; MS (ESI, m/z): 491 [M + H]+; Anal. calcd. for C29H18N2O4S: C, 71.01; H, 3.70; N, 5.71. Found: C, 71.23; H, 3.55; N, 5.57.
3-(6-Bromo-8-methoxy-2-oxo-2H-chromen-3-yl)-5-phenylindeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-one (IVj). 6-Bromo-3-(2-bromoacetyl)-8-methoxy-2H-chromen-2-one (1 mmol, 275.99 mg) was used as reagent. Green solid (86%, 489.7 mg); mp 279–281°C; IR: 1732 (C=Olactone), 1703 (C=Oindanone), 1644 (C=N), 1558 (C=C), 1243 (C–O–C), 723 (C–Br); 1H NMR: 3.97 (s, 3H, OCH3), 6.35 (s, 1H, H4pyrimidine), 6.98–7.00 (m, 2H, Ar–H and H2thiazole), 7.16 (t, 3H, J = 7.2 Hz, Ar–H), 7.23 (d, 1H, J = 7.2 Hz, Ar–H), 7.32 (t, 2H, J = 8.0 Hz, Ar–H), 7.40–7.55 (m, 3H, Ar–H), 7.56 (s, 1H, Ar–H), 7.84 (s, 1H, H4coumarin); MS (ESI, m/z): 570 [M + H]+; Anal. calcd. for C29H17BrN2O4S: C, 61.17; H, 3.01; N, 4.92. Found: C, 61.05; H, 3.28; N, 4.79.
3-(6,8-Dinitro-2-oxo-2H-chromen-3-yl)-5-phenyl-indeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-one (IVk). 3-(2-Bromoacetyl)-6,8-dinitro-2H-chromen-2-one (1 mmol, 357.07 mg) was used as reagent. Yellow solid (82%, 451.41 mg); mp 287–289°C. IR: 1738 (C=Olactone), 1697 (C=Oindanone), 1619 (C=N), 1588 (C=C), 1564, 1348 (NO2); 1H NMR: 6.40 (s, 1H, H4pyrimidine), 7.00–7.02 (m, 2H, Ar–H and H2thiazole), 7.15 (d, 2H, J = 6.8 Hz, Ar–H), 7.23 (d, 1H, J = 6.8 Hz, Ar–H), 7.31 (d, 2H, J = 7.2 Hz, Ar–H), 7.41 (d, 2H, J = 7.6 Hz, Ar–H), 7.69 (d, 1H, J = 9.2 Hz, Ar–H), 8.14 (s, 1H, H4coumarin), 8.51 (s, 1H, Ar–H), 8.65 (s, 1H, Ar–H); MS (ESI, m/z): 551 [M + H]+; Anal. calcd. for C28H14N4O7S: C, 61.09; H, 2.56; N, 10.18. Found: C, 61.21; H, 2.37; N, 10.01.
3-(3-Oxo-3H-benzo[f]chromen-2-yl)-5-phenylindeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-one (IVl). 2-(2-Bromoacetyl)-3H-benzo[f]chromen-3-one (1 mmol, 317.13 mg) was used as reagent. Red solid (83%, 423.77 mg); mp 282–284°C; IR: 1737 (C=Olactone), 1657 (C=Oindanone) 1625 (C=N), 1561 (C=C); 1H NMR: 6.44 (s, 1H, H4pyrimidine), 6.97 (s, 1H, H2thiazole), 7.06 (t, 2H, J = 7.2 Hz, Ar–H), 7.23 (d, 1H, J = 7.2 Hz, Ar–H), 7.33–7.44 (m, 4H, Ar–H), 7.62–7.74 (m, 5H, Ar–H), 7.84 (t, 1H, J = 8.0 Hz, Ar–H), 8.11 (t, 1H, J = 7.2 Hz, Ar–H), 8.24–8.38 (m, 1H, Ar–H), 8.40 (s, 1H, H4coumarin); 13C NMR: 189.12, 169.49, 158.65, 153.77, 142.36, 141.09, 139.07, 135.08, 134.99, 134.33, 131.95, 130.29, 129.96, 128.98, 128.82, 128.74, 128.64, 128.26, 126.49, 126.20, 122.40, 120.46, 119.20, 116.51, 115.75, 112.53, 112.20, 104.54, 59.37; MS (ESI, m/z): 511 [M + H]+; Anal. calcd. for C32H18N2O3S: C, 75.28; H, 3.55; N, 5.49. Found: C, 75.43; H, 3.39; N, 5.31.
Antimicrobial Activity
Antimicrobial screening was carried out using the agar well diffusion method [39, 40]. Minimum inhibitory concentration (MIC, µg/mL) values for antibacterial activity and zone of inhibition (ZOI, mm) values for antifungal activity were measured, and the data were compared with the standard drugs streptomycin and clotrimazole respectively. ZOI values were measured at 150 µg/mL for compounds (IVa–l) and 30 µg/mL for standard drug. Gram-positive strains included Streptococcus pyogenes and Staphylococcus aureus; gram-negative, Klebsiella pneumonia and Pseudomonas aeruginosa. Fungal strains included Candida glabrata, Candida albicans, Aspergillus parasiticus, and Aspergillus niger.
REFERENCES
Guo, H., Eur. J. Med. Chem., 2019, vol. 164, pp. 678–688.
Furst, A.L. and Francis, M.B., Chem. Rev., 2019, vol. 119, pp. 700–726.
Zhang, B., Eur. J. Med. Chem., 2019, vol. 168, pp. 357–372.
Richardson, L.A., PLoS Biol., 2017, vol. 15, p. e2 003 775.
Naylor, N.R., Atun, R., Zhu, N., Kulasabanathan, K., Silva, S., Chatterjee, A., Knight, G.M., and Robotham, J.V., Antimicrob. Resist. Infect. Control., 2018, vol. 7, p. 58.
Davies, J. and Davies, D., Microbiol. Mol. Biol. Rev., 2010, vol. 74, pp. 417–433.
Janardhan, B., Manjulatha, K., Srinivas, B., Rajitha, B., Muralikrishna, N., and Sadanandam, A., RSC Adv., 2014, vol. 4, pp. 22 866–22 874.
Dong, C., Zhi-Hua, Z., Yu, C., Xin-Jia, Y., Shi-Ti, Z., Liang-Jing, Z., Li-Hong, M., Fang, L., and Bing-Jie, F., Med. Chem. Res., 2016, vol. 25, pp. 292–302.
Ashok, M., Holla, B.S., and Kumari, N.S., Eur. J. Med. Chem., 2007, vol. 42, pp. 380–385.
Pan, B., Huang, R., Zheng, L., Chen, C., Han, S., Qu, D., Zhu, M., and Wei, P., Eur. J. Med. Chem., 2011, vol. 46, pp. 819–824.
Suresh, L., Sagar Vijay Kumar, P., Poornachandra, Y., Ganesh Kumar, C., Babu, N.J., and Chandramouli, G.V., Bioorg. Med. Chem., 2016, vol. 24, pp. 3808–3817.
Hassan, G.S., El-Messery, S.M., and Abbas, A., Bioorg. Chem., 2017, vol. 74, pp. 41–52.
Holla, B.S., Rao, B.S., Sarojini, B.K., and Akberali, P.M., Eur. J. Med. Chem., 2004, vol. 39, pp. 777–783.
Mohamed, S.F., Flefel, E.M., Amr, Ael-G., and Abd El-Shafy, D.N., Eur. J. Med. Chem., 2010, vol. 45, pp. 1494–1501.
Abdel Moty, S.G., Hussein, M.A., Abdel Aziz, S.A., and Abou-Salim, M.A., Saudi Pharm. J., 2016, vol. 24, pp. 119–132.
Ramesh, L.S., Charusheela, A.B., and Jyothi, B.W., Med. Chem. Res., 2013, vol. 22, pp. 1884–1892.
Tozkoparan, B., Ertan, M., Kelicen, P., and Demirdamar, R., Farmaco, 1999, vol. 54, pp. 588–593.
Tozkoparan, B., Ertan, M., Krebs, B., Lage, M., Kelicen P., and Demirdamar, R., Arch. Pharm., 1998, vol. 331, pp. 201–206.
Al-Omary, F.A., Hassan, G.S., EI-Messery, S.M., and EI-Subbagh, H.I., Eur. J. Med. Chem., 2012, vol. 47, pp. 65–72.
Fatima, S., Sharma, A., Saxena, R., Tripathi, R., Shukla, S.K., Pandey, S.K., Tripathi, R., and Tripathi, R.P., Eur. J. Med. Chem., 2012, vol. 55, pp. 195–204.
Danel, K., Pedersen, E.B., and Nielsen, C., J. Med. Chem., 1998, vol. 41, pp. 191–198.
Maddila, S., Damu, G., Oseghe, E., Abafe, O., Rao, C.V., and Lavanya, P., J. Korean Chem. Soc., 2012, vol. 56, pp. 334–340.
Balkan, A., Uma, S., Ertan, M., and Wiegrebe, W., Pharmazie., 1992, vol. 47, pp. 687–688.
Liu, S., Shang, R., Shi, L., Wan, D.C., and Lin, H., Eur. J. Med. Chem., 2014, vol. 81, pp. 237–244.
Hui, Z., Lan-mei, C., Lin-lin, Z., Si-jie, L., David, C.C.W., Huang-quan, L., and Chun, H., Arkivoc, 2008, vol. xiii, pp. 266–277.
Kolb, S., Mondesert, O., Goddard, M.L., Jullien, D., Villoutreix, B.O., Ducommun, B., Garbay, C., and Braud, E., ChemMedChem., 2009, vol. 4, pp. 633–648.
Khobragade, C.N., Bodade, R.G., Dawane, B.S., Konda, S.G., and Khandare, N.T., J. Enzyme Inhib. Med. Chem., 2010, vol. 25, pp. 615–621.
Feng, Y., Ding, X., Chen, T., Chen, L., Liu, F., Jia, X., Luo, X., Shen, X., Chen, K., Jiang, H., Wang, H., Liu, H., and Liu, D., J. Med. Chem., 2010, vol. 53, pp. 3465–3479.
El-Bayouki, K.A. and Basyouni, W.M., J. Sulfur Chem., 2010, vol. 31, pp. 551–590.
Nagarajaiah, H., Khazi, I., and Begum, N.S., J. Chem. Sci., 2012, vol. 124, pp. 847–855.
Kappe, C.O., Eur. J. Med. Chem., 2000, vol. 35, pp. 1043–1052.
Quan, Z.J., Zhang, Z., Wang, J.K., Wang, X.C., Liu, Y.J., and Ji, P.Y., Heteroat. Chem., 2008, vol. 19, pp. 149–153.
Bozsing, D., Sohar, P., Gigler, G., and Kovacs, G., Eur. J. Med. Chem., 1996, vol. 31, pp. 663–668.
Kulakovi, I.V., Nurkenovi, O.A., Turdybekov, D.M., Issabaevai, G.M., Mahmutova, A.S., and Turdybekov, K.M., Chem. Heterocycl. Compd., 2009, vol. 45, pp. 856–859.
Gali, R., Banothu, J., Porika, M., Velpula, R., Hnamte, S., Bavantula, R., Abbagani, S., and Busi, S., Bioorg. Med. Chem. Lett., 2014, vol. 24, pp. 4239–4242.
Gali, R., Banothu, J., Gondru, R., Bavantula, R., Velivela, Y., and Crooks, P.A., Bioorg. Med. Chem. Lett., 2015, vol. 25, pp. 106–112.
Gondru, R., Peddi, S.R., Manga, V., Khanapur, M., Gali, R., Sirassu, N., and Bavantula, R., Mol. Divers., 2018, vol. 22, pp. 943–956.
Sahu, S.K., Mishra, A., and Behera, R.K., Indian J. Heterocycl. Chem., 1996, vol. 6, pp. 91–94.
Semra, I., Filiz, S., and Ferdag, C., Int. J. Nat. Eng. Sci., 2007, vol. 1, pp. 59–61.
Mert, S., Kasimogullari, R., Ica, T., Colak, F., Altun, A., and Ok, S., Eur. J. Med. Chem., 2014, vol. 78, pp. 86–96.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Busi Siddhardha, Department of microbiology, Pondicherry University, for antimicrobial screening. Dr. Rajitha thanks the Department of Chemistry, Gayatri Vidya Parishad College of Engineering (A), A.P., for providing research facilities.
Funding
Arya C.G. thanks the MHRD for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Corresponding author: phone: 0091-0891-2535788; e-mail: janardhan@nitc.ac.in.
Rights and permissions
About this article
Cite this article
Rajitha, G., Arya, C.G., Janardhan, B. et al. 3-Aryl/Heteryl-5-Phenylindeno[1,2-d]thiazolo[3,2-a]pyrimidin-6(5H)-ones: Synthesis, Characterization, and Antimicrobial Investigation. Russ J Bioorg Chem 46, 612–619 (2020). https://doi.org/10.1134/S106816202004007X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202004007X