Abstract—
The recent study reported the designing of substituted 3-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one derivatives and assessed computationally to calculate the bioactivity and physicochemical properties. The substituted 3-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one derivatives represented the bioactivity score in the zone for an active drug molecule and were in compliance with the Lipinski Rule of five. Then the synthesis, characterization, and biological screening as antimicrobial potential and percent viability of cells were carried out for the substituted 3-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one derivatives. The zone of inhibition and minimum inhibitory concentration (MIC) findings portrayed that the compounds-(IV) and compound-(V) possessed better antimicrobial activity than the reference drug ciprofloxacin, while the significant antimicrobial potential was observed by other members of the series. The molecular docking studies were performed to assist the in vitro antimicrobial results and the findings exhibited that significant H-bonding in between the substituted 3-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one derivatives and the residues of GlcN-6-P-synthase, like ASP 474 (I–IX), SER 316 (I–VI), ASN 522 (I–IX), TRP 313 (V) with good binding affinity ranging –7.7 to –6.8 kcal/mole. The compounds represented the less toxic effects to the HepG2 cells and the percent viability of the cells ranging from 93–98%, 73–78% and 70–76% up to 3.125, 50 , 100 mmol/L respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Development of resistance to the available antimicrobial agents is not only the matter of great health concern but also the major threat to human health [1]. The regular increase in the resistance prompted researchers to find some new, safe and potent antimicrobial agents [2]. Piperonyl nucleus has been observed to be part of many derivatives possessing many pharmacological properties like antiplasmodial, antimicrobial, antitumor, anticonvulsant, antihypertensive, anticancer, antiamoebic, monoamine oxidase-b inhibitors, antileishmanial, trypanocidal agents, DNA binding and antibacterial, antifungal, Anti-Alzheimer, etc. [3–15]. On the other hand pyrimidine, a six-member aromatic nucleus with Nitrogen heteroatom at 1st and 3rd position, have been found to show versatile pharmacological potentials like antimicrobial, anti-inflammatory, antioxidant, anticancer, antimalarial, antiplasmodial, antileishmanial, antihypertensive, antitubercular, anti-HIV, antidiabetic, antiviral, antipyretic, analgesic, inhibitors of MnK1, MnK2, JAK3, ALK2, cSrc Kinase, and VEGFR-2-tyrosine kinase, the antagonist to CB2 neutral, SSRI and 5-HT1A receptor, adenosine receptor, GPR119 agonist, protease inhibitors, etc [16–31]. Aiming the versatile biological applications of derivatives of thiazolidin-4-one a number of studies performed such as antimicrobial, Anti-fungal, GABA receptor, antioxidant and anti-inflammatory, anti-HIV, antihyperglycemic and antidyslipidemic, anti-tumor, antimycobacterial, anti-glioma activity activities [32–41]. Recently we reported the design Computational, synthesis, and biological screening of some thiozolidin-one derivatives possessing indole and pyridine nucleus [16].
RESULTS AND DISCUSSION
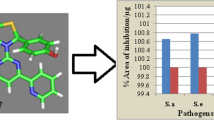
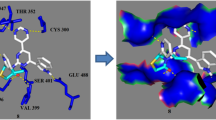
The 1,3-thiazolidin-4-one derivatives bearing piperonal and pyrimidine moieties (I–IX) was designed by ChemDraw Ultra 8.0 and screened for bioactivity score and physicochemical properties by molinspiration. The findings represented that the bioactivity score of all the designed compounds were lying in the zone of active drug molecule as enzyme inhibitor as well as the bioactivity score of compound-4 and 5, lying in the zone of active drug molecule as GPCR ligand. While the bioactivity score for other members were lying under the zone of the moderately active drug molecule, physicochemical properties for the designed compounds (I–IX) were observed in accordance with the Lipinski rule, Table 1. All the computationally bioactive compounds (I–IX), were then synthesized following the procedure as drawn in Fig. 1, that possessed four steps. First step involved the synthesis of (2E)-1-(1,3-benzodioxol-5-yl)-3-(pyridin-2/3/4-yl)prop-2-en-1-one (I), by the condensation reaction between Piperonal and 2- or 3- or 4- acetyl pyridine in ethanol sodium hydroxide under refluxing. In the second step (2E)-1-(1,3-benzodioxol-5-yl)-3-(pyridin- 2- or 3- or 4-yl)prop-2-en-1-one (A) was reacted with guanidine hydrochloride in presence of sodium to yield 4-(1,3-benzodioxol-5-yl)-6-(pyridin-2- or 3- or 4-yl)pyrimidin-2-amine (B). The third step dealt with the condensation reaction between the amine functional group of 4-(1,3-benzodioxol-5-yl)-6-(pyridin-2- or 3- or 4-yl)pyrimidin-2-amine (B) and 2, 3,4-pyrdine carboxaldehyde to yield the (E)-N-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2- or 3- or 4-yl)pyrimidin-2-yl]-1-(pyridin-2- or 3- or 4- yl)methanimine (C). The aimed compounds (I-IX) were prepared by refluxing the (E)-N-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2- or 3- or 4-yl)pyrimidin-2-yl]-1-(pyridin-2- or 3- or 4-yl)methanimine (C), thioglycolic acid in DMF and zinc chloride. Structural confirmation of the synthesized compounds was performed by the help of various spectroscopic methods like FT-IR, NMR (1H and 13C), Mass spectroscopy etc. The absence of FT-IR band at 1720 cm–1 due to the presence of HC=O and the presence of bands around 1653–1660 and 1407–1451 cm–1 due to (C=O) and (C=C) respectively confirmed the condensation between piperonal and 2/3/4-acetyl pyridine (A). The absence of bands around 1653–1660 cm–1 and 1407–1451 cm–1 due to (C=O) and (C=C) respectively and the simultaneous appearance of bands around 3290–3312 cm–1 because of amine group confirmed the synthesis of compound-B. The FTIR spectra of compound-C exhibited the absence of bands around 3290–3312 cm–1 due to the NH2 functional group and the appearance of the bands around 1622–1644 cm–1. FT-IR spectra of the synthesized compounds (I–IX), represented the characteristic band in the range 1050–1097, 1611–1635 and 1671–1710 cm–1, due to the presence of (C–N), (C=N) and (C=O) respectively. The appearance of the double doublets at 3.525–3.901 ppm due to H(1), 4.230–4.267 ppm due to H(2), 5.750–5.604 ppm due to H(3), confirmed the synthesis of I. While the absence of all these signals and the appearance of singlets for NH2 around 5.532–5.592 ppm recommended the formation of B. The 1H NMR spectra of compound C represented the absence of singlets around 5.532–5.592 ppm and the appearance of singlets around 8.129–8.204 ppm because of the HC=N that further confirmed the synthesis of compound C. Further structural confirmation of synthesized compounds (I–IX) was carried out by 1H NMR spectra, by the presence of characteristic singlet around 6.009–6.190 ppm and 2.709–2.823 ppm due to the presence of (O–CH2–O), (CH2-thiazolidinone nucleus) respectively and the disappearance of the singlet around 8.129–8.204 ppm confirmed the cyclization of Schiff bases to thiazolidin-one derivatives. Additionally, the structures were also confirmed by the 13C NMR spectra that exhibited the signals around 53.87–55.40 ppm due to (CH2), 100.88–101.33 ppm due to (O–C–O), 160.15–160.88 ppm because of (C=N), 162.10–163.44 ppm due to (C=N), 163.37–164.23 ppm due to (C=N), 164.39–165.06 ppm due to (C=N), 170.10–171.63 ppm due to (C=O). The compounds (I–IX) were assessed for antimicrobial therapeutic effects against the microbes (S. aureus (ATCC-25923), S. epidermidis (ATCC-29887), E. coli (ATCC-25922), P. mirabilis (ATCC-25933)). The microbial therapeutic effect was recorded in terms of zone of inhibitions and Minimum inhibitory concentrations and the results were compared with “Ciprofloxacin,” Table 2. The percent area of inhibition per microgram of the compounds was also calculated and represented in Fig. 2. The results revealed that compound-4 and compound-5 were found to represent better antimicrobial potential than ciprofloxacin against all the treated gram-positive and gram-negative pathogens, while other members of the series were also represented the significant antimicrobial potential. 1,3-thiazolidin-4-one bearing piperonal and pyrimidine moieties (I–IX) and the reference ciprofloxacin was assessed for percent viability of the cells against human hepatocellular carcinoma cell line (HepG2). The increasing concentrations (3.125–100 mmol/L) of the compounds (I–IX) and ciprofloxacin was treated with a sub-confluent the population of HepG2 cells and the number of viable cells was calculated according to the MTT protocol. Viable cells possess mitochondria that contained the succinate dehydrogenase system which reduces the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to water-insoluble purple formazan crystals, that after solubilization can be detected by spectrophotometrically. Only the viable cells will be able to produce formazan crystal, therefore, it can be hypothesized that the amount of formazan produced is directly proportional to the number of cells (viable). The percent viability of cells was found concentration-dependent as reported in Fig. 3, and the findings revealed that all the compounds (I–IX), as well as ciprofloxacin exhibited the viability ranging 93–98%, 73–78%, and 70–76% up to the concentrations 3.125, 50 , 100 mmol/L respectively. To support the obtained antimicrobial findings, the in silico molecular docking assessment was performed for all the designed compounds (I–IX), against the receptor GlcN-6-P-synthase, (PDB: 2VF5). To understand the level of H-bonding between the compounds (ligands) (I–IX) and the amino acid residues of the receptor GlcN-6-P-synthase by AutoDock Tool-1.5.6, AutoDock Vina, and the pymol. The structures were designed by ChemDraw Ultra 12.0 to generate the smiles that were copied and converted to the PDB files using an online smiles translator of all the ligands (I–IX). PDBQT were prepared using the AutoDock Tool-1.5.6, then docking was performed using AutoDock Vina to produced the docked file of ligand and receptors. Pymol was used to draw the docking image and the molecular docking images represented that H-bonding in between the compounds (I–IX) and the residues of GlcN-6-P-synthase, like ASP 474 (I–IX), SER 316 (I–VI), ASN 522 (I–IX), TRP 313 (V), on the other hand, ciprofloxacin exhibited the H-bonding with the residues THR 352, THR 302, LYS 485 and SER 401 Fig. 4. No similar H-bonding was observed by the compounds (I–IX) and ciprofloxacin. Besides this, the designed compounds (I–IX) were observed to possess the significant binding affinities that are as followed –7.7 to –7.0 (I), –7.7 to –7.3 (II), –7.7 to –7.0 (III), –7.6 to –7.0 (IV), –7.7 to –7.2 (V), –7.7 to –6.9 (VI), –7.7 to –7.1 (VII), –7.7 to –7.0 (VII), –7.7 to –6.8 (IX). Docked images of the most active compounds (IV–V) were also drawn exhibiting the different patterns of binding as publication, mesh, and the surface of the protein GlcN-6-P-synthase, (PDB: 2VF5) Fig. 5.
EXPERIMENTAL
ChemDraw Ultra 8.0 and ChemSketch were employed for preparing the structures of the compounds (I–IX) and ciprofloxacin and to generate the smiles for drug likeness analysis that was performed on the Molinspiration. The necessary chemicals, and reagents (pyridine-2-carboxaldehyde, pyridine-3-carboxaldehyde, pyridine-4-carboxaldehyde, 2-acetylpyridine, 3-acetylpyridine, 4-acetylpyridine, piperonal, guanidine hydrochloric acid, isopropyl alcohol, NaOH, glacial acetic acid, ethanol, Muller–Hinton agar, antibiotic agar, ciprofloxacin, dimethylsulfoxide, MTT, DMEM, FBS ) were obtained from the chemical companies like Sigma Aldrich, Merck, Germany, HIMEDIA and HyClone Laboratories, Logan, UT, USA. Progress of the reaction was observed by the help of thin-layer chromatographic plates. Analytical techniques to confirm the structures was performed by Heraeus Vario EL III analyzer for elemental analysis, Perkin-Elmer model 1600 FT-IR RX1 instrument for FT-IR spectra, Spectra Bruker Avance 300 MHz spectrometer for NMR (1H or 13C) spectra, Micromass Quattro II triple quadrupole mass spectrometer for mass spectra.
COMPUTATIONAL SCREENING
Chem Draw Ultra 8.0/Chem Sketch, the software was used for drawing (the structures and schematic diagram) and to generate the smiles that were used for the estimation of bioactivity and physicochemical score with the help mol inspiration (an online available software) [42–50].
Chemistry
General method for the preparation of (2E)-1-(1,3-benzodioxol-5-yl)-3-(pyridin-2/3/4-yl)prop-2-en-1-one (A). Piperonal (10 mmol) and the 2,3,4-acetyl pyridine (10 mmol) were added to the round bottom flask in 50 ml of ethanol, and was set on refluxing for 3 hours followed by the dropwise addition of 20% sodium hydroxide solution to yield (2E)-1-(1,3-benzodioxol-5-yl)-3-(pyridin-2/3/4-yl)prop-2-en-1-one (A). The reaction status was observed by thin-layer chromatographic plates under UV- cabinet. On completion, the yellow precipitate was obtained by filtration and dried under vacuum and recrystallized from diethyl ether [16]. IR νmax (cm–1): 1653–1660 (C=O), 1407–1451 (C=C); 1H NMR (CDCl3) δ (ppm): 3.525–3.901 H (1), 4.230–4.267 H (2), 5.750–5.604 H (3), 5.991–6.090 (s, 2H, O–CH2–O).
General method for the preparation of 4-(1,3-benzodioxol-5-yl)-6-(pyridin-2/3/4-yl)pyrimidin-2-amine (B). Guanidine hydrochloride (11 mmol), Na metal (11 mmol) was mixed in 50 mL of isopropanol in a round bottom flask, and set on refluxing for 3 hours. After 3 hours the (2E)-1-(1,3-benzodioxol-5-yl)-3-(pyridin-2/3/4-yl)prop-2-en-1-one (A) (10 mmol) was added to the mixture and set on refluxing for 8 h to yield the desired product (B). The reaction status was observed by the thin layer chromatographic plates under UV- cabinet and finally the desired product was obtained as precipitate that was filtered, dried and re-crystallized [16]. IR νmax (cm–1): 3290–3312 (NH2); 1H NMR (CDCl3) δ (ppm): 5.532–5.592 (NH2), 5.935–5.988 (s, 2H, O–CH2–O).
The general method for the preparation of (E)-N-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2/3/4-yl)pyrimidin-2-yl]-1-(pyridin-2/3/4-yl)methanimine (C). 4-(1,3-benzodioxol-5-yl)-6-(pyridin-2/3/4-yl)pyrimidin-2-amine (10 mmol) and pyridine-2,3 and 4-carboxaldehyde (10 mmol) were mixed in ethanol (50 mL), in a round bottom flask and set on refluxing followed by the addition of some droplets of glacial acetic acid. The solid precipitate was observed that was filtered and dried under vacuum and recrystallized using a proper solvent [8]. IR νmax (cm–1): 1622–1644 (HC=N); 1H NMR (CDCl3) δ (ppm): 8.129–8.204 (s, 1H, HC=N), 5.883–5.990 (s, 2H, O–CH2–O).
General procedure for the synthesis of 1,3-thiazolidin-4-one bearing piperonal pyrimidine moiety (I–IX). (E)-N-[4-(1,3-benzodioxol-5-yl)-6-(pyridin-2/3/4-yl)pyrimidin-2-yl]-1-(pyridin-2/3/4-yl)methanimine (10 mmol) and thioglycolic acid (10 mmol) was dissolved in DMF (50–60 mL) and added a small pinch of anhydrous ZnCl2 set on refluxing for 24 h. The reaction status was observed by thin-layer chromatographic plates under UV- cabinet, on completion of the final mixture was poured to the cold water to form a precipitate that was dried under vacuum to obtain desired compounds (I–IX) [32].
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-2-yl)-pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one (I). Yield: 90%; mp: 222–224°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1059 (C–N), 1614 (C=N), 1689 (C=O), 2942 (CH–Ar); 1H NMR (CDCl3) δ (ppm): 2.80 (s, 2H, H1, H2), 6.01 (s, 2H, H15, H16), 6.89–7.10 (m, 3H, H4, H5, H6), 7.21–7.31 (m, 4H, H8, H9, H10, H11), 7.38 (d, 1H, J = 8.7, H7), 7.48 (d, 1H, J = 5.4, H13), 7.50 (d, 1H, J = 7.8, H14), 7.53 (s, 1H, H3), 7.63 (s, 1H, H12); 13C NMR DMSO-d6 (ppm): 55.40 (CH2), 101.32 (O–C–O), 106.31, 109.22, 111.17, 112.33, 113.61, 114.41, 115.70, 120.50, 120.81, 121.49, 122.68, 123.40, 124.91, 130.50, 131.62, 132.54, 160.88 (C=N), 163.03 (C=N), 163.41 (C=N), 164.99 (C=N), 170.63 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-3-yl)-pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one (II). Yield: 86%; mp: 218–220°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1065 (C–N), 1623 (C=N), 1671 (C=O), 2960 (CH–Ar); 1H NMR (CDCl3) δ (ppm): 2.79 (s, 2H, H1, H2), 6.11 (s, 2H, H15, H16), 6.92–7.18 (m, 4H, H8, H9, H10, H11), 7.24–7.35 (m, 3H, H5, H6, H7), 7.37 (s, 1H, H4), 7.40 (d, 1H, J = 12.9 Hz, H13), 7.49 (d, 1H, J = 8.4 Hz, H14), 7.54 (s, 1H, H3), 7.61 (s, 1H, H12); 13C NMR DMSO-d6 (ppm): 54.32 (CH2), 100.88 (O–C–O), 107.27, 108.90, 110.20, 112.66, 114.02, 114.95, 115.88, 120.09, 120.94, 121.48, 123.10, 123.88, 124.17, 129.50, 130.90, 132.00, 160.24 (C=N), 162.10 (C=N), 163.53 (C=N), 165.06 (C=N), 170.10 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-4-yl)pyrimidin-2-yl]-2-(pyridin-2-yl)-1,3-thiazolidin-4-one (III). Yield: 85%; mp: 215–217°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1073 (C–N), 1620 (C=N), 1683 (C=O), 2985 (CH–Ar); 1H NMR (CDCl3) δ (ppm): 2.79 (s, 2H, H1, H2), 6.01 (s, 2H, H15, H16), 6.89–7.10 (m, 3H, H4, H5, H6), 7.21–7.31 (m, 4H, H8, H9, H10, H11), 7.38 (d, 1H, J = 11.7 Hz, H7), 7.48 (d, 1H, J = 7.2 Hz, H13), 7.50 (d, 1H, J = 13.5 Hz, H14), 7.53 (s, 1H, H12), 7.63 (s, 1H, H3); 13C NMR DMSO-d6 (ppm): 54.95 (CH2), 101.34 (O–C–O), 106.98, 107.80, 110.07, 111.50, 113.22, 114.26, 115.30, 120.41, 121.28, 122.15, 122.90, 123.10, 124.44, 130.33, 131.08, 132.44, 160.31 (C=N), 162.90 (C=N), 164.07 (C=N), 164.90 (C=N), 169.88 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-3-yl)-1,3-thiazolidin-4-one (IV). Yield: 92%; mp: 220–222°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1066 (C–N), 1611 (C=N), 1680 (C=O), 2990 (CH–Ar); 1H NMR (CDCl3) δ (ppm): 2.77 (s, 2H, H1, H2), 6.19 (s, 2H, H15, H16), 6.80–7.08 (m, 4H, H4, H5, H6, H7), 7.14–7.28 (m, 4H, H8, H9, H10, H11), 7.34 (d, 1H, J = 13.2 Hz, H13), 7.51 (d, 1H, J = 9.9 Hz, H14), 7.58 (s, 1H, H12), 7.67 (s, 1H, H3); 13C NMR DMSO-d6 (ppm): 55.35 (CH2), 101.12 (O–C–O), 105.50, 107.22, 110.03, 111.22, 112.30, 113.41, 115.11, 120.20, 120.91, 121.41, 122.67, 123.10, 124.31, 130.27, 131.37, 132.44, 160.51 (C=N), 163.42 (C=N), 164.10 (C=N), 164.82 (C=N), 170.55 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-3-yl)pyrimidin-2-yl]-2-(pyridin-3-yl)-1,3-thiazolidin-4-one (V). Yield: 85%; mp: 219–221°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1083 (C–N), 1627 (C=N), 1695 (C=O), 2975 (CH–Ar); 1H NMR (CDCl3) δ (ppm): 2.70 (s, 2H, H1, H2), 6.09 (s, 2H, H15, H16), 6.87–7.00 (m, 4H, H4, H5, H6, H7), 7.09–7.21 (m, 3H, H8, H9, H10), 7.28 (s, 1H, H11), 7.39 (d, 1H, J = 9 Hz, H13), 7.42 (d, 1H, J = 13.2 Hz, H14), 7.50 (s, 1H, H12), 7.55 (s, 1H, H3); 13C NMR DMSO-d6 (ppm): 54.67 (CH2), 101.33 (O–C–O), 106.80, 108.31, 110.42, 111.80, 112.42, 113.60, 114.84, 120.60, 121.10, 121.90, 122.90, 123.90, 124.30, 129.50, 131.70, 132.85, 160.15 (C=N), 162.90 (C=N), 163.37 (C=N), 164.59 (C=N), 171.63 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-4-yl)pyrimidin-2-yl]-2-(pyridin-3-yl)-1,3-thiazolidin-4-one (VI). Yield: 90%; mp: 217–219°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1050 (C–N), 1618 (C=N), 1679 (C=O), 2937 (CH–Ar); 1H NMR (CDCl3) δ (ppm): 2.77 (s, 2H, H1, H2), 6.04 (s, 2H, H15, H16), 6.81–7.12 (m, 4H, H4, H5, H6, H7), 7.21–7.31 (m, 4H, H8, H9, H10, H11), 7.39 (d, 1H, J = 7.2 Hz, H13), 7.45 (d, 1H, J = 12 Hz, H14), 7.51 (s, 1H, H12), 7.61 (s, 1H, H3); 13C NMR DMSO-d6 (ppm): 55.08 (CH2), 101.22 (O–C–O), 106.90, 107.37, 110.81, 112.80, 113.79, 114.83, 115.76, 120.70, 121.80, 122.30, 123.36, 124.40, 124.94, 130.64, 131.87, 132.19, 160.37 (C=N), 162.23 (C=N), 163.50 (C=N), 164.86 (C=N), 170.93 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-2-yl)pyrimidin-2-yl]-2-(pyridin-4-yl)-1,3-thiazolidin-4-one (VII). Yield: 82%; mp: 225–227°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1080 (C–N), 1633 (C=N), 1690 (C=O), 2878 (CH–Ar); 1H NMR (CDCl3) δ (ppm): 2.81 (s, 2H, H1, H2), 6.10 (s, 2H, H15, H16), 6.88–7.10 (m, 4H, H4, H5, H6, H7), 7.19–7.26 (m, 4H, H8, H9, H10, H11), 7.30 (d, 1H, J = 8.7 Hz, H13), 7.39 (d, 1H, J = 12 Hz, H14), 7.50 (s, 1H, H12), 7.60 (s, 1H, H3); 13C NMR DMSO-d6 (ppm): 54.91 (CH2), 101.10 (O–C–O), 106.34, 107.27, 110.55, 111.80, 112.50, 1143.30, 114.77, 119.50, 120.30, 121.73, 122.66, 123.90, 124.85, 130.50, 131.89, 132.64, 160.18 (C=N), 163.24 (C=N), 164.23 (C=N), 165.07 (C=N), 171.17 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-3-yl)pyrimidin-2-yl]-2-(pyridin-4-yl)-1,3-thiazolidin-4-one (VIII). Yield: 95%; mp: 228–230°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1097 (C–N), 1630 (C=N), 1710 (C=O), 2908 (CH–Ar); 1H NMR (CDCl3) δ (ppm): 2.82 (s, 2H, H1, H2), 6.10 (s, 2H, H15, H16), 6.89–7.07 (m, 4H, H4, H5, H6, H7), 7.12–7.29 (m, 3H, H9, H10, H11), 7.34 (s, 1H, H8), 7.43 (d, 1H, J = 14.4 Hz, H13), 7.49 (d, 1H, J = 7.8 Hz, H14), 7.53 (s, 1H, H12), 7.57 (s, 1H, H3); 13C NMR DMSO-d6 (ppm): 55.20 (CH2), 101.18 (O–C–O), 105.33, 107.20, 110.27, 112.30, 113.29, 114.21, 115.90, 121.10, 122.38, 123.10, 123.90, 124.06, 125.19, 130.74, 131.77, 132.88, 160.33 (C=N), 163.27 (C=N), 163.98 (C=N), 164.39 (C=N), 170.10 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
3-[4-(1,3-Benzodioxol-5-yl)-6-(pyridin-4-yl)pyrimidin-2-yl]-2-(pyridin-4-yl)-1,3-thiazolidin-4-one (IX). Yield: 96%; mp: 227–229°C; Grey yellow crystals; Anal. calc. for C24H17N5O3S: C 63.29, H 3.76, N 15.38; Found: C 63.32, H 3.82, N 15.41; IR νmax (cm–1): 1069 (C–N), 1635 (C=N), 1700 (C=O), 2989 (CH-Ar); 1H NMR (CDCl3) δ (ppm): 2.80 (s, 2H, H1, H2), 6.00 (s, 2H, H15, H16), 6.78–6.98 (m, 4H, H4, H5, H6, H7), 7.13–7.21 (m, 4H, H8, H9, H10, H11), 7.31 (d, 1H, J = 12.3 Hz, H13), 7.39 (d, 1H, J = 11.4 Hz, H14), 7.43 (s, 1H, H12), 7.53 (s, 1H, H3); 13C NMR DMSO-d6 (ppm): 53.87 (CH2), 101.25 (O–C–O), 106.33, 109.29, 111.41, 112.15, 113.70, 114.55, 115.52, 120.20, 120.19, 121.31, 122.72, 123.57, 124.35, 130.00, 131.47, 132.63, 160.28 (C=N), 163.44 (C=N), 163.97 (C=N), 164.90 (C=N), 170.33 (C=O); ESI-MS (m/z): [M+ + 1] 456.11 (456.15).
BIOLOGY
Antimicrobial Screening
Disc diffusion protocol with necessary modification was followed to assess the antimicrobial potential of the synthesized compounds (I–IX) against, the gram-positive and gram-negative pathogens (S. aureus (ATCC-25923), S. epidermidis (ATCC-29887), E. coli (ATCC-25922), P. mirabilis (ATCC-25933)). The preparation of the suspension of the bacterial cells was performed by McFarland Protocol, the suspensions were further added to the antibiotic agar and transferred to the agar plate under the UV-laminar hood. On the other hand, the synthesized compounds I–IX were subjected to the preparation of the stock the solution that was prepared by dissolving 1 mg of each in 100 mL of DMSO. To estimate the antimicrobial effect of these compounds, the paper discs of 6 mm diameter were prepared and dipped into the solutions of each compound and were placed on the agar plate to find out the zone of inhibition. Dilutions of the synthesized compounds were made to estimate the MIC, Minimum concentration of the test compounds to inhibit the growth of the pathogen, MIC, by macro dilution method [51–58].
Assessment of Percent of Cell Viability
The percent viability of the cells for the prepared compounds 1–10 was performed against HepG2 (Human hepatocellular carcinoma). The cells were grown Dulbecco’s modified Eagle’s medium with 10% heat-activated fetal bovine serum, and (100 units/mL penicillin, 100 mg/mL streptomycin, and 2.5 mg/mL amphotericin B) and incubated at 37°C in an atmosphere containing (95% air/5% CO2) [59, 60].
Molecular Docking
Aautodock-tools 1.5.6, Autodock-Vina, and Pymol software was employed to perform the molecular docking assessment against the GlcN-6-P-synthase, (PDB: 2VF5) as per the method described in [16, 50, 51, 61, 62].
CONCLUSION
1,3-Thiazolidin-4-one derivatives bearing piperonal and pyrimidine moiety (I–IX), were designed and screened for bioactivity score and physicochemical properties. The bioactivity score of the designed compounds was lying under the zone of the active drug molecule. The bioactive compounds (I–IX) were then synthesized and characterized by various spectroscopic methods like FT-IR, NMR (1H and 13C), Mass spectroscopy. The compounds were then tested for antimicrobial potential against gram-positive and gram-negative bacteria and the results portrayed that compound (IV–V) represented better antimicrobial potential than standard drug ciprofloxacin. To assist the in vitro antimicrobial results the molecular docking studies were performed and revealed the significant H-bonding with the receptor GlcN-6P-Synthase with good binding affinity. Further studies in this platform will guide to develop a potential chemo-core that can be used to cure antimicrobial infections.
REFERENCES
Babu, K.R., Eeshwaraiah, B., Aravind, D., Harshadas, M.M., Rallabaldi, M.R., Apurba, B., and Rakeshwar, B., Monatsh Chem., 2008, vol. 139, pp. 179–181. https://doi.org/10.1007/s00706-007-0772-5
Kulandaivelu, U., Padmini, V.G., Suneetha, K., Shireesha, B., Vidyasagar, J.V., Rao, T.R., Jayaveera, K.N., Basu, A., and Jayaprakash, V., Arch. Pharm., 2011, vol. 344, pp. 84–90. https://doi.org/10.1002/ardp.201000201
Rohit, K., Shabana, I.K., Aparna, B., Mohit, T., and Diwan, S.R., Eur. J. Med. Chem., 2017, vol. 131, pp. 126–140. https://doi.org/10.1016/j.ejmech.2017.03.007
Hanwen, W., Fei, M., Shuaishuai, N., Feifei, C., Baoli, L., Xiaoxia, Q., Linghao, H., Manjiong, W., Xinyu, Z., Jin, Z., Lefu, L., and Jian, L., Eur. J. Med. Chem., 2018, vol. 145, pp. 235–251. https://doi.org/10.1016/j.ejmech.2017.12.090
Leite, A.C.L., da Silva, K.P., de Souza, I.A., de Araujo, J.M., and Brondali, D.J., Eur. J. Med. Chem., 2004, vol. 39, pp. 1059–1065. https://doi.org/10.1016/j.ejmech.2004.09.007
Prasanthi, G., Prasad, K.V.S.R.G., and Bharathi, K., Eur. J. Med. Chem., 2013, vol. 66, pp. 516–525. https://doi.org/10.1016/j.ejmech.2013.06.006
Moreira Leal, C., Lopes Pereira, S., Kummerle, A.E., Moreira Leal, D., Tesch, R., de Sant'Anna, C.M.R., Fraga, C.A.M., Barreiro, E.J., Takashi Sudo, R., Zapata-Sudo, G., Eur. J. Med. Chem., 2012, vol. 55, pp. 49–57. https://doi.org/10.1016/j.ejmech.2012.06.056
Arshad, M., Bhat, A.R., Pokharel, S., Ki,m, J-E., Lee, E.J., Athar, F., and Choi, I., Eur. J. Med. Chem., 2014, vol. 71, pp. 229–236. https://doi.org/10.1016/j.ejmech.2013.11.008
Wani, M.Y., Bhat, A.R., Azam, A., Choi, I., and Athar, F., Eur. J. Med. Chem., 2012, vol. 48, pp. 313–320. https://doi.org/10.1016/j.ejmech.2011.12.033
Chavarria, D., Fernandes, C., Silva, V., Silva, C., Gil-Martins, E., Soares, P., Silva, T., Silva, R., Remião, F., Oliveira, P. J., and Borges, F., Eur. J. Med. Chem., 2020, vol. 185, p. 111 770. https://doi.org/10.1016/j.ejmech.2019.111770
Singh, I.P., Jain, S.K., and Kour, A., Eur. J. Med. Chem., 2010, vol. 4, p. 33.
da Silva Ferreira, W., Freire-de-Lima, L., Barbosa Saraiva, V., Alisson-Silva, F., Mendonca-Previato, L., Previato, J.O., Echevarria, A., and Freire de Lima, M.E. Bioorg. Med. Chem., 2008, vol. 16, pp. 2984–2991.
Gupta, S.D., Rao, G.B., Bommaka, M.K., Raghavendra, N.M., Aleti, S., Arab. J. Chem., 2016, vol. 9, pp. 1878–5352. https://doi.org/10.1016/j.arabjc.2014.08.004
Wang, S., Bao, L., Song, D., Wang, J., and Cao, X., Bioorg. Med. Chem. Lett., 2019, vol. 29, p. 126 661. https://doi.org/10.1016/j.bmcl.2019.126661
Brum, J.O.C., Neto, D.C.F., de Almeida, J.S.F.D., Lima, J.A., Kuca, K., França, T.C.C., and Figueroa-Villar, J.D. Int. J. Mol. Sci., 2019, vol. 20, p. 3944. https://doi.org/10.3390/ijms20163944
Arshad, M., J. Iran Chem. Soc., 2020, vol. 17, pp. 1305–1315. https://doi.org/10.1007/s13738-020-01855-9
Ghoneim, A.A., El-Farargy, A.F., Elkanzi, N.A.A. J. Iran Chem. Soc., 2019, vol. 16, pp. 319–325. https://doi.org/10.1007/s13738-019-01768-2
Hamid, A.M.A., Shehta, W., J. Iran Chem. Soc., 2018, vol. 15, pp. 2771–2779. https://doi.org/10.1007/s13738-018-1464-2
Bhosle, M.R., Andil, P., Wahul, D., Bondle, G.M., Sarkate, A., Tiwari, S.V., J Iran Chem Soc., 2019, vol. 16, pp. 1553–1561. https://doi.org/10.1007/s13738-019-01633-2
Ślifirski, G., Król, M., Kleps, J., Podsadni, P., Belka, M., Bączek, T., Siwek, A., Stachowicz, K., Szewczyk, B., Nowak, G., Bojarski, A., Kozioł, A. E., Turło, J., Herold, F., Eur. J. Med. Chem., 2019, vol. 180, pp. 383–397. https://doi.org/10.1016/j.ejmech.2019.07.027
da S. Falcao, E.P., de Melo, S.J., Srivastava, R.M., de A. Catanho, M.T.J., and Do Nascimento, S. C., Eur. J. Med. Chem., 2006, vol. 41, pp. 276–282. https://doi.org/10.1016/j.ejmech.2005.09.009
Kotaiah, Y., Nagaraju, K., Harikrishna, N., Venkata Rao, C., Yamini, L., Vijjulatha, M., Eur. J. Med. Chem., 2014, vol. 75, pp. 195–202. https://doi.org/10.1016/j.ejmech.2014.01.006
Kaur, H., Balzarini, J., Kock, C. de., Smith, P.J., Chibale, K., Singh, K., Eur. J. Med. Chem., 2015, vol. 101, pp. 52–62. https://doi.org/10.1016/j.ejmech.2015.06.024
Maurya, S.S., Bahuguna, A., Khan, S.I., Kumar, D., Kholiya, R., Rawat, D.S., Eur. J. Med. Chem., 2019, vol. 162, pp. 277–289. https://doi.org/10.1016/j.ejmech.2018.11.021
Lee, H.W., Kim, B.Y., Ahn, J.B., Kang, S.K., Lee, J.H., Shin, J.S., Ahn, S.K., Lee, S.J., Yoon, S.S., Eur. J. Med. Chem., 2005, vol. 40, pp. 862–874. https://doi.org/10.1016/j.ejmech.2005.03.019
Verbitskiy, E.V., Cheprakova, E.M., Slepukhin, P.A., Kravchenko, M.A. Skornyakov, S.N., Rusinov, G.L., Chupakhin, O.N., Charushin, V.N., Eur. J. Med. Chem., 2015, vol. 97, pp. 225–234. https://doi.org/10.1016/j.ejmech.2015.05.007
Hafez, H.N., Hussein, H.A.R., El-Gazzar, Abdel-Rahman B.A., Eur. J. Med. Chem., 2010, vol. 45, pp. 4026–4034. https://doi.org/10.1016/j.ejmech.2010.05.060
Zhu, M., Ma, L., Zhou, H., Dong, B., Wang, Y., Wang, Z., Zhou, J., Zhang, G., Wang, J., Liang, C., Cen, S., Wang, Y., Eur. J. Med. Chem., 2020, vol. 185, p. 111 866. https://doi.org/10.1016/j.ejmech.2019.111866
Keri, R. S., Hosamani, K. M., Shingalapur, R. V., Hugar, M. H., Eur. J. Med. Chem., 2010, vol. 45, pp. 2597–2605. https://doi.org/10.1016/j.ejmech.2010.02.048
Jin, X., Merrett, J., Tong, S., Flower, B., Xie, J., Yu, R., Tian, S., Gao, L., Zhao, J., Wang, X., Jiang, Tao, Proud, C. G., Eur. J. Med. Chem., 2019, vol. 162, pp. 735–751. https://doi.org/10.1016/j.ejmech.2018.10.070
Shehab, W.S., El-Shwiniy, W.H., J. Iran. Chem. Soc., 2018, vol. 15, p. 431. https://doi.org/10.1007/s13738-017-1244-4
Arshad, M., Khan, M. S., Nami, S. A. A., Ahmad, D., Rus. J. Gen. Chem., 2018, vol. 88, pp. 2154–2162. https://doi.org/10.1134/S1070363218100213
Carradori, S., Bizzarri, B., D’Ascenzio, M., Monte, C. D., Grande, R., Rivanera, D., Zicari, A., Mari, E., Sabatino, M., Patsilinakos, A., Rino Ragno, R., and Daniela Secci, D., Eur. J. Med. Chem., 2017, vol. 140, pp. 274–292. https://doi.org/10.1016/j.ejmech.2017.09.026
Pejović, A., Denić, M.S., Stevanović, D., Damljanović, I., Vukićević, M., Kostova, K., Tavlinova-Kirilova, M., Randjelović, P., Stojanović, N.M., Bogdanović, G.A., Blagojević, P., D’hooghe, M., Radulović, N.S., and Vukićević, R.D., Eur. J. Med. Chem., 2014, vol. 83, pp. 57–73. https://doi.org/10.1016/j.ejmech.2014.05.062
Koppireddi, S., Komsani, J.R., Avula, S., Pombala, S., Vasamsetti, S., Kotamraju, S., and Yadla, R., Eur. J. Med. Chem., 2013, vol. 66, pp. 305–313. https://doi.org/10.1016/j.ejmech.2013.06.005
Rawal, R.K., Tripathi, R., Katti, S.B., Pannecouque, C., and De Clercq, E., Eur. J. Med. Chem., 2008, vol. 43, pp. 2800–2806. https://doi.org/10.1016/j.ejmech.2007.12.015
Raza, S., Srivastava, S.P., Srivastava, D.S., Srivastava, A.K., Haq, W., and Katti, S.B., Eur. J. Med. Chem., vol. 63, 2013, pp. 611–620. https://doi.org/10.1016/j.ejmech.2013.01.054
Revelant, G., Huber-Villaume, S., Dunand, S., Kirsch, G., Schohn, H., and Hesse, S., Eur. J. Med. Chem., 2015, vol. 94, pp. 102–112. https://doi.org/10.1016/j.ejmech.2015.02.053
Barbosa, V.A., Baréa, P., Mazia, R.S., Ueda-Nakamura, T., da Costa, W.F., Foglio, M.A., Ruiz, A.L.T.G., de Carvalho, J.E., Vendramini-Costa, D.B., Nakamura, C., and Sarragiotto, M.H., Eur. J. Med. Chem., 2016, vol. 124, pp. 1093–1104. https://doi.org/10.1016/j.ejmech.2016.10.018
Trotsko, N., Golus, J., Kazimierczak, P., Paneth, A., Przekora, A., Ginalska, G., and Wujec, M., Eur. J. Med. Chem., 2020, vol. 189, p. 112 045. https://doi.org/10.1016/j.ejmech.2020.112045
da Silva, D.S., da Silva, C.E.H., Soares, M.S.P., Juliana Hofstatter Azambuja, de Carvalho, T.R., Zimmer, G.C., Frizzo, C.P., Braganhol, E., Spanevello, R.M., and Cunico, W., Eur. J. Med. Chem., 2016, vol. 124, pp. 574–582. https://doi.org/10.1016/j.ejmech.2016.08.057
Alodeani, E.A., Arshad, M., and Izhari, M.A. Asian Pac.J. Health Sci. 2015, vol. 2, pp. 41–47. https://www.apjhs.com/pdf/8-Antileishmanial-screening-physicochemical-properties-and-drug-likeness-of-pyrazole-carbaldehyde-derivatives.pdf.
Alodeani, E.A., Arshad, M., and Izhari, M.A., Eur. J. Pharm. Med. Res., 2015, vol. 2, pp. 324–328. http://www.ejpmr.com/admin/assets/article_issue/ 1448880734.pdf.
Alodeani, E.A., Arshad, M., and Izhari, M.A. Asian Pac. J. Trop. Biomed., 2015, vol. 5, pp. 676–683. https://doi.org/10.1016/j.apjtb.2015.04.010
Alodeani, E.A., Arshad, M., and Izhari, M.A. Eur. J. Pharm. Med. Res., 2015, vol. 2, pp. 296–301. http://www.ejpmr.com/admin/assets/article_issue/ 1446625932.pdf.
Arshad, M. and Shadab, M., Eur. J. Pharm. Med. Res., 2017, vol. 4, pp. 364–368. http://www.ejpmr.com/ home/abstract_id/2154.
Arshad, M. and Shadab, M., Eur. J. Pharm. Med. Res. 2017, vol. 4, pp. 447–454. https://www.ejpmr.com/home/abstract_id/2267.
Arshad, M., Eur. J. Pharm. Med. Res., 2017, vol. 4, pp. 511–517. http://www.ejpmr.com/admin/assets/article_issue/1512459098.pdf.
Arshad, M., Russ. J. Gen. Chem., 2018, vol. 88, pp. 1886–1891. https://doi.org/10.1134/S1070363218090207
Arshad, M., Khan, M.S., and Nami, S.A.A., Russ. J. Gen. Chem., 2019, vol. 89, pp. 1851–1858. https://doi.org/10.1134/S1070363219090202
Arshad, M., Int. J. Pharm. Sci. Res., 2018, vol. 9, pp. 35–41. https://www.ijpsr.info/docs/IJPSR18-09-02-017.pdf.
Alodeani, E.A., Arshad, M., Izhari, M.A. Europ.J. Biomed. and Pharm. Sci., 2014, vol. 1, pp. 504–527. http://www.ejbps.com/admin/assets/article_issue/ volume_1_december_issue_3/1419598913.pdf.
Arshad, M., Bhat, A.R., Hoi, K.K., Choi, I., and Athar, F, Chin. Chem. Lett., 2017, vol. 28, pp. 1559–1565. https://doi.org/10.1016/j.cclet.2016.12.037
Arshad, M., Khan, M.S., Nami, S.A.A., and Ahmad, D., SNAppl. Sci., 2019, vol. 1, pp. 1–8. https://doi.org/10.1007/s42452-019-0571-8
Iram, N.E., Khan, M.S., Jolly, R., Arshad, M., Alam Alam, P., Khan, R.H., and Firdaus, F., J. Photochem. Photobiol. B: Biol., 2015, vol. 153, pp. 20–32. https://doi.org/10.1016/j.jphotobiol.2015.09.001
Nami, S.A.A., Arshad, M., Shakir, M., Khan, M.S., Alam, M., Lee, D.-U., Park, S., and Sarikavakli, N., Polym. Adv. Technol., 2015, vol. 26, pp. 1627–1638. https://doi.org/10.1002/pat.3591
Nami, S.A.A., Khan, M.S., Arshad, M., Raza M.A., and Khan, I., Polym. Adv. Technol., 2017, vol. 28, pp. 10–19. https://doi.org/10.1002/pat.3846
Kareema, A., Laxmi, Arshad M., Nishat, N., J. Photochem. Photobiol. B: Biol., 2016, vol. 160, pp. 163–171. https://doi.org/10.1016/j.jphotobiol.2016.03.030
Gupta, M.K., Neelakantan, T.V., Sanghamitra, M., Tyagi, R.K., Dinda, A., Maulik, S., Mukhopadhyay, C.K., and Goswami, S.K., Antioxid. Redox Signal., 2006, vol. 8, pp. 1081–1093.
Mosmann, T., J. Immunol. Methods., 1983, vol. 65, p. 55. https://doi.org/10.1016/0022-1759(83)90303-4
Nayab, P.S., Arif, R, and Arshad, M., Heterocycl.Lett., 2015, vol. 5, pp. 223–239. https://www.heteroletters.org/issue25/PDF/Paper-9.pdf.
Arshad, M., SNAppl. Sci., 2020, vol. 2, p. 467. https://doi.org/10.1007/s42452-020-2243-0
ACKNOWLEDGMENTS
The author, Dr. Mohammad Arshad, is highly thankful to the Dean, College of Medicine, Al-Dawadmi, Shaqra University, Kingdom of Saudi Arabia for his cooperation to accomplish this work.
Funding
There was no funding allotted for the study the authors performed the study using their own resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest.
Additional information
Corresponding author: phone: +966559712511; e-mail: mohdarshad1985@gmail.com; m.arshad@su.edu.sa.
Rights and permissions
About this article
Cite this article
Mohammad Arshad Design, Drug-Likeness, Synthesis, Characterization, Antimicrobial Activity, Molecular Docking, and MTT Assessment of 1,3-Thiazolidin-4-one Bearing Piperonal and Pyrimidine Moieties. Russ J Bioorg Chem 46, 599–611 (2020). https://doi.org/10.1134/S1068162020040056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020040056