Abstract—
The natural nitrogen-15 abundance method does not always make it possible to calculate the rate of symbiotic nitrogen fixation by legumes and needs to be improved. Five legume species typical for the alpine belt of the Teberda Nature Reserve (Anthyllis vulneraria, Astragalus levieri, Hedysarum caucasicum, Oxytropis kubanensis, and Trifolium polyphyllum) have been grown from seeds under conditions of laboratory vegetation experiment. The results show that nodules on the roots of these plants are formed at early stages of their development; Trifolium polyphyllum does not form nodules either under high-mountain conditions or during growth in the laboratory. The natural 15N abundance in the leaves of legume plants in alpine ecosystems makes it possible to calculate the contribution of atmospheric N2 to nitrogen nutrition as early as the first year of their development, while the isotopic nitrogen composition of the roots does not allow this parameter to be determined. The calculation of atmospheric nitrogen fixation rate should take into account isotope fractionation between symbiotic bacteria (nodules) and the host plant; otherwise, the proportion of fixed nitrogen in plant nutrition may be underestimated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Symbiotic nitrogen fixation is essential for the functioning of ecosystems. Although the estimates of the global input of fixed atmospheric N2 to natural terrestrial ecosystems may differ severalfold depending on what calculation methods are used (from 195 [1] to 128 [2] or even 44 Tg N per year [3]), they nevertheless indicate that large amounts of nitrogen are bound from the atmosphere and involved in the biological cycle. In particular, plants with symbiotic nitrogen fixation in high mountains prevail at first succession stages during glacier melting, thereby contributing to the accumulation of nitrogen in the soil and increasing its availability for future colonizing plants [4].
The quantitative assessment of symbiotic nitrogen fixation based on analysis of the natural isotopic composition of plant nitrogen began to be widely used in the 1970s–1980s. The method is based on the concept that the concentration of 15N isotope in nitrogen-containing soil components usually differs from its concentration in atmospheric N2. As a result, the isotopic composition of N in nitrogen-fixing species that take it up from both soil and atmosphere usually differs from that in species that use only its soil sources. This makes it possible to calculate the contribution of nitrogen fixation to the nitrogen nutrition of plants using the principle of isotope mixing and isotopic mass balance [5]. The advantages and limitations of this method for estimating the proportion of fixed nitrogen in plant nutrition and its contribution to the total soil nitrogen pool in different ecosystems have been characterized in field studies and vegetation experiments [5, 6].
As shown in a series of studies [4, 7–12], the process of symbiotic nitrogen fixation in high mountains (where nitrogen availability to plants is generally low) is active under conditions of low temperatures, acid soils, and low phosphorus availability and provides a significant proportion of nitrogen nutrition for legumes (30–100%). The contribution of legumes to the nitrogen supply for the ecosystem (74–810 mg/m2 per year) depends mainly on their proportion in the biomass of phytocenosis [7, 8, 13]. Legume plants that actively fix atmospheric nitrogen increase its availability in the soil, thereby influencing other plants in alpine phytocenosis [14].
Although an increasing number of attempts are made to improve the quantitative assessment of nitrogen that enters the soils of natural ecosystems as a result of symbiotic nitrogen fixation [15], a number of issues still require clarification. Theoretically, the calculation should use the weighted average δ15N value for the whole plant; however, this is often hardly possible during the study of perennial legumes forming strong deep root systems under natural ecosystem conditions. In addition, there are almost no data on the isotopic composition of nitrogen in different parts of legume plants (leaves, roots, and nodules) in high-mountain ecosystems, and the proportion of fixed nitrogen in this case is often estimated from the δ15N value in the aboveground plant parts [7, 12]. The rate of nodule formation on the roots of perennial alpine legumes is also unknown, as well as the degree to which the nitrogen isotopic composition in the roots and nodules influences the estimate of the contribution of nitrogen fixation to their nutrition. To resolve these issues, we carried out a laboratory vegetation experiment on growing legume plants of the alpine belt of the Northwestern Caucasus from seeds and employed the method of natural 15N abundance to assess the activity of the symbiotic fixation of atmospheric nitrogen.
OBJECT AND METHODS
We cultivated five legume species that are typical of habitats with the poorest (lichen heaths) and richest (geranium–hedysarum meadows) amount of mineral nutrients in the alpine belt of the Teberda Reserve (Northwestern Caucasus). The former include Trifolium polyphyllum, Anthyllis vulneraria, Astragalus levieri, and Oxytropis kubanensis; the latter include Hedysarum caucasicum. A mixture of quartz sand (50% by weight) and humus horizon of the soil from the alpine lichen heath (50%) was used as a cultivation substrate; the properties of the mixture were described in detail previously [16]. The seeds of each species were planted in early May in ten growing pots with a volume of 0.8 L. In July (at the age of 2.5 months) and August (4 months), plant samples were taken for analysis from five pots in each of the two periods. The plants extracted from the substrate were divided into the aboveground and belowground parts, and the roots were thoroughly washed with distilled water and dried. The dried samples were weighed, ground in a Retsch MM 200 vibration mill, and analyzed for the nitrogen content and isotopic composition on a Thermo Flash 1112 elemental analyzer and a Thermo Delta V Plus isotope mass spectrometer at the Common Use Center of the Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences.
In two species, Anthyllis vulneraria and Hedysarum caucasicum, which formed a sufficient number of nodules, their nodules were separated from the roots, weighed, and analyzed for the content and isotopic composition of nitrogen as described above.
The contribution of nitrogen fixation (Nbiol, %) to the plant nitrogen was calculated by the formula
where δ15Ncontr is the 15N natural abundance in the control plant species, which does not have symbiotic N2 fixation; δ15Nfix is the 15N natural abundance in the nitrogen-fixing species; δ15N0 is the 15N natural abundance in the nitrogen-fixing species grown in a nitrogen-free medium (taking into account isotope fractionation during nitrogen fixation).
The alpine ecosystems of the Teberda Nature Reserve provide a unique possibility of using as a control species the legume T. polyphyllum, which does not form symbiosis with nitrogen-fixing bacteria but is taxonomically and functionally similar to nitrogen-fixing legumes [12]. This excludes the problem of uncertainty in the selection of control species [5].
The δ15N0 value is not determined experimentally in most studies, and the authors refer to the values available from the literature (0 to –1‰) [4, 10, 12]. Recent studies confirm that this value for legume plants is generally close to the above range, although it may slightly differ between the aboveground and belowground plant parts [15]. Nbiol was calculated at δ15N0 = –0.6‰; this value proved to be most consistent with the results obtained for legumes in the alpine lichen heath under field conditions using the methods of natural 15N abundance and 15N isotopic label dilution [12].
All the data were processed to calculate mean values and estimate the significance of observed differences according to Student’s t-test.
RESULTS AND DISCUSSION
Formation of Plant and Nodule Biomass
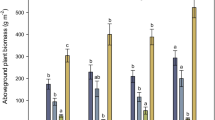
Different species produced different amounts of biomass (Fig. 1, Table 1). Anthyllis vulneraria grew most intensely, forming plants with an average biomass of about 90 mg in the aboveground part and about 20 mg in the belowground part after 2.5 months. The aboveground biomass produced by H. caucasicum and T. polyphyllum was 2.5 times lower. Similarly to A. vulneraria, the belowground biomass of T. polyphyllum was approximately 4.5 times lower than its aboveground biomass, while the aboveground and belowground biomasses of H. caucasicum were almost equal. The biomass was the lowest in two species that most actively fixed atmospheric nitrogen under field conditions [12], namely, A. levieri and O. kubanensis. The aboveground parts of these plants weighted about 14 mg and their belowground biomass was only 3–4 mg.
The biomass of some plant species increased by the age of 4 months, while that of other species remained unchanged (Table 1). The latter included both A. vulneraria, which was actively growing during the first 2.5 months, and O. kubanensis, whose growth, on the contrary, was poor during this period. Other species increased their biomass by a factor of 1.5–2 both in aboveground and belowground parts. Therefore, young legume plants in the first year differ from perennial plants by a low growth rate of their belowground biomass (according to the results of excavations of the H. caucasicum root system under field conditions, the average aboveground to belowground biomass ratio was 1 : 5).
Nodules differing in number and size were formed on the roots of young plants of all species except one, T. polyphyllum (Fig. 1, Table 1). In our previous study, T. polyphyllum did not form nodules and showed no symbiotic nitrogen fixation under the conditions of alpine lichen heath [12]; these features were also not observed when the phosphorus availability was increased and the soil acidity was reduced [17]. In turn, this research showed that this species did not form nodules even at higher growing temperatures than the temperature of field conditions. This confirms the phenomenon unique for herbaceous extratropical legumes: T. polyphyllum can be considered the only known exception to the generally accepted concept that all of them are obligate nitrogen fixing symbiotrophs [18]. In particular, all close relatives of T. polyphyllum intensely fix nitrogen at high elevations of other mountain systems, e.g., T. alpinum in the Swiss Alps [19] and T. dasyphyllum in the Colorado Rocky Mountains [7, 20].
Neither the number nor the size of nodules formed on 2.5-month-old plants changed after 1.5 months. Their numbers on the roots were the highest in A. vulneraria (15–25 nodules per plant), lower in H. caucasicum (3–8) and still lower in A. levieri and O. kubanensis (1–4 nodules per plant) (Table 1). The abundant spherical nodules were small (with an average diameter of 1 mm and weight of 0.1 mg) on A. vulneraria roots, while the rare ellipsoidal nodules were noticeably larger on the roots of other plant species, reaching a length of 3–5 mm at an average weight of 0.2–0.3 mg. The nodules of H. caucasicum were the largest (Fig. 2, Table 1). A tenfold difference was revealed between the weight of small nodules of A. vulneraria and weight of large nodules of O. kubanensis growing in nature [12].
Concentration and Isotopic Composition of Nitrogen
The nitrogen concentration in the aboveground part of 2.5-month-old plants varied from 2.89% (in T. polyphyllum) to 2.96–4.24% (in other species) (Table 2). These values proved to be higher than those for plants growing under natural conditions (2.05 and 2.64–3.30%, respectively [12]). Unlike in the aboveground part, the nitrogen concentration in the roots of T. polyphyllum was not minimal (2.94%). It was lower in two other nitrogen-fixing species: 1.76% in H. caucasicum and 2.76% in A. vulneraria; at the same time, it was higher for O. kubanensis and A. levieri (3.30 and 3.83%, respectively). According to our unpublished data, the nitrogen concentration was significantly lower not only in the aboveground part of plants growing under field conditions but also in the perennial roots of legume plants in alpine ecosystems: the minimum concentration was 0.93% in the roots of T. polyphyllum and 1.92–2.03% in the roots of other legumes.
The nodules of A. vulneraria and H. caucasicum had the highest nitrogen concentrations: 4.94 and 4.59%, respectively.
The concentration of nitrogen decreased in the aboveground parts of all plant species at the age of 4 months and this level was fully suitable for plants growing in nature. Trifolium polyphyllum still had the minimum value (1.92%), while the N concentration for the other species was 2.10 to 3.48%. The nitrogen concentration in the roots changed to a lower extent; it significantly decreased only in T. polyphyllum (up to 2.23%) and A. vulneraria (up to 2.33%), remained unchanged in three other species, and was still the lowest in the roots of H. caucasicum (1.81%).
The nitrogen isotopic composition did not significantly differ between the aboveground and belowground organs of all legume species (except the roots of A. vulneraria) at the age of 2.5 months and was close to the isotopic composition of atmospheric nitrogen: the δ15N values varied from –0.52 to –0.37‰. The δ15N value for T. polyphyllum proved to be closest to the atmospheric value (–0.08‰ for the aboveground part and +0.03‰ for the roots). The δ15N value was 4.95‰ in the roots of A. vulneraria.
There were some age-related changes in the nitrogen isotopic composition of plants. On the whole, they were characterized by a trend towards decrease in the δ15N value (approximately by 1‰); however, this decrease was statistically significant only in O. kubanensis, the roots of A. vulneraria, and the aboveground parts of T. polyphyllum. The δ15N value still remained highly positive for the roots of A. vulneraria (3.21‰). Compared to other parts of plants, the nodules were significantly enriched in heavy nitrogen isotope (the δ15N value was 8.45‰ for A. vulneraria and 4.70‰ for H. caucasicum). It was previously shown that nitrogen in nodules was often (but not always) enriched in 15N isotope (δ15N could exceed 10‰) and its accumulation in nodules was associated with bacterial cells [5]. This accumulation corresponds to the general pattern of microbial nitrogen enrichment with 15N, which was shown for both total soil microbial biomass [21–23] and for the mycelium and fruit bodies of ectomycorrhizal fungi [24, 25].
The mechanism responsible for the accumulation of 15N in microbial cells is greater discrimination of heavy isotope during the dissimilation of nitrogen by microorganisms, compared to its assimilation. The efficiency of this mechanism depends on the ratio of carbon and nitrogen availability to microorganisms, which controls the activity of nitrogen dissimilation [22, 23]. Similarly to mycorrhizal symbiosis, microbial nitrogen dissimilation in symbiotic nitrogen fixation is expressed in the prevailing transfer of 14N isotope to the host plant and in the accumulation of 15N in the biomass of microorganisms. In this case, the fractionation of isotopes between symbionts decreases with a decrease in the efficiency of symbiosis, which is confirmed by the examples of both mycorrhizal [24, 26] and nitrogen fixing symbioses [5].
Efficiency of Symbiotic Nitrogen Fixation
The deviations of δ15N value from 0‰ in legume plants are regarded as a consequence of the contribution of soil sources to nitrogen nutrition, taking into account that their isotopic composition differs from atmospheric nitrogen. These differences make it possible to calculate the proportion of nitrogen assimilated by plants as a result of nitrogen fixation (biological nitrogen) [5].
However, the results of determining the nitrogen isotopic composition in 2.5-month-old plants did not allow us to calculate the contribution of biological nitrogen to the nitrogen pool of young legume plants. The reason was that the δ15N values for the control species (T. polyphyllum), which does not form symbiosis with nitrogen-fixing bacteria, were closest to the isotopic composition of atmospheric nitrogen both in the aboveground (–0.08‰) and in the belowground (0.03‰) parts of the plant.
This was no longer a problem with plants reaching the age of 4 months. When the δ15N of the aboveground part of the plants were used for calculation, the contribution of fixed atmospheric nitrogen to the nutrition was 55% for A. levieri, 34% for O. kubanensis, and 15% for H. caucasicum (see Table 2). Higher values were previously obtained under field conditions for the first two species (about 70% according to the results of determining the 15N natural abundance and over 90% in the experiment with isotope label dilution [12]).
The exception is A. vulneraria, for which the δ15N value in the aboveground part is the same (–1.19‰) as that for T. polyphyllum, which does not make it possible to estimate the contribution of nitrogen fixation to the nutrition of A. vulneraria. Under natural conditions, the role of nitrogen fixation was also significantly lower in the nitrogen supply to A. vulneraria compared to other species, despite the active formation of nodules [12].
Unlike the aboveground parts, the nitrogen isotopic composition in the roots does not make it possible to calculate the contribution of nitrogen fixation to the nitrogen nutrition of legumes in the alpine belt during the first year of their growth, since the δ15N value for the roots of T. polyphyllum was closest to the atmospheric value not only at the plant age of 2.5 months but also at the age of 4 months.
The result of estimating the contribution of nitrogen fixation to the nitrogen nutrition of plants proved to be unexpected. It shows that the species that most actively form nodules (A. vulneraria and H. caucasicum) use less biological nitrogen for their nutrition. This seems to be even more unusual if we take into account that the nodules of A. vulneraria and H. caucasicum are significantly enriched in 15N isotope and this enrichment is directly correlated with the efficiency of nitrogen fixation [5].
Discussing the relatively low involvement of atmospheric nitrogen in the nutrition of A. vulneraria in our previous study [12], we paid attention to the fact that the depth of the root system in this plant is basically lower, which could affect the result of estimation. Since this factor is mitigated in vegetation experiments, we believe that the involvement of nitrogen fixation in the nitrogen nutrition of plants that actively form nodules could be underestimated because of another factor, namely, isotope fractionation between symbionts.
Since nodules account for less than 10% of total nitrogen in a plant (3 and 6% for A. vulneraria and H. caucasicum), it is usually considered that their enrichment in 15N isotope does not lead to noticeable 15N-depletion of the nitrogen pool in the plant. The δ15N value for a legume plant proves to be closer to atmospheric nitrogen [27, 28] and the fractionation effect can be ignored during the calculation of the contribution of nitrogen fixation to its nutrition [5].
However, it is quite obvious that the accumulation of heavy nitrogen isotope in nodules results in slightly negative δ15N values in other plant parts due to isotope fractionation between nodule bacteria and the host plant. For example, if we assume that the nitrogen pool of bacteria is 5% of total nitrogen in the plant and the δ15N value for this pool is 6.0‰, the fractionation of isotopes will lead to a decrease in the δ15N value for the plant by 0.3‰, compared to atmospheric nitrogen. When the δ15N values significantly differ between the legume and control species (3–5‰), this effect will actually hardly influence the calculated portion of fixed nitrogen. However, if the difference is low (1–2‰), which is often observed for plants in alpine and subalpine ecosystems [7, 10, 12], the ignoring of fractionation may lead to a significant (10–20%) underestimate of the contribution of nitrogen fixation to legume nutrition. It is obvious that isotope fractionation between symbionts should be taken into account in such cases.
In addition, A. vulneraria is a special case where a heavy nitrogen isotopic composition is characteristic not only of the nodules but also of the roots as a whole. The heavier isotopic composition of nitrogen in the roots is usually explained by isotope fractionation between mycorrhizal fungi (part of their mycelium is in the roots) and the host plant [26, 28]. The specific reason for the accumulation of 15N in the roots of A. vulneraria is unknown; however, it is quite obvious that the fractionation of isotopes between the parts of this plant leads to the formation of the lightest isotopic composition of nitrogen in its aboveground part (among all the nitrogen-fixing legume species included in our study). When the efficiency of nitrogen fixation is calculated by the δ15N value in the aboveground part of the plant, the isotope fractionation does not make it possible to determine N2 fixation for A. vulneraria under conditions of vegetation experiment and probably underestimates this parameter for the plant studied under natural conditions [12]. The “heavy” roots in A. vulneraria account for 16% of the total nitrogen pool, which decreases the δ15N value by 0.5‰ for the aboveground part of the plant.
The best way to calculate the exact proportion of symbiotic nitrogen fixation in the nutrition of legume plants is to use the weighted average δ15N value for the whole plant. This can be easily done under vegetation experiment and is more problematic during the analysis of crops and much more problematic during the study of natural ecosystems. Our calculations of the weighted average δ15N significantly corrected the Nbiol value: it was increased from 0 to 51% for A. vulneraria and from 15 to 32% for H. caucasicum (see Table 2). In both cases, higher estimate are reliable, since this increase is based on the use of statistically much higher δ15N values in the plant roots (A. vulneraria) and nodules (A. vulneraria and H. caucasicum) in the calculations. The decrease in Nbiol for the other two legume species is not so obvious, since it is determined by lower (however, with a statistically insignificant difference) δ15N values in the roots and by the absence of data on the δ15N for nodules.
CONCLUSIONS
Perennial legumes in the alpine belt of the Northwestern Caucasus have symbiotic nitrogen fixation, which is manifested in the first year of their growth. The fractionation of isotopes between symbionts can lead to the formation of a “light” isotopic composition of nitrogen in the aboveground plant parts and cause underestimation in calculating the proportion of symbiotically fixed nitrogen. The best way to correctly estimate this proportion is to use the weighted average δ15N value for the whole plant (including nodules). Since this poses a significant methodological problem under field conditions (especially in natural ecosystems), it is expedient to determine the isotopic composition of nitrogen in the leaves, roots, and nodules of the plant and, if necessary (when there are nitrogen pools with significantly different isotopic compositions), to make corrections for isotope fractionation while calculating the contribution of symbiotic nitrogen fixation to the nitrogen nutrition of the plant.
REFERENCES
Cleveland, C.C., Townsend, A.R., Schimel, D.S., et al., Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems, Glob. Biogeochem. Cycle, 1999, vol. 13, pp. 623–645.
Galloway, J.N., Dentener, F.J., Capone, D.G., et al., Nitrogen cycles: Past, present, and future, Biogeochemistry, 2004, vol. 70, pp. 153–226.
Vitousek, P.M., Menge, D.N.L., Reed, S.C., and Cleveland, C.C., Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems, Philos. Trans. R. Soc. Lond. B, 2013, vol. 368, article 20130119.
Wang, J., He, Q., Wu, Y., et al., Effects of pioneer N2-fixing plants on the resource status and establishment of neighboring non-N2-fixing plants in a newly formed glacier floodplain, eastern Tibetan Plateau, Plant Soil, 2020. https://doi.org/10.1007/s11104-020-04462-y
Shearer, G. and Kohl, D.H., N2-fixation in field settings: Estimation based on natural 15N abundance, Aust. J. Plant Physiol., 1986, vol. 13, pp. 699–756.
Andrews, M., James, E.K., Sprent, J.I., et al., Nitrogen fixation in legumes and actinorhizal plants in natural ecosystems: Values obtained using 15N natural abundance, Plant Ecol. Divers., 2011, vol. 4, pp. 131–140.
Bowman, W.D., Schardt, J.C., and Schmidt, S.K., Symbiotic N2-fixation in alpine tundra: Ecosystem input and variation in fixation rates among communities, Oecologia, 1996, vol. 108, pp. 345–350.
Arnone, J.A. III, Symbiotic N2 fixation in a high alpine grassland: Effects of four growing seasons of elevated CO2, Funct. Ecol., 1999, vol. 13, pp. 383–387.
Holzmann, H.-P. and Haselwandter, K., Contribution of nitrogen fixation to nitrogen nutrition in an alpine sedge community (Caricetum currulae), Oecologia, 1988, vol. 76, pp. 298–302.
Jacot, K.A., Lüscher, A., Nösberger, J., and Hartwig, U.A., Symbiotic N2 fixation of various legume species along an altitudinal gradient in the Swiss Alps, Soil Biol. Biochem., 2000, vol. 32, pp. 1043–1052.
Makarov, M.I., Malysheva, T.I., Ermak, A.A., et al., Symbiotic nitrogen fixation in the alpine community of a lichen heath of the Northwestern Caucasus region (the Teberda Reserve), Euras. Soil Sci., 2011, vol. 44, no. 12, pp. 1381–1388.
Makarov, M.I., Onipchenko, V.G., Malysheva, T.I., et al., Determinants of 15N natural abundance in leaves of co-occurring plant species and types within an alpine lichen heath in the Northern Caucasus, Arct. Antarct. Alpine Res., 2014, vol. 46, pp. 581–590.
Jacot, K.A., Lüscher, A., Nösberger, J., and Hartwig, U.A., The relative contribution of symbiotic N2 fixation and other nitrogen sources to grassland ecosystems along an altitudinal gradient in the Alps, Plant Soil, 2000, vol. 225, pp. 201–211.
Soudzilovskaia, N.A., Aksenova, A.A., Makarov, M.I., et al., Legumes affect alpine tundra community composition via multiple biotic interactions, Ecosphere, 2012, vol. 3, UNSP 33.
Gentili, F.G. and Huss-Danell, K., The δ15N value of N2 fixing actinorhizal plants and legumes grown with N2 as the only nitrogen source, Symbiosis, 2019, vol. 79, pp. 213–219.
Grishina, L.A., Onipchenko, V.G., Makarov, M.I., and Vanyasin, V.A., Changes in properties of mountain-meadow alpine soils of the Northwestern Caucasus under different ecological conditions, Euras. Soil Sci., 1993, vol. 25, no. 9, pp. 1–12.
Makarov, M.I., Lavrenov, N.G., and Onipchenko, V.G., et al., Nitrogen nutrition of plants in an alpine lichen heath under the conditions of soil enrichment with biogenic elements, Russ. J. Ecol., 2020, vol. 51, no. 2, pp. 99–106.
Sprent, J.I., Biological nitrogen fixation associated with angiosperms in terrestrial ecosystems, in Nutrient Acquisition by Plants: Ecological Studies (Analysis and Synthesis), vol. 181, Berlin: Springer, 2005, pp. 89–115.
Gigon, A., Positive interaktionen in einem alpinen Blumenpolster, Ber. d. Reinh.-Tüxen-Ges., 1999, vol. 11, pp. 321–330.
Thomas, B.D. and Bowman, W.D., Influence of N2‑fixing Trifolium on plant species composition and biomass production in alpine tundra, Oecologia, 1998, vol. 115, pp. 26–31.
Dijkstra, P., Ishizu, A., Doucett, R.R., et al., 13C and 15N natural abundance of the soil microbial biomass, Soil Biol. Biochem., 2006, vol. 38, pp. 3257–3266.
Dijkstra, P., LaViolette, C.M., Coyle, S.C., et al., 15N enrichment as an integrator of the effects of C and N cycling on microbial metabolism and ecosystem function, Plant Soil, 2008, vol. 115, pp. 189–198.
Coyle, J.S., Dijkstra, P., Doucett, R.R., et al., Relationships between C and N availability, substrate age, and natural abundance 13C and 15N signatures of soil microbial biomass in a semiarid climate, Soil Biol. Biochem., 2009, vol. 41, pp. 1605–1611.
Hobbie, E.A., Jumpponen, A., and Trappe, J., Foliar and fungal 15N : 14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: Testing analytical models, Oecologia, 2005, vol. 146, pp. 258–268.
Hobbie, E.A. and Hobbie, J.E., Natural abundance of 15N in nitrogen-limited forests and tundra can estimate nitrogen cycling through mycorrhizal fungi: A review, Ecosystems, 2008, vol. 11, pp. 815–830.
Makarov, M.I., Buzin, I.S., Tiunov, A.V., et al., Nitrogen isotopes in soils and plants of tundra ecosystems in the Khibiny Mountains, Euras. Soil Sci., 2019, vol. 52, no. 10, pp. 1195–1206.
Handley, L.L., Diazotrophy and δ15N biology and environment, Proc. Royal Ir. Acad., 2002, vol. 102, pp. 49–51.
Hobbie, E.A., Macko, S.A., and Williams, M., Correlations between foliar δ15N and nitrogen concentrations may indicate plant–mycorrhizal interactions, Oecologia, 2000, vol. 122, pp. 273–283.
Funding
This study was supported by the Russian Science Foundation, project no. 16-14-10208.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by D. Zabolotny
Rights and permissions
About this article
Cite this article
Makarov, M.I., Onipchenko, V.G., Malysheva, T.I. et al. Symbiotic Nitrogen Fixation by Legumes in Alpine Ecosystems: a Vegetation Experiment. Russ J Ecol 52, 9–17 (2021). https://doi.org/10.1134/S1067413621010094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1067413621010094