Abstract
For the estimation of the tritium activity in environmental samples, the triple to double coincidence ratio (TDCR) method is standardized for the liquid scintillation counter (LSC). Variation of TDCR with tritium activities, optimization of the region of interest for low-level measurement of tritium, the effect of different quenching agents on the TDCR values, and application of TDCR method for the quantification of ionization quench factor (kB) are discussed in this paper. Relative standard deviation in TDCR values at tritium activity levels of 15–140 Bq/L is found to be less than 1%. The the quench curve equation does not depend on the type of quenching agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Tritium is a pure beta emitter with an endpoint energy of 18.6 keV, and its half-life is 12.3 years. Tritium is produced naturally in the upper layer of the atmosphere by cosmic ray interaction with atmospheric nitrogen. In nuclear reactors, tritium is produced by ternary fission and neutron reactions with heavy water (D2O), boron, and lithium [1]. Activities of tritium in environmental samples are measured using either a liquid scintillation counter (LSC) or gas proportional counters (GPC) [2]. LSC is mostly used for tritium measurements among all other available techniques because of easy sample preparation.
In LSC, the efficiency of the system varies from sample to sample depending on quenching agents present in the samples, so the system must be calibrated for efficiency at different quench levels. The quench curve describing the relationship between the quench level in the sample and the counting efficiency should be established for the LSC system. The quench levels present in each sample should be monitored to calculate the efficiency values. Different techniques successfully employed to monitor the quench levels in the samples are reported in the literature [3, 4].
To avoid the quench curve construction or the standard method of activity addition for efficiency calibration, efficiency tracing techniques like triple to double coincidence ratio (TDCR) method and the CIEMAT/NIST (Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas/National Institute of Standards and Technology) method are widely used in many laboratories [5, 6]. The TDCR value is defined as the ratio between the triple coincidence count rate to overall double coincidence count rate, and for a large number of detected events the TDCR value converges to the efficiency value [7]. Many metrology laboratories that designed and built the TDCR method based on HIDEX instrument used the experimental TDCR values for the absolute standardization. However, the application note of the commercially available HIDEX devices does not consider the experimental TDCR as efficiency factor. The use of experimental TDCR values as efficiency factor can result in 15% variation in the activity measurements [8, 9].
National Henri Becquerel Laboratories (LNHB) developed software to determine the detection efficiency values based on the experimental TDCR values. To use the TDCR model, the ionization quenching factor (kB), which describes the nonlinearity in the light emission from the scintillator, should be determined. The influence of kB on the activity calculated from the derived efficiency was studied; it has been found that kB is more important for low energy beta-emitting radionuclides like 3H and 63Ni [10]. The kB factor essentially depends on the liquid scintillation cocktail composition. Here we report the construction of the quench curve using TDCR as a quench indicating parameter, the dependence of the quench curve on quenching agents, the variation of TDCR with tritium activity levels, and also the optimization of the ionization quench factor (kB) for the UltimaGold LLT cocktail.
EXPERIMENTAL
HIDEX 300SL Liquid Scintillation Counter. Hidex 300SL LSC contains a detection assembly of three photomultiplier tubes (PMTs), which are kept at 120° angle apart, so as to cover the isotropic geometry. NaI(Tl) based scintillation detector is used as a guard counter. Background reduction in the system is achieved with 70 mm thick lead shielding around the PMTs and sample vial, and the guard counter is operated in the anticoincidence counting mode. The system contains an automatic sample changer with a robotic arm that moves in X, Y, and Z directions. The robotic arm picks up the vials by applying the tray vacuum and places the vial above the detection chamber using X and Y movement. Finally, the vial is inserted in the detection chamber using the Z-axis movement. All these movements are controlled using stepper motors and position limiting sensors.
Standards and reagents. In this study, we used diisopropylnaphthalene (DIN) solvent-based Ultima Gold LLT grade cocktail. For sample counting, vials of 20 mL volume to match with the dimension of the PMTs are employed. The optimum sample (tritiated water) to cocktail volume ratio used is 8 : 12 [11]. The standard tritiated water solution of specific activity 39.96 kBq/g with an uncertainty of 1.6% (Czech Metrology Institute) was used to calibrate the instrument. Carbon tetrachloride (CCl4) and nitromethane (analytical reagent grade, purity ≥99%) solutions were applied as quenching agents.
Methodology for ionization quench factor (kB) determination. The ionization quenching factor is defined as the reduction in light emissions due to high ionization density along the path of the scintillator. The fluorescent yield L(E) produced with the interaction of charged particles with liquid scintillators is a nonlinear function of the particle energy. Birks expressed the specific fluorescence, dL/dx, the number of photons emitted per unit distance along the path, using Eq. (1):
where ŋ0 is the scintillation yield; dE/dx, stopping power of the scintillator for one beta particle of energy E; and kB, ionization quenching parameter.
The ionization quenching function Q(E) used for calculating the counting efficiency in CIEMAT/NIST or TDCR method is
The computation of the Q(E) function requires the optimum kB value, stopping power of energies less than 1 keV, and the atomic composition and density of liquid scintillators. The optimum kB values of the LSC system are determined by varying the detection efficiency. The LSC detection efficiency can be varied either by chemical quenching or by using grey filters [12]. For the present study, the optimum kB value is determined from the chemical quenching using CCl4.
Calculation of detection efficiencies using the TDCR07c software. The TRCD07c software developed in LNHB [13] is used to calculate the detection efficiencies as well as the figure of merit (FOM) values for LSC with three PMTs. The physical models used in the TDCR07c program are Poisson statistics for the light emissions, Birks law of nonlinearity in the light emission, stopping power of electrons as per the ICRP report 37, and Fermi model for the beta spectrum calculations. The kB values depend on the scintillator composition. The average composition of commercially available liquid scintillators reported in the literature [14] is used in the simulation.
RESULTS AND DISCUSSION
Variation of TDCR with low activity. The TDCR, triple coincidence to double coincidence count rate ratio of the samples, depends on the sample count rate. Water samples with low tritium activity in the range 15–140 Bq/L, prepared in plastic vials at the optimum sample to the cocktail ratio, were counted in LSC for 5 h. The variation of TDCR with the activity is shown in Fig. 1. The relative standard deviation in TDCR values at different activity levels was less than 1%.
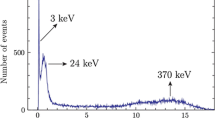
Region of interest for low-level measurements. The tritium spectra recorded at low level activities, including the background spectrum, are shown in Fig. 2. The figure of merit (FOM) as defined using Eq. (3) is used to optimize the region of interest (ROI) of tritium for low activity measurements:
where S is the sample net count rate (cps), and B, background count rate (cps).
The optimum window was chosen where the FOM values are maximized. Table 1 shows the FOM values at different ROI regions. The optimum window for tritium is 26–200 channels. Compared to the tritium full window (0–300 channels), the reduction in optimized window for in the net count rates of source and background spectra is 6.53 and 34.15%, respectively,
Quench curve. Quench standards are prepared and counted in LSC using commercial Ultima Gold LLT cocktail for the same activity at different quench levels at the optimum sample to cocktail volume ratio. CCl4 is added to quench standards as a quenching agent in the range 0–50 µL. The quench curve (TDCR vs. LSC efficiency) is depicted in Fig. 3. The efficiency of the system for tritium without external quenching is found to be 0.410 cps/Bq, but with increasing the quench levels, TDCR and efficiency values tend to decrease. The double coincidence efficiency is defined as the number of light signals detected per unit activity per unit time in any two PMTs, whereas the triple coincidence efficiency is defined as the number of light signals detected per unit activity per unit time in all three PMTs with a coincidence time frame of 35 µs. The total efficiency is the summation of double and triple coincidence efficiency values. The variation of the double and triple coincidence efficiency with TDCR values is shown in Fig. 3. As can be seen, with an increase in the quench levels, the reduction in the triple coincidence efficiency is more pronounced compared to the double coincidence efficiency.
Comparison of quench curves. Along with CCl4, standards were prepared using nitromethane as a quenching agent and counted in LSC. Nitromethane was added in amounts in the range 0–150 µL. Figure 4 compares the efficiency vs. TDCR curves for the two quenching agents studied. As can be seen, the TDCR values are found to be low for nitromethane compared to CCl4 at the same amounts of the quenching agents added. The relationship between the efficiency and TDCR for both quenching agents can be fitted by a linear equation with R2 = 0.99 and negligible difference in the intercept values. That is, the quench curve constructed by the TDCR method to determine the efficiency is independent of the quenching agent type.
Detection efficiencies and determination of optimal kB value. The experimental TDCR values for different quench levels at the optimum sample to cocktail ratio are applied in TRCD07c software for calculating the detection efficiency. The detection efficiency and the logical sum of the double coincidence values determined for each kB value using the TRCD07c software are varied from 0.007 to 0.015 in steps of 0.001 cm/MeV at different TDCRs. The activities are estimated using the derived efficiencies for each kB value at different TDCRs. The normalization factor (NF) is defined as the ratio of the activity determined from the derived efficiency to the actual activity. This factor becomes nearly unity at the optimum kB value. Figure 5 shows the variation of the normalization factor with kB values at TDCR of 0.381; as can be seen, the ratio varies from 0.96 to 1.024. Similarly, the normalization factor calculated at different TDCR values for each kB values is plotted in Fig. 6. As can be seen, the normalization factor is nearly unity for all the TDCR values at the kB value of 0.012 cm/MeV. The optimum kB value determined for the Ultima Gold LLT cocktail is within the range of literature values (0.011–0.014 cm/MeV) for tritium in different commercial scintillators [15]. The optimum kB value is used for determining the efficiency from the measured TDCRs at different quench levels using TRCD07c software.
CONCLUSIONS
The variation of the efficiency obtained from the experimental TDCR used as quench indicating parameter for activity estimation is studied with different quenching agents, and the efficiency derived from the quench curves does not vary significantly. The relative standard deviation in TDCR values at different tritium activity levels is 1%. The optimum window for low-level measurements of tritium is 26–200 channels, and the background reduction in the optimized window is 34.15% compared to the full window of tritium. The ionization quench factor used for the determination of the efficiency factor is optimized and found to be 0.012 cm/MeV for Ultima Gold LLT grade cocktail.
REFERENCES
Management of Waste Containing Tritium and Carbon: IAEA Tech. Rep. Ser. no. 421, Vienna: IAEA, 2004.
Xiaolin, Hou, J. Radioanal. Nucl. Chem., 2018, vol. 318, p. 1597. https://doi.org/10.1007/s10967-018-6258-6
L’Annunziata, M.F. and Kessler, M.J., Liquid Scintillation and Analysis: Principles and Practice, Handbook of Radioactivity Analysis, Amsterdam: Elsevier, 2012.
Edler, R., Available Quench Parameters in TriCarb LSC’s, LSC Application Note, Rodgau-Jügesheim: Perkin Elmer, 2015.
Broda, R., Appl. Radiat. Isot., 2003, vol. 58, no. 5, p. 585. https://doi.org/10.1016/S0969-8043(03)00056-3
Kossert, K., Broda, R., Cassette, P., Ratel, G., and Zimmerman, B.E., Metrologia, 2015, vol. 52, no. 3, p. 172. https://doi.org/10.1088/0026-1394/52/3/S172
Wanke, C., Kossert, K., and Nahle, O.J., Appl. Radiat. Isot., 2012, vol. 70, no. 9, p. 2176. https://doi.org/10.1016/j.apradiso.2012.02.097
HIDEX Oy, TDCR Quench Correction with the HIDEX 300 SL.DOC 413–001 Version 1.0. https://lablogic.com/data/file/e/a/TDCR%20quench%20correction%20with%20the%20HIDEX%20300%20SL%20Application%20Note.1427706354.pdf
Lecompte, Y., Rosset, M., Loess, J., Chianea, N., Jourquin, F., Cazoulat, A., and Cassette, P., Radiat. Prot. Dosim., 2020, no. 2, pp. 148–161. https://doi.org/10.1093/rpd/ncz270
Broda, R., Cassette, P., and Kossert, K., Metrologia, 2007, vol. 44, p. 36.
Arun, B., Vijayalakshmi, I., Sivasubramanian, K., and Jose, M.T., Radiochemistry, 2019, vol. 61, no. 1, pp. 61–65. https://doi.org/10.1134/S1066362219010090
Cassette, P., Broda, R., Hainos, D., and Terlikowska, T., Appl. Radiat. Isot., 2000, vol. 52, no. 3, p. 643.
Cassette, P., Detection Efficiency Calculation for Pure Beta Radionuclides: LNHB TDCR07c Program, 2017. http://www.lnhb.fr/icrm_lsc_software/
http://www.nucleide.org/ICRM_LSC_WG/2010_LSC_cocktails_elementary_composition.pdf
Broda, R., Małetka, K., Terlikowska, T., and Cassette, P., Appl. Radiat. Isot., 2002, vol. 56, p. 285. https://doi.org/10.1016/S0969-8043(01)00202-0
ACKNOWLEDGMENTS
The authors are thankful to Dr. R. Venkatesan, Head, RESD and to Mrs. I. Vijalakshmi of Safety Quality and Resource Management Group for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Arun, B., Viswanathan, S., Subramanian, V. et al. Study of Triple to Double Coincidence Method for Tritium Measurements. Radiochemistry 63, 221–226 (2021). https://doi.org/10.1134/S1066362221020120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362221020120