Abstract
Wildfire is a crucial event in the regulation of the structure and function of forest ecosystems. The effects of fire on soil microorganisms is still poorly understood. Here, we compared soil properties and bacterial communities between burnt and unburnt soils on sunny and shady slopes 4 and 13 years after a fire in a warm temperate forest ecosystem at Zhenshan Mountain in Shandong, eastern China. Soil physicochemical properties and enzyme activity were more affected by fire than by slope aspect. Fire significantly altered bacterial β-diversity but did not affect bacterial α-diversity. Co-occurrence networks showed that fire decreased the complexity, edge number, average degree, and average clustering coefficient of the bacterial communities. Available nitrogen content was the major factor explaining the differences in bacterial communities between the burnt and unburnt samples. Moreover, the impacts of fire varied with slope aspect and recovery time. The relative abundance of Spartobacteria, Gammaproteobacteria, TK10, and JG30-KF-CM66 differed significantly between sunny and shady slopes in burnt soil, and were all significantly correlated with soil pH. Differences in soil pH mediated by slope aspect drove the variation in soil bacterial community structure at burned sites. Within constant slope aspect, the soil bacterial community in burnt soil 4 years after the fire was significantly different from that in unburnt soil, and after 13 years of recovery it was similar to that before the fire. These results indicate that the slope aspect should be considered when predicting the response of soil microbial communities to fire.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Fire in forest ecosystems is a major disturbance, as it not only modifies the species composition of aboveground plants but also notably affects the physical and chemical properties and microbial community of underground soil [16]. Soil microbes are a key to fundamental ecological processes that occur in ecosystems, including organic matter decomposition and nutrient cycling [34]. The disturbance of soil microbial communities by fire affects essential ecosystem functions such as carbon sequestration, nutrient cycling, net ecosystem productivity, and climate regulation [1, 33, 48]. Therefore, it is vital to understand how fire affects underground microbial communities that are responsible for driving essential ecosystem processes.
Fire has direct and indirect effects on soil microbial communities. On the one hand, fire breaks biomolecules by directly exposing soil microbes to extremely high temperatures, decreasing bacterial and fungal biomass [41, 42]. Microbes differ in their sensitivity to fire-induced heat, and certain heat-resistant taxa may benefit from reduced competition, leading to drastic alterations in the soil microbial community structure compared to the pre-fire community state [28, 32]. On the other hand, fires reduce living plants and alter soil physicochemical properties, including consumed organic matter and increased soil pH, which indirectly affects microbial diversity and community structure [10, 30]. Previous studies have shown that fire disturbance modifies the microbial community diversity and phylogenetic structure [9, 30]. Moreover, fire reduces the relative abundance of most functional genes involved in carbon degradation and nitrogen cycling [40]. Soil microbes are essential for the re-establishment of soil biogeochemical processes that contribute to forest recovery [2]. Therefore, understanding the response of soil microorganisms to fire will provide essential knowledge for biogeochemical cycles, ecosystem recovery, and forest management [20].
The response of soil microbes to fire is inconsistent. It is affected by vegetation type, fire severity, fire duration, time after fire, topography, climate, and soil sampling depth. High severity burning substantially reduces the biomass and diversity of soil bacteria [13, 23, 45]. Moreover, the soil microbial community varies over time as it recovers after the fire. Numerous studies have investigated the effects of fire severity and recovery time on microbial communities [8, 23, 45, 46]. For example, fire in Canadian boreal forests increased bacterial alpha diversity and altered bacterial beta diversity during the early recovery stage (<5 yrs since fire), but did not affect bacterial communities in the middle (8–20 yrs) and late (>30 yrs) recovery stages [48]. In fact, variations in topography and microclimate often lead to heterogeneity in fire severity across burned areas. However, little is known about the effects of topography on the microbial community in burnt soils.
Slope aspect, as an important topographic factor in forest ecosystems, is well documented to cause variability in soil microclimates (solar radiation, temperature, and soil humidity). In the northern hemisphere, the southern slope receives more solar radiation than the northern slope, resulting in a higher temperature and lower soil moisture in the southern slope [5, 25]. The slope aspect also has a significant effect on the spatial variability of the physical and chemical properties of soil. For instance, sunny slope sites in the alpine meadow on the Qinghai-Tibetan Plateau had higher soil temperature, organic carbon, and underground biomass, whereas shady slope sites had higher soil moisture, total nitrogen, and aboveground biomass [38]. These changes in soil microclimates and physicochemical characteristics caused by different slope aspects lead to variations in the microbial community. Soil bacterial diversity was lowest on the north-facing aspect compared to the south-facing aspect and flat area in a boreal forest of the Greater Khingan Mountains [14]. The abundance, diversity, and community composition of N-cycling microbes differ dramatically between the south- and north-facing slopes [17]; thus, slope aspect significantly affected the soil microbial community. However, little is known about how slope aspect affects the soil microbial community during fire disturbance. In particular, it remains to be studied whether soil microbial communities and their recovery after fire vary on different slope aspects and at different times on the same slope.
Compared to temperate and subtropical forest ecosystems [49], there is a lack of studies on fire disturbance in warm temperate forest ecosystems in China, whereas drought and rainless springs often lead to fires. Therefore, we selected warm temperate forest ecosystems to study the effects of fire on soil microbial communities in order to test the following hypotheses: (1) fire would alter bacterial diversity and community composition, (2) the effects of fire on the bacterial community would vary with slope aspect, and (3) on the same slope aspect, the soil bacterial community may show varied recovery patterns in the short- and long-term. To test these hypotheses, we examined soil properties and bacterial communities from burned and adjacent non-burned sites on sunny and shady slopes. Further understanding of the response pattern of bacteria to fire under the varied microtopography, and the recovery pattern of soil microorganisms in different slope aspects after fire, will provide theoretical guidance for soil remediation after fire disturbance.
MATERIALS AND METHODS
Study site. The study area was located in the Zhenshan Mountains, north Shandong Peninsula, China (37°30′–37°32′ N, 121°19′–121°21′ E), and had an average elevation of 230–250 m. This area has a continental monsoon climate with an annual mean temperature of 12°C and annual precipitation of 740.3 mm. The dominant tree species in this area were secondary Robinia pseudoacacia and Pinus thunbergii, and the main soil types were brown soil. Forest fires are frequent in spring because of dry weather, high winds, and frequent human activity. There were two wildfires in the Xishan fragment of Zhenshan Mountain in April 2005 and April 2014, which turned litter and vegetation into ash and damaged trees. The burned areas were selected as the study regions, of which the area burned in 2005 was located on a sunny slope, and the burned area in 2014 had both a sunny slope and a shady slope. Nearby non-burned areas were used as controls. Thus, there were five sampling sites: a burned area on the sunny slope burned in 2005 (BSU2005), burned area on the sunny slope burned in 2014 (BSU2014), burned area on the shady slope burned in 2014 (BSH2014), non-burned area on a sunny slope (USU), and non-burned area on a shady slope (USH).
Soil sampling. Soil samples were collected from the five abovementioned areas in April 2018. Four sampling sites were randomly selected in each area and used as replicates. The litter, charred debris, and ash layers were removed before soil sampling, and soil cores (0–10 cm) were collected using a stainless-steel soil collar. A five-point sampling method was used to collect soil samples, and five soil samples were homogenized in the field to form a composite sample then stored in a sterile freezing tube. All soil samples were placed in a cooler with ice and transported to the laboratory as soon as possible. Soil samples were stored at –80°C for DNA extraction and –20°C for determination of soil physicochemical properties.
Soil properties and enzyme activity analysis. Soil pH was determined in the supernatant of 1 : 2.5 soil-water mixtures using a calibrated pH meter (Mettler, Switzerland). Soil organic matter (SOM) content was determined using the K2Cr2O7–H2SO4 oxidation-reduction colorimetric method. Total nitrogen (TN) was measured using a Vario Micro Cube elemental analyzer (Elementar, Germany). Total phosphorus (TP) was measured using the molybdenum blue method after wet digestion with H2SO4 + HClO4. Total potassium (TK) was measured using a flame photometric detector after digesting the sample with HNO3 + HClO4. Available nitrogen (AN) was determined using an alkaline-hydrolyzable diffusion method. Available phosphorus (AP) was extracted using sodium bicarbonate and measured using the molybdenum blue method. Available potassium (AK) was extracted with ammonium acetate and determined using a flame photometric detector. All soil properties were performed according the methods reported by Bao [39].
Soil enzyme activities were determined according to Guan [43]. Briefly, urease activity was measured using indophenol blue colorimetric method and expressed as the amount (mg) of NH3-N/g of dry soil produced during 24 h incubation at 37°C. Phosphatase activity was measured using alkaline phosphatase colorimetric method and expressed as the amount (mg) of phenol produced per gram of dry soil during a 24-h incubation period at 37°C. Sucrase activity was measured by a 3, 5-dinitrosalicylic acid colorimetric assay and expressed as the amount (mg) glucose produced by incubation for 24 h at 37°C. Soil catalase activity was measured by potassium permanganated titration method and the activity unit was expressed as the millilitres of 0.1 mol L−1 KMnO4 solution consumed by 1 g dry soil after adding 10 mL 0.3% hydrogen peroxide solution for 0.5 h.
DNA extraction, amplification, and high-throughput sequencing. DNA was extracted from 0.5 g soil using a FastDNA Spin Kit (MP Biomedical, USA), following the manufacturer’s instructions. DNA concentrations were determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, USA). The V3-V4 hypervariable region of the 16S rRNA gene was amplified using the bacterial universal primers, 338F (5'-ACTCCTACGGGAGGCAGCA-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3'). We set up 20 μL reaction mixtures including 2.0 μL PCR ExTaq Buffer, 1.0 μL of each primer, 2.0 μL dNTP, 0.25 μL ExTaq Polymerase, 0.5 μL DNA (100 ng/mL), and 13.25 μL H2O. The PCR cycling parameters were 95°C for 3 min, followed by 27 cycles of 30 s at 95, 55°C for 30 s, 72°C for 30 s, and a 10 min extension at 72°C. Paired-end sequencing was conducted using an Illumina HiSeq sequencer (Illumina, USA) at Mega Genomics Health Technology Co. Ltd (Beijing, China).
Sequence processing. Raw reads were processed and analyzed using QIIME (v. 1.8.0) [19] and the MOTHUR software package (v. 1.34.4) [35]. Joined paired-end reads were filtered using a series of quality standards such as a quality score higher than 20, lengths between 330 and 450 bp, no ambiguous bases, and homopolymers less than 6 bp. Sequences satisfying these criteria were retained. Chimeric sequences were identified and removed using USEARCH v.61 based on the Greengenes database (released Aug. 2013). Sequences were picked into operational taxonomic units (OTUs) with 97% sequence similarity using UCLUST v.1.2.2 [36]. Singleton sequences were excluded from subsequent analyses. Taxonomy was assigned against the Greengenes reference database, and Archaea, Chloroplast, and unassigned sequences were removed before further analysis.
Statistical analyses. Alpha diversities of bacteria were calculated based on 17 200 (the minimum number of sequences in 20 samples) rarefied sequences, including OTU richness and Simpson, Shannon, and Chao1 indices. For beta diversity analysis, the OTU table was normalized using the edgeR package [27]. The non-metric multidimensional scaling (NMDS) based on the Bray–Curtis distance was used to explore differences in bacterial community structures across all soil samples. Analysis of similarity (ANOSIM) was performed to evaluate significant differences in the bacterial community structure across the soil samples, using the “vegan” package in R (version 4.0.2). A two-way ANOVA test was performed to determine significant differences in soil properties and bacterial relative abundance between the wildfire treatment, slope direction, and recovery time, using SPSS v20.0 (SPSS Inc. USA). Redundancy analysis (RDA) was performed to examine the relationships between environmental variables and bacterial communities, using R. Pearson’s correlation was performed to explore the associations between relative bacterial abundance and soil physicochemical characteristics. Bacterial co-occurrence networks were structured based on a Spearman’s correlation matrix from OTU tables. We visualized bacterial co-occurrence networks using Gephi and analyzed topological parameters, including clustering coefficient, network density, node number, edge number, and average degree.
RESULTS
Soil physicochemical properties and enzyme activity. The soil properties were significantly affected by wildfire, slope aspect, and recovery time after fire (Fig. 1, Table S1). Wildfires significantly reduced soil OM, TN, AN, and AK contents on sunny slopes but not on shady slopes. The effect of slope aspect on soil properties was mainly observed in the non-burned area; soil OM, TN, and AN contents were higher in sunny slope soil than in shady slope soil. However, in the burned area, only a difference in pH was observed between sunny and shady slopes. For burned sites on the sunny slope, the soil after long-term recovery (BSU2005) was characterized by higher contents of AN and AP and lower pH and TK content (P < 0.05) compared with the soil after short-term recovery (BSU2014).
Soil enzyme activity was affected by fire, but was little affected by slope aspect. Soil urease and phosphatase activities at sites burned in 2014 were significantly lower than those at non-burned sites, whereas catalase activity was significantly higher than that in unburnt soil on both shady and sunny slopes. Only in non-burned sites, sucrase activity was significantly affected by slope aspect, and was significantly lower on the sunny slope than on the shady slope (P = 0.043). Considering the recovery of enzyme activity on sunny slopes in burnt soil, the activities of urease and catalase in soil at the early burned site (BSU2005) were significantly different from those at the recently burned site BSU2014, but not significantly different from those in the unburnt soil USU, suggesting that soil enzyme activity had basically recovered to the pre-fire situation by 13 years after the fire.
Bacterial community composition and α-diversity. A total of 760 556 sequences (after quality filtering) were generated from 20 soil samples and clustered into 2817 OTUs for further analyses. Good’s coverage ranged from 0.986 to 0.991, indicating that the identified sequences represented the majority of bacterial sequences in the soil samples. A total of 28 bacterial phyla were identified among all sampled soils (Fig. 2). Proteobacteria were the most frequent (30.98%), followed by Actinobacteria (26.93%), Acidobacteria (18.86%), Chloroflexi (8.45%), and Verrucomicrobia (3.46%). Within Proteobacteria, most of the sequences were affiliated with Alphaproteobacteria (22.77%), including Rhizobiales (13.90%) and Rhodospirillales (6.28%). Gemmatimonadetes, Firmicutes, Saccharibacteria, Planctomycetes, Bacteroidetes, and Armatimonadetes comprised the minor fractions.
Soil bacterial α-diversity indices were not obviously affected by fire, slope aspect, or recovery time (Fig. 3). Only the Shannon index was significantly lower in BSU2005 than in BSU2014.
Variation in bacterial alpha diversity between burnt and unburnt soils on sunny and shady slopes. Boxes with different lower-case letters are significantly different (P < 0.05). BSU represents burned soil on the sunny slope; USU represents unburned soil on the sunny slope; BSH represents burned soil on the shady slope; USH represents unburned soil on the shady slope.
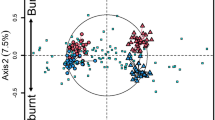
Difference in bacterial community structure. At the OTUs level, the results of NMDS and the Bray–Curtis similarity indices revealed that the bacterial community structure was significantly affected by fire, slope aspect, and recovery time. The bacterial community structure in the burnt soils differed significantly from that in the unburnt soils (Fig. 4, Table 1) while the effect of slope aspect on bacterial community structure followed different patterns in burnt and unburnt soil. In the burned area, the soil bacterial community structures on the sunny and shady slopes were significantly different (P = 0.029), but this difference was not observed in the non-burned area (P = 0.086). The early-burned soil on the sunny slope clearly differed from the recently burnt soil samples (P = 0.034) but clustered with the unburnt soil (P = 0.057) in the NMDS plot, which suggested that the bacterial community structure of burnt soil gradually recovered to that of unburnt soil with the extension of recovery time.
We further analyzed the significant taxa that produced the differences between the groups in bacterial community structure. Parcubacteria and TM6_Dependentiae were significantly lower in burnt soils of 2014 than in unburnt control soils (Table S2). Fire decreased the relative abundance of Gammaproteobacteria and Thermomicrobia at the class level in burned sites compared with non-burned sites. The differences in Gammaproteobacteria were mainly attributed to the order Xanthomonadales, including Xanthomonadaceae and Xanthomonadales_Incertae_Sedis. Moreover, at the order level, Subgroup_10, Subgroup_7 (Acidobacteria; Acidobacteria), and TRA3-20 (Proteobacteria; Betaproteobacteria) increased after fire, whereas JG30-KF-AS9 (Chloroflexi; Ktedonobacteria), Sphaerobacterales (Chloroflexi; Thermomicrobia), and Legionellales (Proteobacteria; Gammaproteobacteria) decreased after fire.
The effect of slope aspect on soil bacterial community composition was significant, especially in the burned sites. In the burned area, the bacterial community on the sunny slope was characterized by higher Spartobacteria, TK10, and JG37-AG-4, while Gammaproteobacteria, JG30-KF-CM66, and OPB35_soil_group were higher in shady slope soils. The differences in Spartobacteria and Gammaproteobacteria between sunny and shady slope soils were mainly attributed to Chthoniobacterales (order DA101_soil_group) and Xanthomonadales (family Xanthomonadales_Incertae_Sedis). Moreover, there were significantly fewer Acidobacteriales and more Micromonosporales at the order level in sunny slope soils.
The soil burned in 2005 was characterized by a higher relative abundance of Gammaproteobacteria and JG30-KF-CM66, whereas the 2014 burned soil was characterized by higher Deltaproteobacteria and Anaerolineae. At the order level, Frankiales, Xanthomonadales, SC-I-84, and JG30-KF-CM66 were significantly more abundant in BSU2005 than in BSU2014. The difference in Frankiales was mainly in Acidothermaceae family Acidothermus genus (1.31% vs. 4.28%, P = 0.022). The family Xanthomonadales_Incertae_Sedis in the order Xanthomonadales was significantly more abundant in BSU2005 than in BSU2014.
Co-occurrence network complexity of bacteria. Wildfires decreased the complexity of the bacterial co-occurrence network (Fig. 5). Although the number of nodes was similar between burnt and unburnt soil samples, wildfire reduced the edge number, average degree, and average clustering coefficient (Table 2). The slope aspect also affected the complexity of the network. The complexity of the network among the bacterial communities was higher on shady slopes than on sunny slopes, and the edge number, average degree, and average clustering coefficient of the co-occurrence network of the bacterial communities were higher on shady slopes than on sunny slopes. However, the effect of the recovery time on the complexity of the bacterial co-occurrence network was not significant. The clustering coefficient and network density are slightly higher for the BSU2005 sample than for the BSU2014 sample, whereas the node number, edge number, and average degree were lower for the BSU2005 sample than for the BSU2014 sample.
Correlations between bacterial community and soil properties. Pearson correlation analysis was performed to examine the associations between soil parameters and bacterial taxa and alpha diversity in the 20 soil samples (Fig. 6). Alpha diversity estimators of bacteria were positively correlated with pH (r > 0.528, P < 0.017), except for the Simpson index (r = –0.655, P = 0.002). The Shannon index was positively correlated with the TK content (r = 0.462, P = 0.040). However, the Ace and Chao1 indices were significantly correlated with AP content (r < –0.471, P < 0.036).
The RDA revealed that the bacterial community structure among all 20 burnt or unburnt soils co-varied significantly with the two geochemical parameters (Fig. 7). The AN content was the major factor explaining the differences in bacterial communities between burnt and unburnt samples, whereas pH was the major factor explaining community variations between sunny and shady slope samples.
DISCUSSION
Soil properties were more affected by fire than by slope aspect. After the fire, soil pH remained unchanged or decreased, and was related to slope aspect or recovery time. Most previous studies found an increase in soil pH after fire [29, 30], whereas others observed no significant changes or decreases [4, 26]. The effect of fire on soil pH mainly depends on the fire severity [16, 18]. Adkins et al. [16] found that light burning had no effect on soil pH; however, the pH values in the medium- and high-intensity burned areas were higher than that in the control area. Similarly, Alcañiz [26] suggested that fire events that did not alter soil pH were low-intensity and low-severity prescribed fires applied periodically (every two years or more). In addition, the variation in pH was inconsistent on sunny and shady slopes after fire, which was probably attributable to the higher soil moisture being more conducive to vegetation recovery on the shady slope, thus increasing organic acids secreted by plants and reducing soil pH.
Fire decreased SOM and nitrogen content, as also reported in previous study, which may be attributed to the effect of fire on vegetation, decreasing the resource of organic matter from litter [21]. Moreover, fire consumes and alters SOM at the surface of the soil through combustion, which results in the volatilization of carbon and other nutrients [15]. SOM serves as a reservoir of available nutrients, particularly nitrogen [15]. Previous research has shown that the amount of nitrogen lost during a fire is directly proportional to the amount of combusted organic matter [24]. However, the effect of fire on soil properties varied with the slope aspect. The contents of OM, TN, AN, and AK in the burnt soil were significantly lower than those in the unburnt soil on the sunny slope but were similar in burnt and unburnt soil on the shady slope. This may be because the effect of fire on shady slopes is less than that on sunny slopes, and the soil on shady slopes recovers faster after fire. Shady slopes receive lower solar radiation and retain higher soil water content, which are conducive to the recovery of plants and the enhancement of microbial activity, and thus may accelerate the recovery of soil nutrients.
Enzyme activity in the soil was greatly affected by fire, but slightly affected by the slope aspect. The negative effects of fire on soil urease and phosphatase activities over the short term (4 years) after fire were consistent with a previous study in thermic oak-pine forests [30], and could be explained by direct enzyme denaturation occurring when the temperature exceeds 60 to 70°C, and the complete destruction of soil enzymes at 180°C [44]. Moreover, urease activity reflects the capacity of soil organic nitrogen to be converted to available nitrogen. In this study, the reduction in SOM after fire could be responsible for the observed decrease in urease activity; this idea is supported by the significant correlation between urease activity and SOM content. Fire-related declines in phosphatase activity were also observed elsewhere in forest soils, where they were attributed to changes in organic carbon and reduced SOM quality [37], a factor that also appears likely in our case. In this study, soil catalase activity increased slightly after fire, which may be the result of oxidative stress caused by soil chemical changes due to fire [31]. The effect of fire on soil enzyme activity mainly occurred within a short time (4 years) after fire. The soil enzyme activity 13 years after fire was similar to that in the unburnt soil, suggesting that soil enzyme activity gradually recovered to the pre-fire situation.
Fire affects the β-diversity of bacteria, but not the α‑diversity. Wildfire did not significantly affect bacterial α-diversity in this study, which is consistent with the observations of a previous study on the impact of fire in the Chinese Great Khingan Mountains across a 29-year chronosequence [46]. However, other studies have shown that the variation in soil bacterial diversity is inconsistent at different recovery stages after fire. In Mediterranean ecosystems, bacterial diversity increased during the early stages of post-fire recovery and gradually decreased to pre-fire conditions over a 20-year wildfire chronosequence [9]. In contrast, some studies have shown that fire reduces soil bacterial diversity in the short period after fire [23]. These differences are probably related to different fire severities, ecosystem types, and climatic conditions [48].
Wildfire had a significant influence on soil bacterial community composition. Recent burning of soil is characterized by a decrease in the relative abundance of Gammaproteobacteria, Thermomicrobia, Parcubacteria, and TM6_Dependentiae. The decreased relative abundance of these taxa in burnt soils is probably because these groups are heat-intolerant and can be killed directly by burning. Alternatively, nutrients, such as organic matter, are decreased by burning, which is not conducive to the survival of these taxa. Thermomicrobia, for example, although hyperthermophilic, grows at an optimum temperature of 70 to 75°C and a maximum temperature of 80°C [11]. Forest fires can increase soil temperatures up to 200 degrees, making it difficult for Thermomicrobia to survive. The decrease in Gammaproteobacteria was mainly attributed to Xanthomonadale, which is one of the largest and most important groups of bacterial phytopathogens [3]. The decrease in relative abundance of Xanthomonadale may be attributed to two reasons. First, fire directly kills the host plant of pathogenic bacteria, thus reducing the relative abundance of plant pathogenic bacteria, such as Xanthomonadale. Second, many groups of Xanthomonadales are related to carbon degradation, such as the genera Dokdonell, Mizugakiibacter, Rhodanobacter, and Rudaea, which use various carbon substrates as energy sources. In this study, the reduction of organic matter content by fire may have reduced the carbon sources required for the survival of these groups, resulting in a decrease in the relative abundance of these taxa.
The effects of fire on the soil microbial community varied with time. The bacterial community structure in the burnt soil after 4 years of recovery was significantly different from that in the unburnt soil, but the bacterial community almost recovered to pre-fire conditions by 13 years after the fire. This phenomenon has been observed in previous studies where the bacterial community altered dramatically in the early recovery stage (<5 years since fire) and returned to a similar composition to the controls after 8 years [48]. The relative abundance of Gammaproteobacteria decreased significantly in burnt soil compared with unburnt soil for 4 years after fire but increased significantly by 13 years after fire, which may be due to the restoration of vegetation providing more hosts for Xanthomonadale pathogens. Moreover, vegetation can provide organic matter to the soil for utilization by bacteria and promoting their survival, which may explain the increased relative abundances of Rhodanobacter, Mizugakiibacter, and Lysobacter in long-term recovery soil samples. In general, bacteria are not only affected by host plants and nutrient status but are also influenced by environmental conditions (e.g., soil pH). The Acidothermus genus (Frankiales) is suitable for growth in acidic environments, and the low pH of burnt soil in 2005 could be beneficial to its growth.
Slope aspect impacts on bacterial community are driven by pH. Slope aspect is known to affect the hydrothermal condition of the soil and thus indirectly affects the distribution of the microbial community. Sunny slopes are characterized by stronger solar radiation, higher evaporation, and less moisture, while shady slopes are characterized by the opposite, which results in the differences in plant species and belowground microbial communities [38]. Previous studies have observed that slope aspect had a significant effect on the soil bacterial community composition and specific functional taxa [22, 47]. Unexpectedly, an impact of slope aspect on the soil bacterial community was only observed in the burned sites in our study. Although soil properties were different between the sunny and shady slopes in non-burned areas, they did not appear to affect the distribution of the soil bacterial community.
There were significant differences in the relative abundance of bacterial taxa between sunny and shady slopes and between burnt and unburnt soils, considering the main effects of slope aspect and burn on soil bacteria on their own. For instance, Thermomicrobia, FBP, Gemmatimonadetes, Gammaproteobacteria, and Saccharibacteria were significantly affected by fire, whereas Anaerolineae, Caldilineae, and TM6_Dependentiae were significantly affected by slope aspect (Table S3). However, considering the interaction of slope aspect and burning, the microbial groups significantly affected by fire changed little, indicating that slope aspect might interfere with the effect of fire on soil microorganisms.
The sole phylum of soil bacteria occurring on both sunny and shady slopes in burnt soil was Verrucomicrobia, which was mainly represented by the Spartobacteria class and DA101_soil_group family. Spartobacteria is the most abundant class in the phylum Verrucomicrobia, which is often observed in forest and grassland soils [12]. The significant correlation between the relative abundance of Spartobacteria and soil pH on different slopes in this study was probably mainly driven by the distribution of soil pH, supported by Fig. S1. A similar observation that the distribution of Verrucomicrobial, including Spartobacteria class and DA101 genera was strongly influenced by soil pH was made in Changbai Mountain soils [7]. It is well known that soil pH is an important factor affecting bacterial communities, as has been shown in a number of studies [6, 22]. Moreover, differences in taxa, such as Spartobacteria, Gammaproteobacteria, TK10, and JG30-KF-CM66, between sunny and shady slopes in burnt soil were also significantly correlated with soil pH (Fig. S1). These observations, combined with the RDA results, indicated that the difference in soil pH caused by slope aspect was the major factor driving the variation in soil bacterial community structure between the sunny and shady slopes in burnt soil. Similar results, where bacterial community composition and richness were most strongly correlated with soil pH across aspect-related gradients, have been observed in alpine soil [49], but they were rarely found in burnt soil.
CONCLUSIONS
The effects of fire, slope aspect, and recovery time on soil bacterial diversity and community structure were elucidated by determining soil properties and bacterial communities of burned and adjacent non-burned sites on sunny and shady slopes on Zhenshan Mountain. Fire had a significant effect on soil physicochemical properties and enzyme activity, whereas an effect of slope aspect on soil properties was mainly restricted to the non-burned area. Fire had little effect on soil bacterial diversity but had a significant effect on soil bacterial community structure, and the effect varied with slope aspect and recovery time. It is noteworthy that slope aspect drove variation in bacterial communities between sunny and shady slopes in burned areas by changing soil pH. These results suggest that slope aspect should be considered in predicting the response of soil microbial communities to fire. Further studies should increase the number of samples to study the relationship between the bacterial community and soil pH mediated by slope aspect, providing a scientific basis for the restoration of different slopes.
REFERENCES
A. F. Pellegrini, A. Ahlström, S. E. Hobbie, P. B. Reich, L. P. Nieradzik, A. C. Staver, B. C. Scharenbroch, A. Jumpponen, W. R. Anderegg, J. T. Randerson, and R.B. Jackson, “Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity,” Nature 553 (7687), 194–198 (2018). https://doi.org/10.1038/nature24668
A. Fuentes-Ramirez, M. Barrientos, L. Almonacid, C. Arriagada-Escamilla, and C. Salas-Eljatib, “Short-term response of soil microorganisms, nutrients and plant recovery in fire-affected Araucaria araucana forests,” Appl. Soil Ecol. 131, 99–106 (2018). https://doi.org/10.1016/j.apsoil.2018.08.010
A. M. Cutiño-Jiménez, M. Martins-Pinheiro, W. C. Lima, A. Martín-Tornet, O. G. Morales, and C. F. M. Menck, “Evolutionary placement of Xanthomonadales based on conserved protein signature sequences,” Mol. Phylogenet. Evol. 54, 524–534 (2010). https://doi.org/10.1016/j.ympev.2009.09.026
A. Meira-Castro, R. A. Shakesby, J. E. Marques, S. H. Doerr, J. P. Meixedo, J. Teixeira, and H. I. Chaminé, “Effects of prescribed fire on surface soil in a Pinus pinaster plantation, northern Portugal,” Environ. Earth Sci. 73, 3011–3018 (2015). https://doi.org/10.1007/s12665-014-3516-y
B. N. Burnett, G. A. Meyer, and L. D. McFadden, “Aspect-related microclimatic influences on slope forms and processes, northeastern Arizona,” J. Geophys. Res. 113, F03002 (2008). https://doi.org/10.1029/2007jf000789
C. C. Shen, J. B. Xiong, H. Y. Zhang, Y. Z. Feng, X. G. Lin, X. Y. Li, W. J. Liang, and H. Y. Chu, “Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain,” Soil Biol. Biochem. 57, 204–211 (2013). https://doi.org/10.1016/j.soilbio.2012.07.013
C. C. Shen, Y. Ge, T. Yang, and H. Y. Chu, “Verrucomicrobial elevational distribution was strongly influenced by soil pH and carbon/nitrogen ratio,” J. Soils Sediments 17, 2449–2456 (2017). https://doi.org/10.1007/s11368-017-1680-x
C. F. Weber, J. S. Lockhart, E. Charaska, K. Aho, and K. A. Lohse, “Bacterial composition of soils in ponderosa pine and mixed conifer forests exposed to different wildfire burn severity,” Soil Biol. Biochem. 69, 242–250 (2014). https://doi.org/10.1016/j.soilbio.2013.11.010
E. Pérez-Valera, M. Goberna, and M. Verdú, “Fire modulates ecosystem functioning through the phylogenetic structure of soil bacterial communities,” Soil Biol. Biochem. 129, 80–89 (2018). https://doi.org/10.1016/j.soilbio.2018.11.007
G. Certini, “Effects of fire on properties of forest soils: a review,” Oecologia 143, 1–10 (2005). https://doi.org/10.1007/s00442-004-1788-8
G. M. Garrity, J. G. Holt, and J. J. Perry, “Phylum BVII. Thermomicrobia phy. nov.,” in Bergey’s Manual of Systematic Bacteriology, Ed. by D. R. Boone, R. W. Castenholz, and G. M. Garrity (Springer, New York, 2001), pp. 447–448.
G. T. Bergmann, S. T. Bates, K. G. Eilers, C. L. Lauber, J. G. Caporaso, W. A. Walters, R. Knight, and N. Fierer, “The under-recognized dominance of verrucomicrobia in soil bacterial communities,” Soil Biol. Biochem. 43, 1450–1455 (2011). https://doi.org/10.1016/j.soilbio.2011.03.012
H. Ammitzboll, G. J. Jordan, S. C. Baker, J. Freeman, and A. Bissett, “Diversity and abundance of soil microbial communities decline, and community compositions change with severity of post-logging fire,” Mol. Ecol. 30, 2434–2448 (2021). https://doi.org/10.1111/mec.15900
H. Y. Chu, X. J. Xiang, J. Yang, J. M. Adams, K. P. Zhang, Y. T. Li, and S. Yu, “Effects of slope aspects on soil bacterial and Arbuscular Fungal communities in a boreal forest in China,” Pedosphere 26 (2), 226–234 (2016). https://doi.org/10.1016/S1002-0160(15)60037-6
J. A. Hatten and D. Zabowski, “Fire severity effects on soil organic matter from a ponderosa pine forest: a laboratory study,” Int. J. Wildland Fire 19, 613–623 (2010). https://doi.org/10.1071/WF08048
J. Adkins, K. M. Docherty, J. Gutknecht, and J. R. Miesel, “How do soil microbial communities respond to fire in the intermediate term? Investigating direct and indirect effects associated with fire occurrence and burn severity,” Sci. Total Environ. 745, 140957 (2020). https://doi.org/10.1016/j.scitotenv.2020.140957
J. B. Pan, Y. J. Liu, Y. Yang, Z. X. Cheng, X. M. Lan, W. G. Hu, G. X. Shi, Q. Zhang, and H. Y. Feng, “Slope aspect determines the abundance and composition of nitrogen-cycling microbial communities in an alpine ecosystem,” Environ. Microbiol. 24 (8), 3598–3611 (2022). https://doi.org/10.1111/1462-2920.15900
J. E. Knelman, E. B. Graham, N. A. Trahan, S. K. Schmidt, and D. R. Nemergut, “Fire severity shapes plant colonization effects on bacterial community structure, microbial biomass, and soil enzyme activity in secondary succession of a burned forest,” Soil Biol. Biochem. 90, 61–168 (2015). https://doi.org/10.1016/j.soilbio.2015.08.004
J. G. Caporaso, J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, and N. Fierer, “QIIME allows analysis of high-throughput community sequencing data,” Nat. Methods 7, 335–336 (2010). https://doi.org/10.1038/nmeth.f.303
J. Liu, L. P. Qiu, X. Wang, X. R. Wei, H. L. Gao, Y. J. Zhang, and J. M. Cheng, “Effects of wildfire and topography on soil nutrients in a semiarid restored grassland,” Plant Soil 428, 23–136 (2018). https://doi.org/10.1007/s11104-018-3659-9
J. Rodríguez, J. A. González-Pérez, A. Turmero, M. Hernández, A. S. Ball, F. J. González-Vila, and M. E. Arias, “Wildfire effects on the microbial activity and diversity in a Mediterranean forest soil,” Catena 158, 82–88 (2017). https://doi.org/10.1016/j.catena.2017.06.018
J. Y. Wu, B. J. Anderson, H. L. Buckley, G. Lewis, and G. Lear, “Aspect has a greater impact on alpine soil bacterial community structure than elevation,” FEMS Microbiol. Ecol. 93, fiw253 (2017). https://doi.org/10.1093/femsec/fiw253
L. E. Sáenz de Miera, R. Pinto, J. J. Gutierrez-Gonzalez, L. Calvo, and G. Ansola, “Wildfire effects on diversity and composition in soil bacterial communities,” Sci. Total Environ. 726, 138636 (2020). https://doi.org/10.1016/j.scitotenv.2020.138636
L. F. DeBano, D. G. Neary, and P. F. Folliott, Fire’s Effect on Ecosystems (John Wiley and Sons, New York, 1998).
L. Wang, S. P. Wei, R. Horton, and M. A. Shao, “Effects of vegetation and slope aspect on water budget in the hill and gully region of the Loess Plateau of China,” Catena 87, 90–100 (2011). https://doi.org/10.1016/j.catena.2011.05.010
M. Alcañiz, L. Outeiro, M. Francos, J. Farguell, and X. Úbeda, “Long-term dynamics of soil chemical properties after a prescribed fire in a Mediterranean forest (Montgrí Massif, Catalonia, Spain),” Sci. Total Environ. 572, 1329–1335 (2016). https://doi.org/10.1016/j.scitotenv.2016.01.115
M. D. Robinson, D. J. McCarthy, and G. K. Smyth, “edgeR: a Bioconductor package for differential expression analysis of digital gene expression data,” Bioinformatics 26, 139–140 (2010). https://doi.org/10.1093/bioinformatics/btp616
M. Goberna, C. García, H. Insam, M. T. Hernández, and M. Verdú, “Burning fire-prone Mediterranean shrublands: Immediate changes in soils microbial community structure and ecosystem functions,” Microb. Ecol. 64, 242–255 (2012). https://doi.org/10.1007/s00248-011-9995-4
M. Pourreza, S. M. Hosseini, A. S. Sinegani, M. Matinizadeh, and W. A. Dick, “Soil microbial activity in response to fire severity in Zagros oak (Quercus brantii Lindl.) forests, Iran, after one year,” Geoderma 213, 95–102 (2014). https://doi.org/10.1016/j.geoderma.2013.07.024
M. S. Huffman and M. D. Madritch, “Soil microbial response following wildfires in thermic oak-pine forests,” Biol. Fertil. Soils 54, 985–997 (2018). https://doi.org/10.1007/s00374-018-1322-5
M. S. Markovic, B. S. Ilic, D. L. Miladinovic, S. M. Stamenkovic, R. Trajkovic, V. P. Stankov-Jovanovic, and G. T. Djelic, “Activity of a catalase enzyme in plants from the burned areas of the Vidlic Mountain beech forest,” Oxid. Commun. 38 (2), 860–868 (2015).
M. T. Prendergast-Miller, A. B. de Menezes, L. M. Macdonald, P. Toscas, A. Bissett, G. Baker, M. Farrell, A. E. Richardson, T. Wark, and P. H. Thrall, “Wildfire impact: natural experiment reveals differential short-term changes in soil microbial communities,” Soil Biol. Biochem. 109, 1–13 (2017). https://doi.org/10.1016/j.soilbio.2017.01.027
O. M. Butler, J. J. Elser, T. Lewis, B. Mackey, and C. R. Chen, “The phosphorus-rich signature of fire in the soil-plant system: a global meta-analysis,” Ecol. Lett. 21, 335–344 (2018). https://doi.org/10.1111/ele.12896
P. Baldrian, “Microbial activity and the dynamics of ecosystem processes in forest soils,” Curr. Opin. Microbiol. 37, 128–134 (2017). https://doi.org/10.1016/j.mib.2017.06.008
P. D. Schloss, S. L. Westcott, T. Ryabin, J. R. Hall, M. Hartmann, E. B. Hollister, R. A. Lesniewski, B. B. Oakley, D. H. Parks, C. J. Robinson, J. W. Sahl, B. Stres, G. G. Thallinger, D. J. Van Horn, and C. F. Weber, “Introducing Mothur: open-source, platform independent, community supported software for describing and comparing microbial communities,” Appl. Environ. Microbiol. 75 (23), 7537–7541 (2009).
R. C. Edgar, “Search and clustering orders of magnitude faster than BLAST,” Bioinformatics 26, 2460–2461 (2010). https://doi.org/10.1093/bioinformatics/btq461
R. E. J. Boerner and J. A. Brinkman, “Fire frequency and soil enzyme activity in southern Ohio oak-hickory forests,” Appl. Soil Ecol. 23, 137–146 (2003). https://doi.org/10.1016/S0929-1393(03)00022-2
R. Xue, Q. Yang, F. H. Miao, X. Z. Wang, and Y. Y. Shen, “Slope aspect influences plant biomass, soil properties and microbial composition in alpine meadow on the Qinghai-Tibetan Plateau,” J. Soil Sci. Plant Nutr. 18 (1), 1–12 (2018). https://doi.org/10.4067/s0718-95162018005000101
S. D. Bao, Analysis of Soil Agrochemistry (China Agriculture Press, Beijing, 2000).
S. H. Yang, Q. S. Zheng, Y. F. Yang, M. T. Yuan, X. Y. Ma, N. R. Chiariello, K. M. Docherty, C. B. Field, J. L. M. Gutknecht, B. A. Hungate, A. Niboyet, X. Le Roux, and J. Z. Zhou, “Fire affects the taxonomic and functional composition of soil microbial communities, with cascading effects on grassland ecosystem functioning,” Global Change Biol. 26 (2), 431–442 (2020). https://doi.org/10.1111/gcb.14852
S. R. Dooley and K. K. Treseder, “The effect of fire on microbial biomass: a meta-analysis of field studies,” Biogeochemistry 109, 49–61 (2012). https://doi.org/10.1007/s10533-011-9633-8
S. R. Holden, A. Gutierrez, and K. K. Treseder, “Changes in soil fungal communities, extracellular enzyme activities, and litter decomposition across a fire chronosequence in Alaskan Boreal forests,” Ecosystems 16, 34–46 (2013). https://doi.org/10.1007/s10021-012-9594-3
S. Y. Guan, Soil Enzyme and Its Research Methods (Agricultural press, Beijing, 1986). https://doi.org/10.1128/AEM.01541-09
V. Fernández-García, J. Miesel, M. J. Baeza, E. Marcos, and L. Calvo, “Wildfire effects on soil properties in fire-prone pine ecosystems: indicators of burn severity legacy over the medium term after fire,” Appl. Soil Ecol. 135, 147–156 (2018). https://doi.org/10.1016/j.apsoil.2018.12.002
W. K. Li, S. K. Niu, X. D. Liu, and J. M. Wang, “Short-term response of the soil bacterial community to differing wildfire severity in Pinus tabulaeformis stands,” Sci. Rep. 9, 1148 (2019). https://doi.org/10.1038/s41598-019-38541-7
W. Q. Su, C. X. Tang, J. H. Lin, M. J. Yu, Z. M. Dai, Y. Luo, Y. Li, and J. M. Xu, “Recovery patterns of soil bacterial and fungal communities in Chinese boreal forests along a fire chronosequence,” Sci. Total Environ. 805, 150372 (2022). https://doi.org/10.1016/j.scitotenv.2021.150372
W. Y. Liu, F. Wang, Y. M. Sun, L. Yang, H. H. Chen, W. J. Liu, B. Zhu, C. M. Hui, and S. W. Wang, “Influence of dragon bamboo with different planting patterns on microbial community and physicochemical property of soil on sunny and shady slopes,” J. Microbiol. 58 (11), 906–914 (2020). https://doi.org/10.1007/s12275-020-0082-8
Z. M. Dai, X. F. Lv, B. Ma, N. Chen, S. X. Chang, J. H. Lin, X. H. Wang, W. Q. Su, H. T. Liu, Y. L. Huang, C. Z. Hu, Y. Luo, R. A. Dahlgren, and J. M. Xu, “Concurrent and rapid recovery of bacteria and protist communities in Canadian boreal forest ecosystems following wildfire,” Soil Biol. Biochem. 163, 108452 (2021). https://doi.org/10.1016/j.soilbio.2021.108452
Z. W. Wu, H. S. He, R. E. Keane, Z. L. Zhu, Y. Q. Wang, and Y. L. Shan, “Current and future patterns of forest fire occurrence in China,” Int. J. Wildland Fire 29, 104–119 (2020). https://doi.org/10.1071/WF19039
ACKNOWLEDGMENTS
This study was funded by the National Natural Science Foundation of China (31770581), Fundamental Research Projects of Science & Technology Innovation, Development Plan in Yantai City (no. 2022JCYJ029) and Taishan Scholars Program of Shandong Province (no. tsqn201812097).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zhu, P., Liu, W., Sun, Z. et al. Soil Bacterial Community Response to Fire Varies with Slope Aspect at Zhenshan Mountain, East China. Eurasian Soil Sc. 56, 599–610 (2023). https://doi.org/10.1134/S1064229322602104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229322602104