Abstract
Soil fauna can serve as an excellent tool for ecological assessment of soil quality. The earthworm Eisenia fetida L. is widely used as a bioindicator organism to assess the toxicity of metals, metalloids, and other pollutants. Many studies have shown that the concentrations of metals and metalloids toxic to earthworms are an order of magnitude lower in artificially contaminated soils than in industrially contaminated soils. The novelty of this study is that toxicity estimates were made using native industrially contaminated soils. The results of the two experiments demonstrate the potential use of earthworms for ecological assessment of soils contaminated with metals and metalloids due to copper mining activities in central Chile. The main contaminant in these soils was copper, but arsenic, commonly found in copper ore, was also present in the contaminated soils. In the short-term bioassay, E. fetida earthworms avoided the soil in response to increasing copper content. However, in long-term experiments, arsenic proved to be more toxic to earthworm reproduction, while copper had little effect. In this study, we present toxicity thresholds for copper and arsenic to E. fetida in industrially contaminated native soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Soil fauna can be an excellent tool for ecotoxicological assessment of soil quality [14, 23]. The earthworm Eisenia fetida L. has been widely used as a bioindicator organism in toxicity screening for a variety of contaminants, including metals and metalloids [30]. The species is a representative of a broad group of earthworms [28] that play an important role in the functioning of terrestrial ecosystems [29]. This species is particularly suited for toxicity studies compared to other Lumbricidae because it is easy to culture, has a short time to sexual maturity, and reproduces well under laboratory conditions [28]. Therefore, Eisenia fetida is recognized and widely used as a standard species in soil toxicity testing. A study by [16] showed that Eisenia fetida is as sensitive to chemicals as other worm species, although other studies have shown it to be less sensitive to zinc and lead [15, 38].
Many studies have shown that the concentrations of metals and metalloids toxic to earthworms in artificially contaminated soils are an order of magnitude lower than those in industrially contaminated soils [35]. This difference is attributed to the fact that metal toxicity depends on the residence time of metals in the soil, a lengthy process called “aging” [21]. Scientists often talk of the importance of using industrially (rather than artificially) contaminated soils for biological toxicity testing with earthworms [25]. However, in most cases, this does not go beyond lip service [34]. The novelty of this study is the use of field-collected industrially contaminated soils for toxicity assessment.
This paper reports the results of two experiments [5, 6] that demonstrated the feasibility of using earthworms for the ecotoxicological evaluation of soils contaminated with metals and metalloids from copper mining in the Valparaíso region, central Chile.
MATERIALS AND METHODS
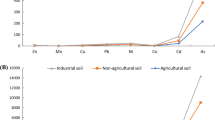
Copper can be considered the most important contaminant in the investigated soils [41], but arsenic, which is usually associated with copper ore, was also present [27]. Copper is an important micronutrient for organisms but is toxic above a certain threshold. Arsenic is not considered a micronutrient and can be toxic to organisms, especially animals [1].
The term “heavy metal” is widely used in the literature, but is not recommended by the International Union of Pure and Applied Chemistry (IUPAC) [7]. Therefore, the terms “metal” (copper) and “metalloid” (arsenic) are used in this work.
The study used alluvial agricultural soils from the Aconcagua river basin that were classified as Eutric Fluvisols [11]. The study also used non-agricultural soils formed on paleosand dunes in the Puchuncaví river basin that were classified as Dystric Arenosols [11]. The sampling sites were chosen to represent a wide range of total soil metal concentrations [26, 40]. Fifty-two and 24 agricultural soils were used for the first and second experiments, respectively (Table 1). Samples of each soil were taken from the top layer (0–20 cm depth). Importantly, all of the samples were examined by the biological test methods described below.
The first experiment was earthworm avoidance of the test substrate [6] performed according to ISO‑17512-1 [10]. The incubation time was 48 h and earthworms were given the option of migrating between the field-collected soil and the artificial control substrate. Thus, the avoidance test evaluated the suitability of the two soil types as potential earthworm habitats.
It is important to note that we made a slight modification to the method because reconnaissance experiments had revealed that the determining factors for earthworm avoidance included not only the presence of toxic substances, but also the organic matter content of the soil and the electrical conductivity of the soil extract [6]. Therefore, the organic matter content and electrical conductivity of the artificial substrate were modified by adding peat and NaCl solution, respectively. Meanwhile, the field-collected soils used in the study were not modified in any way.
The amounts of peat and NaCl solution added to the artificial substrate were selected to mimic the properties of the field-collected soil. By adjusting the physicochemical properties of the artificial substrate, the interferences of organic matter and electrical conductivity were eliminated; thus, the toxicity threshold was correctly determined.
Given the narrow range of pH values of the soils studied (7.1 ± 0.7) [6], pH was not considered a confounding factor. However, further research is needed on the advantages of controlling the pH of the artificial substrate when testing soils with a wide range of pH values. Further research is also needed on the benefits of using other salts (e.g., sea salt) instead of NaCl to control the electrical conductivity of soil extracts.
The second experiment [5] used the number of cocoons produced, i.e., reproductive rate, as the organism response variable. Following the procedures of ISO 11268-2 [10], adult species were incubated in the test soil for 4 weeks. Subsequently, they were transferred to moist filter paper for 24 h to have them empty their guts of soil; the filter paper was changed every 6 h [2]. The elemental content of the earthworm tissue was then analyzed to identify the elements responsible for the toxicity of industrially contaminated soil to earthworms [24].
Note that we use the term “concentration” for the liquid phase of the soil (salt extract in this study) and the term “content” for the solid phase of the soil and for the earthworm tissues [8]. In this study, a 0.1 M solution of KNO3 was used to prepare the salt extract.
Concentrations of Cu and As were measured in a 0.1 M KNO3 extract (soil/solution ratio 1/2.5) [39]. The activity of Cu2+ in the extract was measured using an ion-selective electrode [31]. The results were expressed as pCu2+, the negative logarithm of the free Cu2+ ion activity. To measure total Cu, Pb, Zn, and As contents, samples were digested in boiling nitric acid followed by the addition of perchloric acid [20]. To avoid the volatilization of As during the acid digestion process, a Teflon plug with a 30 cm long glass reflux tube was used [32]. Other chemical properties of the soil were determined by conventional methods [33]. Soil texture was determined by the simplified hydrometer method [37].
Regression analysis was performed between biological responses and soil physicochemical properties [13]. In the second experiment, regression analysis was also performed between biological responses and the content of metals and metalloids in the earthworm tissue. Statistical analysis was conducted with Minitab 18.
A nonlinear regression analysis was performed using the U.S. Environmental Protection Agency Toxicity Analysis Program to calculate the effective concentration (i.e., EC50) at which the response is reduced by 50% compared to the control [42]. Background concentrations of copper and arsenic in uncontaminated soils in the Valparaíso area are 134 mg/kg and 13 mg/kg, respectively [26]. Therefore, to calculate the effective concentrations, the earthworm response for soils with less than 134 mg/kg total copper and less than 13 mg/kg total arsenic was assumed to be 100%.
COPPER TOXICITY THRESHOLDS IN THE AVOIDANCE TEST
One study [6] found that earthworm avoidance was determined by the total Cu content in the soil, while the effects of other elements (Pb, Zn, As) and other Cu pools were negligible. This finding makes it possible to determine toxicity thresholds. It is also consistent with information available in the literature [37], which indicates that total metal concentrations can predict organism responses to the same degree as the bioavailable fraction.
Earthworms in our study were not observed to avoid soils with less than 155 mg/kg total copper. This finding is inconsistent with the conclusions of [3], where earthworm avoidance was observed in soils with about 110 mg/kg total copper. However, the discrepancy is probably due to the high toxicity of copper in the artificially Cu(NO3)2-contaminated soils used in the study of [3]. Our experiments are more relevant from an environmental point of view because we used industrially contaminated soils collected in the field [25].
According to ISO-17512-1 [11], a soil is unsuitable for organisms if it is avoided by more than 80% of earthworms. In our experiment, the total copper content at which 80% of earthworms avoided the target soil was 433 mg/kg (95% confidence interval: 339–528 mg/kg). Thus, a total copper content above 339 mg/kg may be considered the threshold above which the residence time of earthworms in the soil would be limited. Similar values were obtained in a Danish field study [9] in which the only soil contaminant was copper. It was found that earthworm biomass and population density decreased when the total copper content exceeded 300 mg/kg. In both the Danish study and ours, the metal has been present in the soils studied for decades.
It should be noted that very few studies on artificially contaminated soils have determined threshold values for total copper content using earthworms as bioindicators (Table 2) [34]. These studies cover only a very small number of real-world situations and, therefore, do not allow for any broad generalizations to be made.
ARSENIC TOXICITY THRESHOLDS IN THE REPRODUCTION TEST
Stepwise regression analysis showed that the effect of different lead and zinc soil pools on the number of cocoons produced was not statistically significant. In addition, the effect of lead and zinc content in worm tissue was also not statistically significant (p > 0.05). On the other hand, linear regression analysis revealed that the number of cocoons was related to the total arsenic content of the soil (R2 = 0.52, p < 0.05) and to the arsenic content of Eisenia fetida tissue (R2 = 0.45, p < 0.05). However, these relationships were best approximated by plotting a sigmoid curve [42], which allowed us to estimate the effective concentration (Table 3).
In turn, copper concentration in 0.1 M KNO3 extract was poorly correlated with the number of cocoons (R2 = 0.25, p < 0.05). The effects of other soil copper pools (free Cu+2 ion activity in salt extracts and total soil copper content) were not statistically significant (p > 0.05); the effect of copper content in Eisenia fetida tissue was also not statistically significant in a single regression (p > 0.05) and minimally significant in the following multiple regression (p = 0.05): cocoon number = 15.8 – 0.15 As in earthworm tissue—0.05 Cu in earthworm tissue, R2 = 0.58.
Since arsenic and copper do not correlate with each other in the soils studied, we concluded that arsenic in this case is the most toxic element for earthworms, while the effect of copper is less pronounced. This result is somewhat surprising, given that copper was expected to be the most toxic element in soils contaminated by the copper industry.
A study by [5] calculated the bioconcentration factor (ratio of tissue content to soil content) for Eisenia fetida. The mean bioconcentration factor for arsenic was 3.2 and for copper 0.15, suggesting that arsenic may be more toxic than copper. Similarly, the bioconcentration factor for the closely related species Eisenia andrei (considered a subspecies of Eisenia fetida) was higher for arsenic than for copper. This fact suggests that the copper content in Eisenia fetida tissues may be controlled by a specific mechanism of excretion of this element [41]. On the other hand, [19] reported that Eisenia fetida does not excrete arsenic when contaminated earthworms are introduced into clean soil, possibly due to the formation of arsenic and thiol compounds in the worm tissues. The inability to remove arsenic is supported by a similar inability to remove other nonessential elements (such as cadmium and lead), but rapid removal of essential elements such as copper by Eisenia fetida has been documented [41].
In [17] it was shown that the arsenic species As(III) is more toxic to Eisenia fetida than As(V). We [44] found that the proportions of the species As(V) and As(III) in the soils of the Valparaíso region were 75 ± 12% and 12 ± 6%, respectively. Thus, the obtained toxicity thresholds for total arsenic in soil mainly corresponded to the As(V) species. In our review of the literature, we found no information on arsenic toxicity thresholds for earthworms in anthropogenically contaminated soils in the Valparaíso region. Therefore, this study provides new data that can be used to estimate arsenic toxicity thresholds for Eisenia fetida.
Our toxicity thresholds for total arsenic in soil differed significantly from the known toxicity thresholds for arsenic to Eisenia fetida obtained from experiments using artificially contaminated soil. For example, the authors of [20] reported an LC50 of 5.9 mg/kg for total arsenic in artificially contaminated soil during a 4-week experimental period, but in our study no lethal effects were observed with this level of arsenic during this period. In addition, one study [18] reported that the EC50 of total arsenic in soil of 11 mg/kg, similar to the reproduction test, but in our study much higher values of total arsenic in soil (22 mg/kg) were needed to achieve the same effect.
In one of our earlier studies [4], electron probe microscopy analysis showed that the main arsenic-bearing phases in agricultural soils of the Valparaíso region were poorly soluble iron oxides and copper sulfides. Meanwhile, in the aforementioned studies that used artificially contaminated soils, the salts added to the soil were readily soluble (potassium arsenate or sodium arsenate). Therefore, it may be assumed that the differences between the existing arsenic toxicity thresholds for Eisenia fetida in artificially contaminated soils and our results in field-collected anthropogenically contaminated soils were due to the different solubility of the arsenic-bearing phases. This mean that artificially contaminated soils do not adequately reflect real environmental conditions and are of limited ecological significance.
CONCLUSIONS AND PRACTICAL IMPLICATIONS
Short-term experiments showed that Eisenia fetida worms avoided soil with elevated copper content caused by emissions from the copper industry. However, in long-term experiments, arsenic seemed to be more toxic to earthworm reproduction, while the effect of copper was less pronounced. Thus, despite the multi-elemental nature of the soil contamination studied, chemical analysis of earthworm tissue made it possible to identify the elements that are the main culprits responsible for the toxicity of industrially contaminated soil.
Soil contamination is a major concern in determining the potential uses of an area, including the need for reclamation, remediation, or complete removal and replacement of soils, with significant economic costs. The authors argue that regulatory documents in this area need to clearly distinguish between soils in which metals are present but not toxic and soils that pose a significant environmental hazard at similar levels of total metal content. The results of this study provide the necessary information regarding this distinction for soils in the Valparaíso region of Chile. The information obtained can be used for practical purposes to assess and manage the risks posed by anthropogenically contaminated soils.
REFERENCES
D. C. Adriano, Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risk of Metals (Springer–Verlag, New York, 2001).
R. E. Arnold and M. E. Hodson, “Effect of time and mode of depuration on tissue copper concentrations of the earthworms Eisenia andrei, Lumbricus rubellus and Lumbricus terrestris,” Environ. Pollut. 148, 21–30 (2007). https://doi.org/10.1016/j.envpol.2006.11.003
R. E. Arnold, M. E. Hodson, S. Black, and N. A. Davies, “The influence of mineral solubility and soil solution concentration on the toxicity of copper to Eisenia fetida Savigny,” Pedobiologia 47 (5–6), 622–632 (2003). https://doi.org/10.1016/s0031-4056(04)70246-2
G. Ávila, H. Gaete, M. Morales, and A. Neaman, “Reproducción de Eisenia fetida en suelos agrícolas de áreas mineras contaminadas por cobre y arsénico,” Pesqui. Agropecu. Bras. 42 (3), 435–441 (2007). https://doi.org/10.1590/S0100-204X2007000300018
V. Bustos, P. Mondaca, S. Sauvé, H. Gaete, J. L. Celis-Diez, and A. Neaman, “Thresholds of arsenic toxicity to Eisenia fetida in field-collected agricultural soils exposed to copper mining activities in Chile,” Ecotoxicol. Environ. Saf. 122, 448–454 (2015). https://doi.org/10.1016/j.ecoenv.2015.09.009
V. Delgadillo, J. Verdejo, P. Mondaca, G. Verdugo, H. Gaete, M. E. Hodson, and A. Neaman, “Proposed modification to avoidance test with Eisenia fetida to assess metal toxicity in agricultural soils affected by mining activities,” Ecotoxicol. Environ. Saf. 140, 230–234 (2017). https://doi.org/10.1016/j.ecoenv.2017.02.038
J. H. Duffus, ““Heavy metals” a meaningless term? (IUPAC Technical Report),” Pure Appl. Chem. 74, 793–807 (2002). https://doi.org/10.1351/pac200274050793
X. Fuentes-Arderiu, “Concentration and content,” Biochem. Med. 23 (2), 141–142 (2013). https://doi.org/10.11613/bm.2013.017
M. Holmstrup and H. D. Hornum, “Earthworm colonisation of abandoned arable soil polluted by copper,” Pedobiologia 55, 63–65 (2012). https://doi.org/10.1016/j.pedobi.2011.08.005
ISO-17512-1, Report No., 2008.
IUSS Working Group WRB, World Reference Base for Soil Resources 2014, update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106 (Food and Agricultural Organization, Rome, 2015).
L. Konečný, V. Ettler, S. Kristiansen, M. J. Barros Amorim, B. Kříbek, M. Mihaljevič, O. Šebek, I. Nyambe, and J. Scott-Fordsmand, “Response of Enchytraeus crypticus worms to high metal levels in tropical soils polluted by copper smelting,” J. Geochem. Explor. 144, 427–432 (2014). https://doi.org/10.1016/j.gexplo.2013.10.004
M. Kutner, C. Nachtsheim, and J. Neter, Applied Linear Regression Models (McGraw-Hill Education, Boston, 2004).
A. I. Kuznetsova, N. V. Lukina, E. V. Tikhonova, A. V. Gornov, M. V. Gornova, V. E. Smirnov, A. P. Geraskina, N. E. Shevchenko, D. N. Tebenkova, and S. I. Chumachenko, “Carbon stock in sandy and loamy soils of coniferous-broadleaved forests at different succession stages,” Eurasian Soil Sci. 52 (7), 756–768 (2019). https://doi.org/10.1134/s1064229319070081
C. J. Langdon, M. E. Hodson, R. E. Arnold, and S. Black, “Survival, Pb-uptake and behaviour of three species of earthworm in Pb treated soils determined using an OECD-style toxicity test and a soil avoidance test,” Environ. Pollut. 138 (2), 368–375 (2005). https://doi.org/10.1016/j.envpol.2005.03.002
R. Laskowski, P. Kramarz, and P. Jepson, in Handbook of Soil Invertebrate Toxicity Tests (John Wiley & Sons, Chichester, 1998), pp. 21–40.
B.-T. Lee and K.-W. Kim, “Toxicokinetics and biotransformation of As(III) and As(V) in Eisenia fetida,” Hum. Ecol. Risk Assess. 19 (3), 792–806 (2013). https://doi.org/10.1080/10807039.2012.708285
K. Lock and C. R. Janssen, “Toxicity of arsenate to the compostworm Eisenia fetida, the potworm Enchytraeus albidus and the springtail Folsomia candida,” Bull. Environ. Contam. Toxicol. 68, 760–765 (2002). https://doi.org/10.1007/s001280320
K. Maraldo, B. Christensen, B. Strandberg, and M. Holmstrup, “Effects of copper on enchytraeids in the field under differing soil moisture regimes,” Environ. Toxicol. Chem. 25 (2), 604–612 (2006). https://doi.org/10.1897/05-076R.1
J. A. Maxwell, Rock and Mineral Analysis (Interscience Publishers, New York, 1968).
M. B. McBride and M. F. Cai, “Copper and zinc aging in soils for a decade: changes in metal extractability and phytotoxicity,” Environ. Chem. 13 (1), 160–167 (2016). https://doi.org/10.1071/en15057
H. Mirmonsef, H. D. Hornum, J. Jensen, and M. Holmstrup, “Effects of an aged copper contamination on distribution of earthworms, reproduction and cocoon hatchability,” Ecotoxicol. Environ. Saf. 135, 267–275 (2017). https://doi.org/10.1016/j.ecoenv.2016.10.012
M. A. Nadporozhskaya, S. S. Bykhovets, and E. V. Abakumov, “Application of the ROMUL mathematical model for estimation of CO2 emission and dynamics of organic matter in the Subantarctic lithozems,” Eurasian Soil Sci. 55 (4), 413–424 (2022). https://doi.org/10.1134/s1064229322040123
J. Nahmani, M. E. Hodson, and S. Black, “Effects of metals on life cycle parameters of the earthworm Eisenia fetida exposed to field-contaminated, metal-polluted soils,” Environ. Pollut. 149 (1), 44–58 (2007). https://doi.org/10.1016/j.envpol.2006.12.018
J. Nahmani, M. E. Hodson, and S. Black, “A review of studies performed to assess metal uptake by earthworms,” Environ. Pollut. 145 (2), 402–424 (2007). https://doi.org/10.1016/j.envpol.2006.04.009
A. Neaman, P. Valenzuela, J. Tapia-Gatica, I. Selles, A. A. Novoselov, E. A. Dovletyarova, C. Yanez, Y. A. Krutyakov, and J. W. Stuckey, “Chilean regulations on metal-polluted soils: The need to advance from adapting foreign laws towards developing sovereign legislation,” Environ. Res. 185, 109429 (2020). https://doi.org/10.1016/j.envres.2020.109429
P. O’Neill, in Heavy Metals in Soils (Blackie Academic & Professional, London, 1995), pp. 105–121.
OECD-222, Report No. 2074–5761, 2016.
D. Pezzotti, M. Peli, A. Sanzeni, and S. Barontini, “Seasonality of Earthworm Macropores in a Temperate Alpine Area,” Eurasian Soil Sci. 54 (12), 1935–1944 (2021). https://doi.org/10.1134/S1064229321130032
M. A. Pukalchik, V. A. Terekhova, M. M. Karpukhin, and V. M. Vavilova, “Comparison of eluate and direct soil bioassay methods of soil assessment in the case of contamination with heavy metals,” Eurasian Soil Sci. 52 (4), 464–470 (2019). https://doi.org/10.1134/s1064229319040112
J. Rachou, C. Gagnon, and S. Sauvé, “Use of an ion-selective electrode for free copper measurements in low salinity and low ionic strength matrices,” Environ. Chem. 4, 90–97 (2007). https://doi.org/10.1071/EN06036
A. Sadzawka, M. A. Carrasco, R. Demanet, H. Flores, M. L. Mora, A. Neaman, P. Hernández, and M. Sandoval, Métodos de análisis de lodos y de suelos (Sociedad Chilena de la Ciencia del Suelo, Universidad de Concepción, Chillán, 2015).
A. Sadzawka, M. A. Carrasco, R. Grez, M. L. Mora, H. Flores, and A. Neaman, Métodos de análisis recomendados para los suelos de Chile. Serie actas INIA Nº 34, (Instituto de Investigaciones Agropecuarias, Santiago, 2006).
J. Santa-Cruz, P. Peñaloza, M. V. Korneykova, and A. Neaman, “Thresholds of metal and metalloid toxicity in field-collected anthropogenically contaminated soils: a review,” Geogr. Environ. Sustainability 14 (2), 6–21 (2021). https://doi.org/10.24057/2071-9388-2021-023
J. Santa-Cruz, I. I. Vasenev, H. Gaete, P. Peñaloza, Y. A. Krutyakov, and A. Neaman, “Metal ecotoxicity studies with spiked versus field-contaminated soils: literature review, methodological shortcomings and research priorities,” Russ. J. Ecol. 52 (6), 478–484 (2021). https://doi.org/10.1134/S1067413621060126
J. J. Scott-Fordsmand, J. M. Weeks, and S. P. Hopkin, “Importance of contamination history for understanding toxicity of copper to earthworm Eisenia fetida (Oligochaeta: Annelida), using neutral-red retention assay,” Environ. Toxicol. Chem. 19 (7), 1774–1780 (2000). https://doi.org/10.1002/etc.5620190710
B. H. Sheldrick and C. Wang, in Soil Sampling and Methods of Analysis (Canadian Society of Soil Science, Lewis Publishers, Boca Raton, 1993), pp. 499–511.
D. J. Spurgeon and J. M. Weeks, in Advances in Earthworm Ecotoxicology (SETAC Technical Publications Series, Pensacola, 1998), pp. 15–25.
J. W. Stuckey, A. Neaman, R. Ravella, S. Komarneni, and C. E. Martínez, “Highly charged swelling mica reduces free and extractable Cu levels in Cu-contaminated soils,” Environ. Sci. Technol. 42 (24), 9197–9202 (2008). https://doi.org/10.1021/es801799s
J. Tapia-Gatica, I. González-Miranda, E. Salgado, M. A. Bravo, C. Tessini, E. A. Dovletyarova, A. A. Paltseva, and A. Neaman, “Advanced determination of the spatial gradient of human health risk and ecological risk from exposure to As, Cu, Pb, and Zn in soils near the Ventanas Industrial Complex (Puchuncaví, Chile),” Environ. Pollut. 258, 113488 (2020). https://doi.org/10.1016/j.envpol.2019.113488
J. Tapia-Gatica, I. Selles, M. A. Bravo, C. Tessini, W. Barros-Parada, A. Novoselov, and A. Neaman, “Global issues in setting legal limits on soil metal contamination: a case study of Chile,” Chemosphere 290, 133404 (2022). https://doi.org/10.1016/j.chemosphere.2021.133404
US EPA, Toxicity Relationship Analysis Program (TRAP) version 1.3 (United States Environmental Protection Agency, Mid-Continent Ecology Division, 2016).
L. Van Zwieten, J. Rust, T. Kingston, G. Merrington, and S. Morris, “Influence of copper fungicide residues on occurrence of earthworms in avocado orchard soils,” Sci. Total Environ. 329 (1–3), 29–41 (2004). https://doi.org/10.1016/j.scitotenv.2004.02.014
C. Vargas, W. Quiroz, M. Bravo, and A. Neaman, “Stability of arsenic during soil treatment and storage,” J. Chil. Chem. Soc. 60, 2868–2871 (2015). https://doi.org/10.4067/S0717-97072015000300015
ACKNOWLEDGMENTS
The authors are grateful to E.L. Vorobeichik for his valuable comments. The authors would like to express their gratitude to A.L. Savrova for editing the Russian text and to A.A. Tchourakov for the English translation
Funding
This study was supported by FONDECYT project 1200048.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Neaman, A., Yáñez, C. Assessment of the Ecological Status of Soils Contaminated by the Copper Mining Industry in Chile: Earthworms to the Rescue. Eurasian Soil Sc. 56, 69–74 (2023). https://doi.org/10.1134/S1064229322601688
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229322601688