Abstract
Phosphorus plays an important role in double rice cropping systems grown on acidic paddy soil. To improve our knowledge and increase crop utilization of applied phosphorus fertilizers in paddy soils, we investigated changes in the content of soil inorganic phosphorus fractions in response to long-term application of chemical and organic phosphorus fertilizers. In this 35-year-long experiment, paddy soil in a double rice cropping system received one of three different fertilizer treatments: (1) cattle manure, M; (2) a chemical fertilizer of nitrogen (N), phosphorus (P), and potassium (K), NPK; and (3) a combination of NPK and M (NPKM) twice per year. Results showed that the maximum contents of Olsen-P in M, NPK and NPKM stabilized at 12.9, 31.7 and 52.7 mg/kg, respectively over 35 years. In contrast, the proportions of soil inorganic phosphorus content in total phosphorus of M, NPK and NPKM changed from 62.2% at the beginning of the experiment to 53.3, 61.9 and 66.2%, respectively. At equal amounts of accumulated phosphorus surplus, the increasing rates of total inorganic phosphorus content and inorganic phosphorus fractions in NPK were much higher than those in M and NPKM. At an average amount of accumulated phosphorus surplus of 100 kg/ha, the total inorganic phosphorus content of the NPK and NPKM treatments increased by 39.6 and 21.6 mg/kg, respectively. Fertilization mainly decreased the ratio of organic phosphorus to inorganic phosphorus and increased the ratio of aluminum-bound-phosphorus (Al-P) to inorganic phosphorus, especially under the NPK and NPKM treatments. Redundancy analysis showed that total phosphorus and Olsen-P were more closely correlated to iron-bound-phosphorus (Fe-P), calcium-bound-phosphorus (Ca-P) and Al-P fractions. This study suggests that the NPK and NPKM treatments increased phosphorus supply and inorganic phosphorus fractions compared to the manure application. Therefore, to avoid accumulation of a surplus of unabsorbed phosphorus and minimize phosphorus-leaching risk from acidic paddy soil, rates of inorganic phosphorus application, such as the combined application of manure and inorganic phosphorus fertilizer, should be reduced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Phosphorus (P) is one of the most important essential nutrients to plant growth. Phosphate fertilizer plays a major role in crop production and achieving high yields. From 1961 to 2013, the global total P input from applied phosphate fertilizer increased from 40.5 to 66.5% in crop production systems [22]. Extensive use of phosphate fertilizer results in a surplus of P that exceeds crop needs. The excess is sorbed into aggregates by soil cations, such as calcium (Ca2+), iron (Fe3+), and aluminum (Al3+), which builds up soil P pools for crop use or becomes a long-term source of P loss [17, 40]. Consequently, the response characteristics of P pools due to various long-term fertilization methods and improving effective crop use of P in soil has received a lot of attention in research.
Phosphorus availability and its fractions have long been of interest because of their importance in agricultural and aquatic environments. Total P (TP) content, organic matter content and soil pH are major factors that affect available P content in typical paddy soils, and the available P content varies seasonally [35]. Soil inorganic phosphorus (Pi) is the preferred fraction of P in crop P uptake. Having knowledge of the different P fractions within soils is essential to understanding P availability to crops [27]. Inorganic P is the dominant form of P in soil applied with fertilizers, accounting for about 71–83% of total soil P in paddy soils in China [42]. Fractionation of Pi provides an effective approach for investigating soil Pi availability and Pi inter-conversion among soil Pi fractions from different P pools, as well as investigating Olsen-P as an indicator for estimating soil P availability [14].
Different types of applied fertilizer can alter soil physical and chemical properties, which results in changes of Pi forms in soil. Several recent studies have shown that long-term fertilization of soil significantly alters Pi fractions, and the particular method of combined application of manure with inorganic fertilizer is a promising approach to increase labile P pools [8, 14, 36, 39, 42]. Such as soil organic matter (SOM), available P content and pH directly affect the conversion of different stable P fractions in paddy soils [3, 4]. The type of P fertilizer can also alter P fractions by influencing P sorption capacity of paddy soils via direct or indirect effects [41]. Besides extrinsic factors, P fractions change because they will adjust to the current local soil conditions as they naturally reach equilibrium with each other [8].
Not only do changes of soil P occur in one crop growing season, they also change over time with long-term fertilization. Different fertilizer treatments, as well as different phosphate sources, such as chemical and organic fertilizers, and cover crops can produce different patterns of interannual variation among soil P fractions [11, 28, 34]. The content of resin-exchange P as labile P varied slightly in the first three years and then increased significantly thereafter, and content of moderately-labile organic P fractions decreased in the first three years, but increased three years later [2]. However, little is known about the interannual changes in soil P fractions in paddy soil under long-term application of chemical fertilizer and cattle manure.
Soil P balance differs according to the different P source and application rate of P fertilizer [21, 37]. Many studies have found that soil P balance is an important factor influencing soil P levels. Soil Olsen-P and P surplus exhibit a significantly positive linear relationship [34, 40]. Moreover, previous studies have found that, with the continuous application of chemical fertilizers (NP and NPK), soil Olsen-P increased in the absence of P surplus and may be explained by the active transformation of residual soil P (Ca-P, Fe-P, Al-P) into Olsen-P. Their results indicate that the capacity of soil to fix P depends on the P surplus [37]. Different fertilization strategies can result in varied rates of TP accumulation and also create a shift of soil P fractions in the top layer of paddy soils. Knowledge on the effects of fertilization on accumulation rates and shifts of soil P would help our understanding of the effects of P addition on soil P transformations [42]. Unfortunately, there is limited information on a direct quantitative relationship between P surplus and conversion rates of Pi fractions in acidic paddy soil.
The main objectives of this study were to: 1) estimate the effects of cattle manure and other factors on transformations of Pi fractions; (2) quantify the annual patterns of variation in soil TP, soil Olsen-P, and Pi fractions in acidic paddy soil; and (3) quantify the changes of Pi and P balance in response to long-term (35 years) application of chemical fertilizer and cattle manure.
OBJECTS AND METHODS
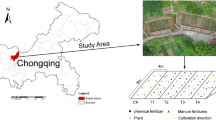
The study was conducted at the National Observation and Research Station of Farmland Ecosystems in Qiyang, Hunan province of southern China (26°45′42″ N, 111°52′32″ E). This region has a subtropical climate with mean annual precipitation of about 1.258 mm and mean annual temperature of 18.4°C (Fig. S1). The elevation is 160 m above sea level. The soil is a typical Ultisol, originally developed from quaternary red clay. Its pH was 5.97 and soil organic carbon (SOC) content was 12.2 g/kg. The initial soil properties measured include, total nitrogen (1.5 g/kg), TP (0.48 g/kg), total potassium (14.2 g/kg), alkali-hydrolysable nitrogen (158 mg/kg), Olsen-P (9.6 mg/kg) and available potassium (65.9 mg/kg).
The experiment began in 1982 and consisted of comparing the effects of application of one of three fertilizer treatments to paddy soils, (1) cattle manure only (M); (2) chemical forms of nitrogen, P and potassium fertilizer (NPK) only; and (3) NPK fertilizer in combination with cattle manure (NPKM). The treatments, each with three replicates, were established in a randomized complete block design. Each plot had an area of 27 m2. Local farming practices were adopted to apply all fertilizer treatments in the experiment. The cropping system was early rice–late rice–winter fallow. Base fertilizer was applied twice per year, once for each of the early- and late-rice crops. Fertilizer application rates are shown in Table 1. Composted cattle manure was thoroughly decomposed and applied at the rate of 22.5 t/ha for the early- and late-rice cropping seasons. Cattle manure contained 3.2 g/kg of N, 2.5 g/kg of P2O5, and 1.5 g/kg K2O. The same rates of N, P and K nutrients were supplied from manure and inorganic fertilizers. Inorganic compound fertilizers were applied as urea (N 46%), superphosphate (P2O5 12%), and potassium chloride (K2O 46%).
During the rice growing season, early rice was transplanted in April and harvested in July while the late rice was transplanted in July and harvested in October. All fertilizers were applied as basal fertilizer prior to transplanting seedlings of both the early- and late-rice crops into plots. After harvesting, rice straw was removed from the field and only roots were left in the field in accordance with the local farming practice.
Soil samples from the plough layer (0–20 cm) were collected once a year, since 1982, after the late rice was harvested. Five soil cores were randomly sampled from each plot using an auger and mixed thoroughly to form a composite sample. These soil samples were air-dried and stored in a sealed glass container at room temperature (25°C). Stored samples from 1982, 1987, 1993, 1998, 2002, 2008, 2012, and 2017 were analyzed for this study. All soil samples were sieved through 2.0‑mm mesh screens to determine contents of available nutrients and through 0.25-mm mesh screens prior to total nutrient and P-fractions analyses.
Soil available P was determined using the Olsen-P [32] method based on extraction of air-dried soil with 0.5 M NaHCO3 at pH 8.5. Total P was determined by the sodium hydroxide melt-molybdenum antimony colorimetric method [30].
Inorganic P was fractionated according to the sequential extraction procedure of Jackson [30]. Sequential chemical fractionation of Pi was performed as follows to obtain each type of bound P (stated in parentheses): extraction with 1 mol/L NH4Cl and 0.5 mol/L NH4F (Al-P), then 1 mol/L NaOH (Fe-P), then 0.3 mol/L Na3C6H5O7·2H2O and Na2S2O4·2H2O (Occluded-P, Oc-P), and lastly, 0.25 mol/L H2SO4 (Ca-P).
Annual P balance of soil from the 0–20 cm plough layer was calculated by subtracting P outputs (P in rice straw and grain) from P inputs (only from applied fertilizers). The accumulated P profit was defined as the sum of annual apparent P balance from 1982 to 2017. The change in soil Pi content and Pi fraction was determined by eq. (1) [34]:
Where Pn is the soil P contents of the nth year and P0 is the contents of initial soil Pi or Pi fraction.
Cumulative recovery efficiency of P (CPRE) was calculated by eq. (2) [12]:
where P uptake included straw and grain P uptake, and P input only accounts for the P sourced from the fertilizer applications of each year.
All statistical analyses were conducted using SPSS 20.0. Analysis of variance was conducted, and significant differences were evaluated using the least significant differences (LSD) test at P ≤ 0.05. Redundancy analysis, a type of linear-constrained ordination based on principal components analysis, was conducted to investigate the relationships between soil properties, climatic factors and Pi fractions using Canoco version 5. All figures were drawn by Sigma-plot 10.0.
RESULTS AND DISCUSSION
Effects of Organic and Inorganic Fertilizers on Olsen-P, TP and Pi Accumulation
After 35 years of biannual fertilization, Olsen-P contents of the M, NPK and NPKM treatments increased 3~10 times from 4.9 mg/kg in 1982 to 12.7, 30.1 and 48.2 mg/kg, respectively, in 2017 (Fig. S2). The data of the relationship of Olsen-P in each treatment with years of fertilization fitted well by the Mitscherlich equation y = a × (1 – exp(–bx)), where a represents the maximum of the dynamic equilibrium process for Olsen-P. Equation estimates of the maximums of Olsen-P for M, NPK and NPKM were approximately 12.9, 31.7 and 52.7 mg/kg, respectively, and all were nearly equal to the measured Olsen-P contents of their respective treatments. We observed more variation in Olsen-P maximums than compared to that of another study which determined a maximum of approximately 15 mg/kg in its NPK treatment of a calcareous soil planted with a rice monoculture system in northern China [14]. The average annual temperature (18°C) at the test site of our study was also higher than that reported in this previous study (9–12°C) [14]. The different physio-chemical properties of the two different environments, crop systems, and soils in which P adsorption and desorption reactions were likely affected by their respective prevailing temperatures and that affect may explain the differences in measured amounts of Olsen-P [23]. For example, mineralization of P from soil organic matter or crop residues depends on soil biological activity, which increases with increasing temperature [29]. Phosphorus adsorption is also influenced by soil mineralogy and soil aggregation. Soil with higher clay content and larger aggregates exhibited greater phosphorus retention [10, 38]. Our acidic paddy soil had higher clay content (45%, unpublished data) than calcareous soil, which was reportedly a silt-loam texture (11.8%) [14].
In our soil, there was no significant difference of accumulated P profit between the NPK and M treatments, which implies that there was no significant difference in rice yield between them. The maximum Olsen-P of NPK and NPKM (31.7 and 52.7 mg/kg) nearly reached the estimated critical level of leaching risk of Olsen-P (30–172 mg/kg) in Chinese rice production systems in the Yangtze River basin [17]. This phenomenon supports that low-P organic manure is a better alternative than chemical fertilizer. Moreover, application of manure results in less yield loss and has low non-point pollution risk [18].
Generally, soil total Pi and TP contents of the different treatments increased with time and at different rates (Fig. 1). Of the M treatment, the annual rate of increase of total Pi estimated for the last 12 years of the study (6.2 mg/(kg y)) rose by about five times of that estimated for the first 23 years of the study (1.3 mg/(kg y)). There were no significant differences found of total Pi between NPK (in the first 16 years of study) and NPKM (in the first 11 years of study); annual rates were similar at 19.7 mg/(kg y). In subsequent years through the end of the study, NPK’s rate decreased to 7.1 mg/(kg y) and NPKM’s rate decreased to 9.5 mg/(kg y). The TP contents of M, NPK and NPKM increased 5.5, 16.3 and 22.1 mg/kg each year, respectively. The amounts of total Pi and TP, and their annual rates of increase ranked the treatments in the following order: NPKM > NPK > M. The results were similar to those of previous studies, where the contents of Olsen-P, TP, and each Pi fraction and cumulative P profit increased with increasing amounts of P inputs in flooded tropical paddy soil [5, 18]. Other studies have demonstrated significant increases in Pi fractions under continuous additions of P fertilizer in paddy soils [19, 26, 39]. But the contents and interannual variation of Pi of M were significantly lower than those of NPK and NPKM. Our results are in line with results of Lee et al. [6] who applied either compost, NPK or NPK combined with compost to paddy soils continuously for 31 years. The total Pi contents of their three treatments increased by 4.3, 6.4 and 10.6 mg/kg each year, respectively. These results suggest that application of chemical P fertilizer or chemical P in combination with organic fertilizer should be applied if a rapid increase of Pi content in soil is needed.
Dynamics of soil total Pi and total P of the three fertilizer treatments. Here and in Figs. 2–4, M, cattle manure only; NPK, chemical nitrogen, phosphorus, and potassium fertilizer; and NPKM, NPK fertilizer in combination with cattle manure. *, **, and *** denote significance at the 5.0, 1.0, and 0.1% levels, respectively.
The percentage of Pi out of TP at the beginning of the experiment was 62.2%, and after 35 years of fertilization, the percentages of soil Pi under M, NPK and NPKM changed to 53.3, 61.9 and 66.2%, respectively. Long-term application of organic fertilizer decreased the proportion of Pi, and long-term combined application of organic and chemical fertilizer increased the proportion of Pi. However, long-term application of chemical fertilizer alone had no effect on the proportion of soil Pi. This observation supports results of the studies reporting that P fertilizer and manure applied for many years led to accumulation of Pi fractions in soil [5, 13]. However, continuous application of M alone decreased the percentage of soil Pi over time. This difference was due to the proportional distribution of soil P pools with different lability and depends on the P sources. Soltangheisi et al. reported that rock phosphate increased the accumulation of Pi as compared to single super phosphate [2]. However, the Pi pool in manure is large and has been reported to vary between 60% and 90% [36]. Moreover, Pi is also readily available to plants because of its high solubility. Meanwhile long-term application of animal manure will reduce soil P adsorption capacity, and increase rates of biologically-mediated turnover of organic P by stimulation of microbial and enzyme activities [1]. In general, manure application reduces the ability of the soil to fix P [24].
Changes of Pi Fraction in the Ploughed Layer
Overall, the contents of Al-P and Fe-P significantly increased with the passage of time in all treatments (Fig. 2). In the organic fertilizer treatment (M), Al-P and Fe-P contents did not change much. The fitted equations estimated that the maximum amounts of Al-P in M, NPK and NPKM were about 32.6, 121.7 and 220.8 mg/kg, respectively. The amount of Fe-P of the M treatment increased annually by 1.5 mg/(kg y). The change in Fe-P content under NPK and NPKM were divided into two stages. Within the first 10 years of NPK fertilization and 18 years of NPKM fertilization, the annual rates of increase of Fe-P were 11.0 and 10.4 mg/(kg y), respectively. Then in the subsequent 10 years of each treatment, Fe-P decreased to 2.8 and 4.1 mg/(kg y), respectively. In the organic fertilizer treatment (M), the contents of Oc-P and Ca-P were not significantly increasing with time. Content of Oc-P of NPK and NPKM annually increased by 1.8 and 2.8 mg/(kg y), respectively. The changes in Ca-P content under M, NPK and NPKM were also divided into two stages. In NPK, Ca-P increased more rapidly during the first eight years of fertilization (2.3 mg/(kg y)), but decreased eight years later (1.1 mg/(kg y)). However, the growth rate of Ca-P in soil treated with NPKM was slower in the first 26 years (1.6 mg/(kg y)), and faster in the subsequent years (4.1 mg/(kg y)).
In all the treatments after 35 years of fertilization, Fe-P was the predominant form of P, followed by Oc-P, Al-P and Ca-P (Fig. 3). The percentage of Oc-P of total Pi decreased gradually with time. In the M, NPK and NPKM treatments, Oc-P decreased by 27.4, 22.5 and 14.7%, respectively. The respective proportions of Al-P, Fe-P, Ca-P out of total Pi increased by 8.8, 13.2, and 16.5% under M; 4.8, 6.4, and 7.6% under NPK; and 1.2, 2.9, and 3.3% under NPKM. Fertilization mainly decreased the percent of Oc-P out of total Pi and increased the percent of Al-P out of total Pi, especially after applying chemical P fertilizer or chemical P fertilizer combined with organic matter.
The contents of Fe–P and Al–P in NPK and NPKM significantly increased with time during the long-term fertilization. Phosphorus solubility in the soil was mainly controlled by Fe-P and Al-P. Therefore, the high content of Al-P may have an important impact on the soil environment [7]. The contents of Fe–P and Ca–P in M did not change much, but Oc–P significantly decreased by 27.4% due to the continuous fertilization. This decrease might be closely related to the decrease of residual P. Based on the extent of isotope excursion among different Pi pools, the application of manure likely caused a shift in P solid-phase partitioning. Another study has shown that the HCl-Pi pool (Ca-P) in long-term P-fertilized soils most likely should be derived from chemical P fertilizer [25].
Correlations between Soil Pi and P Balance
After 35 years of fertilization, the accumulated P profit in soil of NPKM (2168 kg/ha) was significantly (P < 0.01) higher than that of NPK and M, but there was no significant difference between the NPK and M treatments (Fig. 4). The cumulative utilization of P over 35 years for M, NPK and NPKM soils were 52, 58 and 37%, respectively. The contents of total Pi in the NPK and NPKM treatments increased significantly (P < 0.01) with the increase of accumulated profit of P. With an average accumulated P profit of 100 kg/ha, the total Pi content of NPK and NPKM increased by 39.6 and 21.6 mg/kg, respectively. The response of total Pi content to accumulated P profit of the M treatment was different from that of NPK and NPKM treatment. Only when the accumulated P profit exceeded 475 kg/ha did the total Pi content of M increase significantly with the increase of accumulated P profit (P < 0.01). With an average accumulated P profit of 100 kg/ha, the total Pi content of M increased by 24 mg/kg. In addition, at equal levels of accumulated P profit, the increasing rates of total Pi content and Pi fractions in NPK were much higher than those of M and NPKM. Other studies also show that differences in the P availability in paddy soil can be caused by different P fertilization strategies [33]. We suspect that (1) cattle manure has a higher C:P than chemical fertilizer, thus the addition of relatively more C with less P differentially affects the dissolution reactions or (2) soluble P might be taking part in precipitation reactions with Ca and forming secondary Ca phosphate compounds [20]. Application of cattle manure can reduce the rate of change of Pi content with increasing accumulated P profit. In the presence of equal amounts of P surplus, the application of chemical P fertilizer can increase the soil Pi content faster than the application cattle manure. Consequently, chemical sources of fertilizer may exacerbate the environmental pollution risks associated with their application, which are not associated with manure application.
The contents of the Pi fractions, Al-P, Fe-P, Ca-P and Oc-P, in the NPK and NPKM treatments increased significantly with the increase of accumulated P surplus (Table 2). At equal amounts of accumulated P profit, the increasing rates of the Pi fractions in NPK were 2–3 times higher than those of NPKM. However, only the content of Fe-P in M increased significantly with the increase of accumulated P profit (P < 0.05). With an average accumulated P profit of 100 kg/ha, the content of Fe-P in M, NPK and NPKM increased by 5.7, 16.3 and 9.5 mg/kg, respectively and the rate of increase of Fe-P in M was less than that in NPK and NPKM.
Relationships between Soil Properties, Climatic Factors and Pi Fractions
Redundancy analysis determined significant correlations between Pi fractions and soil properties and climatic factors (Fig. 5). Soil properties (pH, SOM, Olsen-P, and TP) significantly influenced the distribution of Pi fractions. Total P and Olsen-P were more closely correlated to the Fe-P, Ca-P and Al-P fractions. The climatic factors of mean annual temperature and mean annual precipitation did not significantly influence interannual Pi fractions. The first axis, RDA-1, explained 84% and the second axis, RDA-2, explained 4% of the total variation. Similar PCA results for carbonate soils were reported by Milić et al. [31]. The availability of soil P depends on the ability of sorbed P, and the sorptive capacity is determined by how many and what kinds of P sorption sites are found in the soil [15, 16]. The most abundant P-sorbing particles are Fe/Al-oxyhydroxides, clay minerals and humic substances. In acid soils, more acidic milieu fosters P sorption to Fe/Al-oxyhydroxides [9]. Similar to the paddy soil in our study, a large area of paddy soils in subtropical China derived from Quaternary red clay also had considerable amounts of Fe and Al oxyhydroxides [43]. Soil P amounts, fractions and dynamics depend on numerous factors, including soil type and its utilization. In agroecosystems, where the P cycle is human-influenced, amounts, availability and dynamics of soil P are largely determined by anthropogenic activities. Furthermore, the climatic factors of mean annual temperature and mean annual precipitation did not significantly influence P fractions (Fig. 5).
Relationships among soil properties, climatic factors and Pi fractions by redundancy analysis. Soil pH, total N, soil organic matter (SOM), Olsen-P, Total-P, mean annual temperature (MAT), and mean annual precipitation are explanatory variables that showed significant effects on Pi fractions (response variables).
CONCLUSIONS
The results of this study demonstrate that fertilization regimes significantly impact soil Olsen-P and Pi fractions in acidic paddy soil. Total P, Olsen-P and Pi contents increased with time in all three types of fertilization treatments. Long-term application of NPK or NPKM produced stronger increases in contents and higher yields than application of M. Total P and Olsen-P were more closely correlated to Fe-P, Ca-P and Al-P fractions. Higher TP, Olsen-P and Pi contents in the plough layer from long-term NPK or NPKM applications than with M application were observed. At the same amount of accumulated P, the increasing rate of total Pi content and Pi fractions in NPK were much higher than those of M and NPKM. The results suggest that P supply capacity was very high when chemical P fertilizer was applied alone at a reasonable level (49 kg P/ha·y). Therefore, replacing a proportion of applied chemical P fertilizer with cattle manure can help reduce surplus P accumulation in soil, as well as reduce the environmental P losses from the acidic paddy soil in southern China.
REFERENCES
A. Sharpley and B. Moyer, “Phosphorus forms in manure and compost and their release during simulated rainfall,” J. Environ. Qual. 22, 597–601 (2000). https://doi.org/10.2134/jeq2000.00472425002900060056x
A. Soltangheisi, M. Rodrigues, M. J. A. Coelho, A. M. Gasperini, L. R. Sartor, and P. S. Pavinato, “Changes in soil phosphorus lability promoted by phosphate sources and cover crops,” Soil Tillage Res. 179, 20–28 (2018). https://doi.org/10.1016/j.still.2018.01.006
A. Waqas, J. Huang, K. L. Liu, Q. Muhammad, N. K. Muhammad, J. Chen, G. Sun, Q. H. Huang, Y. R. Liu, G. R. Liu, M. Sun, C. Li, D. C. Li, A. Sehrish, N. Yodgar, et al., “Changes in phosphorus fractions associated with soil chemical properties under long-term organic and inorganic fertilization in paddy soils of southern China,” PLoS One 14 (5), e0216881 (2019). https://doi.org/10.1371/journal.pone.0216881
A. Waqas, K. L. Liu, Q. Muhammad, J. Huang, Q. H. Huang, Y. M. Xu, A. Sehrish, M. Sajid, M. A. A. Rana, M. Mohsin, and H. M. Zhang, “Long-term mineral fertilization improved the grain yield and phosphorus use efficiency by changing soil P fractions in ferralic cambisol,” Agronomy 9, 784 (2019). https://doi.org/10.3390/agronomy9120784
B. Pratap, K. N. Amaresh, S. Mohammad, T. Rahul, M. Sangita, K. Anjani, R. Rajagounder, B. P. Bipin, L. Banwari, G. Priyanka, K. S. Chinmaya, S. R. Koushik, and K. D. Pradeep, “Effects of 42-year long-term fertilizer management on soil phosphorus availability, fractionation, adsorption–desorption isotherm and plant uptake in flooded tropical rice,” Crop J. 3, 387–395 (2015). https://doi.org/10.1016/j.cj.2015.03.009
C. H. Lee, C. Y. Park, K. D. Park, W. T. Jeon, and P. J. Kim, “Long-term effects of fertilization on the forms and availability of soil phosphorus in rice paddy,” Chemosphere 56, 299–304 (2004). https://doi.org/10.1016/j.chemosphere.2004.02.027
D. Hesterberg, “Macro-scale chemical properties and x-ray absorption spectroscopy of soil phosphorus,” in Synchrotron based Techniques in Soils and Sediments, Ed. by B. Singh and M. Gräfe (Elsevier, Burlington, 2010), Vol. 34, pp. 313–356. https://doi.org/10.1016/S0166-2481(10)34011-6
D. Pizzeghello, A. Berti, S. Nardi, and F. Morari, “Phosphorus forms and P-sorption properties in three alkaline soils after long-term mineral and manure applications in north-eastern Italy,” Agric. Ecosyst. Environ. 141 (1), 58–66 (2011). https://doi.org/10.1016/j.agee.2011.02.011
E. Oburger, D. L. Jones, and W. W. Wenzel, “Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil,” Plant Soil 341, 363–382 (2011). https://doi.org/10.1007/s11104-010-0650-5
H. Cui, Y. Ou, L. X. Wang, H. T. Wu, B. X. Yan, and Y. X. Li, “Distribution and release of phosphorus fractions associated with soil aggregate structure in restored wetlands,” Chemosphere 223, 319–329 (2019). https://doi.org/10.1016/j.chemosphere.2019.02.046
H. Q. Meng, M. G. Xu, J. L. Lu, X. H. He, J. W. Li, X. J. Shi, C. Peng, B. R. Wang, and H. M. Zhang, “Soil pH dynamics and nitrogen transformations under long-term chemical fertilization in four typical Chinese croplands,” J. Integr. Agr. 12 (11), 2092–2102 (2013). https://doi.org/10.1016/S2095-3119(13)60398-6
J. Huang, S. X. Zhang, X. J. Shi, Q. H. Huang, J. Nie, M. G. Xu, and H. M. Zhang, “Change of phosphorus recovery efficiency under long-term fertilization in typical farmland in southern China,” J. Plant Nutr. Ferti. 24 (6), 220–229 (2018). https://doi.org/10.11674/zwyf.18345
J. P. Schmidt, S. W. Buol, and E. J. Kamprath, “Soil phosphorus dynamics during seventeen years of continuous cultivation: fractionation analyses,” Soil Sci. Soc. Am. J. 60, 1168–1172 (1996). https://doi.org/10.2136/sssaj1996.03615995006000040030x
J. Shen, R. Li, F. Zhang, J. Fan, C. Tang, and Z. Rengel, “Crop yields, soil fertility and phosphorus fractions in response to long-term fertilization under the rice monoculture system on a calcareous soil,” Field Crops Res. 86, 225–238 (2004). doihttps://doi.org/10.1016/j.fcr.2003.08.013
J. R. Fink, A. V. Inda, J. Bavaresco, V. Barrón, J. Torrent, and C. Bayer, “Phosphorus adsorption and desorption in undisturbed samples from subtropical soils under conventional tillage or no-tillage,” J. Plant Nutr. Soil Sci. 179, 198–205 (2016). https://doi.org/10.1002/jpln.201500017
J. Wang and G. Chu, “Phosphate fertilizer form and application strategy affect phosphorus mobility and transformation in a drip-irrigated calcareous soil,” J. Plant Nutr. Soil Sci. 178, 914–922 (2015). https://doi.org/10.1002/jpln.201500339
L. L. Hua, J. Liu, L. M. Zhai, B. Xi, F. L. Zhang, H. Y. Wang, H. B. Liu, A. Q. Chen, and B. Fu, “Risks of phosphorus runoff losses from five Chinese paddy soils under conventional management practices,” Agric. Ecosyst. Environ. 245, 112–123 (2017). https://doi.org/10.1016/j.agee.2017.05.015
L. L. Shi, M. X. Shen, C. Y. Lu, H. H. Wang, X. W. Zhou, M. J. Jin, and T. D. Wu, “Soil phosphorus dynamic, balance and critical P values in long-term fertilization experiment in Taihu Lake region, China,” J. Integr. Agric. 14 (12), 2446–2455 (2015). https://doi.org/10.1016/S2095-3119(15)61183-2
M. A. Saleque, U. A. Naher, A. Islam, A. B. M. B. U. Pathan, A. T. M. S. Hossain, and C. A. Meisner, “Inorganic and organic phosphorus fertilizer effects on the phosphorus fractionation in wetland rice soils,” Soil Sci. Soc. Am. J. 68, 1635–1644 (2004). https://doi.org/10.2136/sssaj2004.1635
M. N. Shafqat and G. M. Pierzynski, “Soil test phosphorus dynamics in animal waste amended soils: Using P mass balance approach,” Chemosphere 90, 691– 698 (2013). https://doi.org/10.1016/j.chemosphere.2012.09.050
M. N. Shafqat and G. M. Pierzynski, “The effect of various sources and dose of phosphorus on residual soil test phosphorus in different soils,” Catena 105, 21–28 (2013). https://doi.org/10.1016/j.catena.2013.01.003
M. P. Chen and T. E. Graedel, “A half-century of global phosphorus flows, stocks, production, consumption, recycling, and environmental impacts,” Global Environ. Change 36, 139–152 (2016). https://doi.org/10.1016/j.gloenvcha.2015.12.005
N. Cao, X. P. Chen, Z. L. Cui, and F. S. Zhang, “Change in soil available phosphorus in relation to the phosphorus budget in China,” Nutr. Cycl. Agroecosyst. 94, 161–170 (2012). https://doi.org/10.1007/s10705-012-9530-0
O. B. Rogova, N. A. Kolobova, and A. L. Ivanov, “Phosphorus sorption capacity of gray forest soil as dependent on fertilization system,” Eurasian Soil Sci. 51, 536–541 (2018). https://doi-org-s.caas.cn/10.1134/S1064229318050101
Q. F. Bi, B. X. Zheng, X. Y. Lin, K. J. Li, X. P. Liu, X. L. Hao, H. Zhang, J. B. Zhang, D. P. Jaisi, and Y. G. Zhu, “The microbial cycling of phosphorus on long-term fertilized soil: Insights from phosphate oxygen isotope ratios,” Chem. Geol. 483, 56–64 (2018). https://doi.org/10.1016/j.chemgeo.2018.02.013
Q. Zhang, G. H. Wang, Y. K. Feng, Q. Z. Sun, C. Witt, and A. Dobermann, “Changes in soil phosphorus fractions in a calcareous paddy soil under intensive rice cropping,” Plant Soil 288 (1–2), 141–154 (2006). https://doi.org/10.1007/s11104-006-9100-9
R. W. McDowell and I. Stewart, “The phosphorus composition of contrasting soils in pastoral, native and forest management in Otago, New Zealand: Sequential extraction and 31P NMR,” Geoderma 130 (1–2), 176–189 (2006). https://doi.org/10.1016/j.geoderma.2005.01.020
S. B. Lee, C. H. Lee, K. Y. Jung, K. D. Park, D. Lee and P. J. Kim, “Changes of soil organic carbon and its fractions in relation to soil physical properties in a long-term fertilized paddy,” Soil Tillage Res. 104 (2), 227–232 (2009).
S. C. Sheppard and J. G. Racz, “Phosphorus nutrition of crops as affected by temperature and water supply,” in Proceedings of the Alberta Soil Science Workshop and Western Canada Phosphate Symposium (Alberta, 1980) pp. 159–199.
S. D. Bao, Soil Agriculturalization Analysis (China Agriculture Press: Beijing, 1981) [in Chinese].
S. Milić, J. Ninkov, T. Zeremski, D. Latković, S. Šeremešić, V. Radovanović, and B. Žarković, “Soil fertility and phosphorus fractions in a calcareous chernozem after a long-term field experiment,” Geoderma 339, 9–19 (2019). https://doi.org/10.1016/j.geoderma.2018.12.017
S. R. Olsen, C. V. Cole, F. S. Watanabe and A. Dean, Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (United States Department of Agriculture, Washington, DC, 1954).
S. Sandipan, C. Poulami, T. Jaak, A. Rangasamy, K. Sukjin, and S. Tongmin, “Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils,” Appl. Soil Ecol. 134, 111–115 (2019). https://doi.org/10.1016/j.apsoil.2018.10.016
W. W. Zhang, X. Y. Zhan, S. X. Zhang, H. M. I. Khalid, and M. G. Xu, “Response of soil Olsen-P to P budget under different long-term fertilization treatments in a fluvoaquic soil,” J. Integr. Agric. 18 (3), 667–676 (2019). https://doi.org/10.1016/j.still.2009.02.007
X. Chen, K. Fang, and C. Chen, “Seasonal variation and impact factors of available phosphorus in typical paddy soils of Taihu Lake region, China,” Water Environ. J. 26 (3), 392–398 (2012). https://doi.org/10.1111/j.1747-6593.2011.00299.x
X. Yan, Z. Q. Wei, Q. Q. Hong, Z. H. Lu, and J. F. Wu, “Phosphorus fractions and sorption characteristics in a subtropical paddy soil as influenced by fertilizer sources,” Geoderma 295, 80–85 (2017). https://doi.org/10.1016/j.geoderma.2017.02.012
X. Zhan, L. Zhang, B. Zhou, P. Zhu, S. Zhang, and M. Xu, “Changes in Olsen phosphorus concentration and its response to phosphorus balance in black soils under different long-term fertilization patterns,” PLoS One 10, e0131713 (2015). https://doi.org/10.1371/journal.pone.0131713
Y. Arai and D. L. Sparks, “Phosphate reaction dynamics in soils and soil minerals: a multiscale approach,” Adv. Agron. 94, 135–179 (2007).
Y. Y. Li, R. Yang, R. Gao, H. A. Wei, A. L. Chen, and Y. Li, “Effects of long-term phosphorus fertilization and straw incorporation on phosphorus fractions in subtropical paddy soil,” J. Integr. Agric. 14 (2), 365–373 (2015). https://doi.org/10.1016/S2095-3119(13)60684-X
Z. H. Cao and H. C. Zhang, “Phosphorus losses to water from lowland rice fields under rice–wheat double cropping system in the Tai Lake region,” Environ. Geochem. Health 26, 229–236 (2004). https://doi.org/10.1023/B:EGAH.0000039585.24651.f8
Z. J. Yan, P. P. Liu, Y. H. Li, L. Ma, A. Alva, Z. X. Dou, Q. Chen, and F. S. Zhang, “Phosphorus in China’s intensive vegetable production systems: overfertilization, soil enrichment, and environmental implications,” J. Environ. Qual. 42 (4), 982–989 (2013). https://doi.org/10.2134/jeq2012.0463
Z. M. Lan, X. J. Lin, F. Wang, H. Zhang, and C. R. Chen, “Phosphorus availability and rice grain yield in a paddy soil in response to long-term fertilization,” Biol. Fert. Soils 48, 579–588 (2012). https://doi.org/10.1007/s00374-011-0650-5
Z. T. Gong, G. L. Zhang, and G. B. Luo, “Diversity of Anthrosols in China,” Pedosphere 9, 193–204 (1999). doi CNKI:SUN:TRQY.0.1999-03-000
ACKNOWLEDGMENTS
We acknowledge all staff for their valuable work associated with the long-term fertilization experiment in Qiyang experimental site, and the valuable suggestions of reviewers and the hard work of the editorial department.
Funding
This research was funded by the National Key Research and Development Program of China (2016YFD0300902, 2016YFD0300901), Fundamental Research Funds for Central Non-profit Scientific Institution of China (No. 1610132020023, No.1610132020022, No. 161032019035), and Hengyang Science and Technology Project, Hunan province in China (No. 2019yj010733).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Jing Huang, Qaswar, M., Khan, M.N. et al. Long-Term Application of Chemical and Organic Fertilizers over 35 Years Differentially Affects Interannual Variation in Soil Inorganic Phosphorus Fractions in Acidic Paddy Soil. Eurasian Soil Sc. 54, 772–782 (2021). https://doi.org/10.1134/S1064229321050112
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229321050112