Abstract
A review of approaches to particle-size and microaggregate-size distribution analyses applied in soil science is given. The concepts of the structural organization of soils, primary soil particles, elementary soil particles, and soil microaggregates are considered. Methodological problems, such as the preparation of soil samples for the analyses and interpretation and comparison of the results obtained by different methods, are discussed. The authors suggest the theoretical substantiation of differences between the notions of primary soil particles (soil building units) and elementary soil particles. Primary soil particles are individual mineral particles. Elementary soil particles are solid-phase products of pedogenesis represented by fragments of rocks and minerals and by organomineral and organic particles, all the components of which participate in chemical and physicochemical interactions. Special attention is paid to the existing classifications of soils according to their textures. It is suggested that the upper boundary of the clay fraction in the Russian classification should be shifted from 1 to 2 µm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Soil is a heterogeneous four-phase (liquid, solid, gaseous, and living phases) polydisperse system, the elements of which interact with one another and with the environment and have certain specificity allowing to group them into the structural levels of soil organization. Each of these levels is characterized by its own level-specific interactions, processes, and functions that can be used as criteria for separation of the levels [30]. In this paper, we discuss the levels of elementary soil particles (ESPs) and soil microaggregates.
The most common quantitative characteristic used to describe and assess the degree of dispersion of different soils is their granulometric composition, or particle-size distribution. Particle-size distribution is one of basic physical properties of soils; it is taken into account in dealing with many scientific and applied problems. However, inconsistent terminology and differences in methodological approaches to its determination in different schools of soil science complicate the interpretation of the results and their understanding by specialists in neighboring sciences. At the first glance, the definitions of ESPs and soil textures are simple. However, their careful analysis raises a number of important methodological problems. As argued below, the key problem is the exact definition of ESPs.
In recent decades, new methods of the analysis of particle-size distribution and soil morphology—laser diffractometry, X-ray microtomography, scanning electron microscopy, and mass-spectrometry of secondary ions—have been developed. Instrumental methods of soil preparation for the analyses—ultrasonic dispersion and mechanical screening—have virtually excluded the effect of human factor on the soil pretreatment; it is now possible to standardize and exactly determine the force of action exerted on the soil. In turn, this modifies our perception of the existing notions; some of them have to be refined in order to get a better insight into the investigated problems. In general, soil science has gained a new level in the study of solid soil phase. After the appearance of the method of densimetric fractionation of soils, most of the studies deal with soil structural units separated by this method [46, 79]. Earlier, analogous studies were based on the concept of particle-size (granulometric) soil fractions.
What is the object of particle-size distribution analysis? What is the range of sizes of the particles attributed to ESPs? What lies in the basis of grouping ESPs into size fractions? What should be taken into account in the choice of the method of soil pretreatment to the particle-size and microaggregate-size distribution analyses? Should this method be the same for all the soils? What is the role of pedofeatures (soil neoformations) in the particle-size distribution? Should they be taken into account separately? These are the major questions discussed below.
WHAT IS THE ESP?
Solid soil phase is represented by particles of parent material and the products of its weathering, as well as by organic substances, including plant remains of different degrees of decomposition and organic and organomineral compounds. The physical properties and the chemical and mineralogical compositions of soil particles depend in their size, the type of soil, and the nature of parent material [2, 3, 12, 13, 17, 23, 26, 29, 109, 173, 174]. The sand fraction (>50 µm) is mainly represented by silicate minerals. The silt fraction (2–50 µm) is close in its mineralogical composition to the sand fraction. However, owing to a higher specific surface area, silt particles are often covered by the films of amorphous compounds. The clay fraction (<2 µm) consists of layered aluminosilicates and metal oxides and hydroxides; it may also include nonlayered silicates (quartz, feldspars, etc.). The method of physical fractionation in heavy liquids of known density is successfully applied to distinguish between different pools of soil organic matter (SOM) [46, 79]. Numerous particular methods of this fractionation differ in their impact on the soil solid phase (SSP) and the applied densities of heavy liquids [9, 37, 79, 80, 86, 103, 150, 201]. Usually, three major pools of SOM differing in the character of their interaction with the mineral soil components are distinguished. The first group of free SOM—light-weight fraction—is separated in heavy liquids <1.6–2.0 g/cm3 without the preliminary impact on the SSP. The second group—physically free occluded light fraction—is separated after the preliminary impact on the SSP. The remaining SOM is bound in organomineral complexes and is referred to as the heavy-weight fraction. The density of heavy liquid of 1.6 g/cm3 allows us to isolate the light-weight fraction of SOM in the pure form, whereas the isolation of occluded SOM and other fractions depends on the stability of soil aggregates [74].
Soil particles may have different shapes: spherical, elongated, or platy. These shapes can be described with the use of three parameters: the degree of angularity/roundness, the shape proper, and the character of the surface [88]. Sand particles are evenly developed in all the directions and can be attributed to spherical particles. However, their surface is not quite smooth; it has certain roughness and scores. Most of silt particles also have a spherical shape. Clay particles usually have platy of acicular (elongated) shapes [109]. In general, the smaller the size of the particles, the more pronounced difference of their shape from a spherical shape [136].
The idea that soil particles should be studied in the state maximally close to their natural state is not new. In 1943, Tyulin wrote: “Organic colloids tightly bound to the surface of mineral particles form a single whole with them independently on the nature of these bonds.” [42, p. 4]. Tyulin considered it very important to preserve individuality of soil particles in the course of pretreatment of the soil samples for the analyses. He also argued against the application of chemical reagents for this purpose. From his point of view, the important characteristics of soils are “the quantity and quality of adsorbed organic colloids on the surface of mineral particles” and “the energy of bonds between the organic matter and mineral particles.” Indeed, the investigations of that kind have become most widespread in the next decades of the development of soil science [76, 111, 117, 129, 132, 163, 175, 204, 210]. The study of organomineral interactions is an important direction in soil science [9, 92, 129, 130, 173]. Various physical methods of soil dispersion and fractionation are applied. In particular, the method of ultrasonic dispersion [85] should be mentioned as the method allowing us to determine the energy of bonds of the soil particles [171]. Ultrasonic methods make it possible to study the mechanisms of soil aggregation [82, 84, 85].
Oxides and hydroxides of iron, aluminum, and manganese; amorphous silica; carbonates; gypsum; soluble salts; and specific and nonspecific SOM compounds are the major binding agents in soils. In dependence on the functional role in soil aggregation, three groups of SOM can be distinguished: transient SOM mainly represented by polysaccharides, temporary SOM (plant roots and fungal hyphae), and persistent SOM with organic substances bound with metal ions and sorbed on the surface of soil particles [188].
In the course of soil aggregation under the impact of binding agents, soil structural units of different orders are formed (Figs. 1 and 2). Primary soil particles compose secondary soil particles; in turn, the latter form larger aggregates [12, 102, 188]. The relationships between different structural elements of soil are described in the concepts of the hierarchy of structural levels of soil organization [12, 22, 30, 80] and the hierarchy of soil aggregates [188, 189]. According to Rozanov [30], different structural levels of soil organization are specified by the character of interaction between structural elements [30]. In fact, it is difficult to distinguish between the levels according to the mechanisms of soil aggregation (Fig. 3). Moreover, not all the soils have all the levels of structural organization [81]. There are certain interactions between different structural levels [30]. The most diverse mechanisms of interaction are inherent to the level of soil aggregates. Aggregates can be defined as the integrities of soil particles or microaggregates, the bonds between which are stronger than the bonds with neighboring soil particles [48, 125]. Aggregates are characterized by their size, shape, porosity, mechanical strength, and water stability [24]. Porosity is a distinctive morphological property of aggregates. As for the term microaggregate, there is no unified definition for it. Some researchers consider aggregates <0.25 mm in size as microaggregates [14, 84, 189]; in other studies, microaggregates are specified as aggregates of 0.002 to 0.25 mm in size [188], or as aggregates <0.05 mm in size [10]. Edwards and Bremner [84] argued that microaggregates in base-saturated soils represent dispersion-resistant totality of clay particles and humified organic substances bound by polyvalent metal ions with one another. However, microaggregates are also formed in the unsaturated soils. According to Matthews, aggregates consist of primary particles bound by strong cohesive forces, so that they are not destroyed upon standard sample pretreatment procedures [139]. Microaggregates are more resistant to external impacts than aggregates and are richer in clay particles and organic matter [82]. Water stability of microaggregates is related to the gluing action of organic matter [188]. At the same time, the relationships between the organic matter content and the soil microaggregation are rather ambiguous, because only particular forms of SOM can be “responsible” for the water stability of aggregates [188].
The place of pedofeatures (soil neoformations) in the concept of the hierarchy of soil structural levels is open to discussion. Rozanov [30] argued that most pedofeatures could be considered at the aggregate level. The size of pedofeatures can be comparable with the size of soil particles (e.g., the size of iron nodules may vary from 0.5 to 25 mm [20]. Soil concretions may have different compositions and include elementary mineral particles; they may be also resistant to chemical agents and to ultrasonic dispersion. Carbonates in soils may have different morphologies. In terms of particle-size distribution analysis, their microforms are of particular interest. They may consist of calcite crystals of different sizes—from the cryptograined calcite (<1 µm) to coarse-grained calcite (>1000 µm) [15]. Most often, pedogenic carbonates precipitate as crystals of 2–50 µm in size [96].
The structural level of coatings and pendants is open to argument. Coatings (cutans) form a specific assemblage of pedofeatures that may be present in several horizons [6] and are considered an important morphological element in soils. It is probable that the assemblage of coatings represents a trans-level formation in terms of the structural hierarchy. B.G. Rozanov suggested the term inter-aggregate formation [30], but he did not give a clear definition of this term and the list of phenomena described by it.
In foreign studies, the objects of particle-size distribution analysis are usually referred to as primary soil particles. In Russian literature published in the recent decades, the notion of elementary soil particles is used. Other names—granulometric particles, mechanical particles—are also applied. There are two major approaches to the definition of the objects of particle-size distribution analysis.
Within the framework of the first approach, primary soil particles include separate mineral grains and rock fragments (rock detritus) [56, 139]. Thus, the analyzed phenomena only include the inorganic part of soil [169]. Organic particles are excluded from the group of primary particles. Moreover, newly formed pedogenic substances—organomineral compounds—are also excluded. This approach was realized in the method for determination of particle-size distribution in the mineral soil material [113]. In this method, binding agents—organic colloids, oxides and hydroxides of Fe and Al, and carbonates—are to be removed from the soil before the analysis. At the same time, the impact of pretreatments on the mineral part of the soil should be minimized [164]. The chemical methods of soil pretreatment ensure the stability of the results of particle-size distribution analysis. However, they considerably transform the studied object, so that the results of the analysis do not give adequate characterization of the original soil [151]. They may also alter the properties of the clay fraction [78] and greatly affect the results of the analysis and their qualitative interpretation in dependence on the particular goal of the study [139].
The second approach [12, 22, 30, 48] tends to take into account all the elementary soil particles (ESPs) that are defined as “fragments of rocks and minerals and amorphous compounds, the elements of which are chemically bound together and cannot be destroyed by the routine methods of soil peptization applied as pretreatments to the particle-size distribution analysis” [48, p. 31]. The applied method of peptization should ensure the most compete dispersion of the soil without modifying ESPs. At this level of dispersion, ESPs play the decisive role in the structure and properties of soil [12]. This definition of ESPs generally corresponds to the definition of aggregated particle suggested by Matthews [139], in which the presence of strong cohesive bonds and the resistance of aggregates to “normal” pretreatments, including physical action on soil and soil dispersion with the use of sodium hexametaphosphate (Na6P6O18). As these two terms (ESP and aggregated particle) have a lot in common, it is reasonable to specify the definition of ESP. Indeed, the definition given above brings us the question: which kinds of amorphous compounds are to be taken into account? Amorphous substances are solid substances, the atoms and molecules of which do not compose periodical 3D structures typical of the crystalline state of matter [43]. These substances have “the near range” and do not have “the distant range,” i.e., the regularity in the spatial arrangement of their atoms or molecules is only observed at distances comparable with the distances between the atoms. The term X-ray-amorphous material is also applied. It denotes the amorphous substances proper and the crystals, whose effective size is very small (is less than the area of coherent scattering). According to this definition, amorphous compounds in soil include organic and organomineral compounds, amorphous silica, and a number of other newly formed substances (mainly, oxides and hydroxides of metals). Thus, while defining the object of particle-size distribution analysis, we take into account the inner properties of soil materials rather than the method of soil pretreatment. However, the latter is critically important. In fact, there is no universal method of soil pretreatment for the particle-size distribution analysis suitable for all the soils [75, 153]; the particular standard methods applied in different countries are somewhat variable.
On the one hand, the described approaches to the definition of the object of particle-size distribution analysis are mutually exclusive. In reality, these approaches are aimed at solving somewhat different problems and can be used to describe different hierarchical levels of soil arrangement. The first approach is suitable for soil description at the crystalline–molecular level (according to Rozanov [30]); only mineral particles are considered. The second approach based on the ESP concept tends to characterize the native state of the solid soil phase. It describes a more complex level of soil arrangement. In the case of coarse-textured soils (or parent materials) with the low content of gluing substances, soil dispersion to the level of primary elements without the removal of binding agents is possible. In the case of microaggregated soils (or parent materials), such a state of the soil cannot be achieved without changes in the initial soil state. The structure inherent in parent materials is further transformed in the course of pedogenesis into the soil structure. The degree of this transformation may be different [1].
As already mentioned, the most well-studied soil particles have typical sizes of 2 to 100 µm [81]. Soil particles of less than 2 µm in size often represent microaggregates resistant to ultrasonic treatment rather than individual particles [76].
Often the term clay microstructures is used to denote the particles <20 µm in size [77, 118, 155, 201] along with the term primary soil elements. Thus, in the engineering geology, the terms microtexture and microstructure are often used to describe clayey and loesslike materials and soils containing clay minerals and organic matter in the form of humus, i.e., particles <1–5 µm in size. Such particles rarely occur in the isolated state. Usually they compose “ultramicroaggregates” and “ultramicroblocks” [32, p. 17]. If considerable amounts of organic matter or amorphous substances are present in a soil as binding agents, the role of primary soil elements decreases [178]. Highly aggregated clayey soils behave like sands in terms of their filtration properties [100]. Some important soil physical properties, such as the unsaturated hydraulic conductivity, water retention, soil crusting capacity, tolerance toward erosion, and physical tilth depend on compound particles (domains) rather than on primary soil particles [81]. The notion of “mechanical composition of soils” reflects not only the quantity of soil elements but also the behavior (mechanics) of soils in dependence on their composition. The notions of the “granulometric composition of soils” (soil texture) and “soil particle-size distribution” do not imply the latter meaning.
Indeed, in order to solve some of the theoretical and applied problems, we need to remove organic and other gluing substances from soils before the particle-size distribution analysis. For instance, this is important, if we study the lithological homogeneity/heterogeneity of soils, the genesis of parent material, or the pedogenic transformation of the mineral part of soils with bleached acid eluvial horizons [17, 19, 28, 39]. At the same time, the analysis of many physical properties of soils does not require their special pretreatment. The determination of the classification position, fertility, and erosion resistance of ferrallitic soils does not require complete disintegration of soil particles with the disturbance of their initial stable structure [106]. The presence of carbonates in soils usually contributes to soil microaggregation. After the removal of carbonates, the results of laboratory particle-size distribution analysis do not correspond to the results of field determination of the soil texture. It was suggested that the portion of carbonate clay in such soils should be specially assessed [176]. Particle-size distribution data on carbonate soils (with the CaCO3 content ≥5 vol. %) indicate that the preliminary removal of carbonates changes the grade of soil texture (as determined from these data) in 60% of the studied samples [96]. In this context, a question arises: do particle-size distribution data obtained after the removal of carbonates correspond to the real properties of native soils? [96, 126]. It is also known that particle-size distribution data obtained after the soil dispersion with application of chemical agents do not correlated with the properties of clayey soils of different geneses [11].
An important area of studies in the modern soil physics is the choice and adaptation of the methods allowing us to predict some soil properties on the basis of data on other soil properties [61, 170]. As a result of these studies, pedotransfer soil functions have been developed [67, 68, 97, 206]. We suppose that further refinement of the concept of the hierarchy of structural levels of soil arrangement is necessary to ensure adequate results obtained with the use of pedotransfer functions and other soil models.
GRANULOMETRIC (PARTICLE-SIZE DISTRIBUTION) ANALYSIS OF SOILS
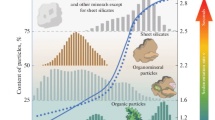
Classification of granulometric (particle-size) elements of soils and classification of soils according to their granulometric composition (texture).Granulometric (particle-size distribution) analysis implies the determination of quantitative ratios between elementary soil particles of different sizes independently from their mineralogical and chemical compositions [48]. In dependence on the particular classification, the range of sizes of the particles attributed to ESPs may vary (Fig. 4). However, all these classification schemes include subdivision into three classes of particles: clay, silt, and sand. Usually, the size of ESPs is less than 2000 µm; in the Russian system, it should be less than 1000 µm; these particles are considered fine earth fractions. Coarser particles—rock debris and separate coarse mineral grains—compose the stony (skeletal) part of soils. The upper boundary of the clay fraction in the Russian classification differs from than in most other classification systems. In Russia, clay particles are defined as particles <1 µm in size; in the international system, these are particles <2 µm. The boundary between silt and sand fractions is set at 20, 50, 60, 63 µm in different classifications. Some classifications suggest a more detailed subdivision of these major classes into subclasses of fine, medium, and coarse fractions. The most detailed subdivision is given in the Russian system (in Fig. 4, subclasses in the clay class—coarse clay (1–0.5 µm), medium clay (0.5–0.01 µm), and colloids (<0.01 µm)—are not shown. Such a detailed subdivision makes it possible to diagnose relatively small changes in the particle-size distribution and perform more detailed studies of the properties of ESPs within their particular fractions.
A detailed history of the development of classification of soil particles according to their sizes can be found in a number of reviews [23, 47, 66, 177]. Mostly, these classifications have the mathematical basis making their use more convenient [110, 128]. An attempt to give physical substantiation of the boundaries between separate size classes of soil particles was made by Atterberg [62]. In his study of physico-mechanical properties of separate fractions separated by elutriation method, Atterberg determined physically meaningful boundaries of 2, 5, and 20 µm. Thus, particles <2 µm are subjected to the Brownian motion and are virtually impermeable for water filtration [191]. However, Atterberg did not study the influence of the properties of particular on the behavior of the mixture of fractions [177]. In 1895, Williams separated the clay (<0.015 mm) and silt fractions according to differences in their densities and cohesive properties. It was suggested that clay particles should be separated as the particles of <2 µm in size; this size boundary was considered to be the lower limit of the occurrence of primary minerals [191]. However, it is known that even the faction <1 µm may contain fine-dispersed quartz [45] and other nonlayered silicates in the soils subjected to intense weathering. The silt fraction has a lower water permeability in comparison with the sand fraction. According to Atterberg. particles <20 µm coagulate under the impact of electrolytes, are invisible for naked eye, and are characterized by the maximum values of capillary rise of water; roots do not penetrate into the pores between such particles [191]. The size limit of 5 µm corresponded to a sharp change in the soil stickiness [47]. Atterberg proved that the water retention capacity of the particles >2 mm in much lower in comparison with that of the particles <2 mm [191]. Kachinskii [23] suggested that the fraction of small gravels (1–3 mm) should be separately distinguished, as the fraction with rapid filtration, low water retention capacity (<3%), and very low values of capillary phenomena in comparison with sands (5–15%). It was shown that the determination of water retention capacity with the use of pedotransfer functions becomes more adequate, if the upper boundary of silt fraction is set at 50 µm instead of 20 µm [152].
A different approach was developed by Berezin [4, 5]. He analyzed integral curves of particle-size distribution obtained by the X-ray–sedimentation method for the soils of different geneses. On all the curves, there was a clear bend in the area of particle size of 5 µm (Fig. 5). This size was set as a boundary between clay (<5 µm) and sand (>5 µm) components of soils. Each of the branches of the integral curve was described by its own equation, and a new classification of soil particle-size groups was suggested on the basis of four quantitative parameters (F5, K, α, and n). The description of the integral curves of particle-size distribution for 19 soils with contrasting differences in their textures made it possible to distinguish between the domains of clay, silt, and clay, the boundaries between differed from the commonly accepted boundaries and ranged from 0.33 to 0.99 µm and from 45.3 to 126.7 µm, respectively [65].
Quantitative ratios between particle-size fractions determine the soil texture. Most of the textural classifications of soils take into account all the three classes (clay, silt, and sand) and are graphically displayed as textural triangles (USDA, ISSS, Australian, and other texture classification systems). In Russia, soil texture is determined according to the classification system suggested by Kachinskii [23], in which only two classes of soil separates—physical clay (particles <10 µm) and physical sand (particles >10 µm)—are taken into account. For the correct classification, the type of soil formation should be known, as somewhat different boundaries between textural classes were established for podzolic, steppe, and solonetzic soils. This was done in order to take into account differences in the qualitative composition of clay in these soils. In fact, this factor is not considered in other classifications [48]. The classification by Kachinskii was mainly developed for practical purposes and considered the major kinds of soils used in agriculture. Strictly speaking, the texture of other soils (not included in these types of pedogenesis) cannot be determined according to this classification. In order to specify soil textural names, Kachinskii supplemented his major classification and suggested that the name of the soil texture should also include the name of the predominant size fraction (e.g., clayey heavy loam for heavy loamy soils with a predominance of clay (<1 µm) particles [30].
The diversity of textural classifications of soils used in the world complicates the comparison of data and the creation of unified data bases. The accuracy of transition from one classification to other classification should depend on the intervals between measured points on the particle-size distribution curves [152]. A method of reclassification of particle-size distribution data presented in the Russian system into the international system was suggested [47]. It is based on the linear interpolation of data on the cumulative particle-size distribution curve within its segment for particles <5 µm, because in this segment the semilogarithmic curve approached the straight line [47]. As shown by the author of this method, the error of recalculation may reach 3–4%. Reclassification of particle-size distribution data is possible after the description of these data by some functions. The type of the function depends on the soil texture [168, 205]. Thus, for fine-textured Bulgarian soils, exponential functions can be used, whereas particle-size distribution in coarse-textured soils is better described by power functions [168]. If a big database is available, the soils with close particle-size distribution (the differences in the contents of separate fractions are within ±0.5%) can be selected, and the averaged data for the missing boundaries between the fractions can be used [152]. This approach proved to be better than the methods of linear interpolation, spline interpolation, and interpolation based on the Gompertz distribution. Spline interpolation gives adequate results, when the distances between known values of fraction boundaries are relatively small [152]. The Gompertz distribution is insufficiently flexible for the description of bimodal particle-size distribution curves and for the soils with the high content of sand fraction [152]. Interpolation based on the Gompertz distribution, as well as spline interpolation, is inadequate for describing particle-size distribution data with separation of only three of four fractions [152].
There are certain linguistic problems in the translation of the terms physical clay (particles <0.01 mm) and physical clay (particles >0.01 mm), because the term clay has a separate and different meaning in the existing classifications of particle sizes [23].
A comparison of particle-size distribution data obtained by different methods poses a separate problem. A question arises: is the same classification of particle-size fractions suitable for different methods? The most common methods are based on sedimentation phenomena (the pipette method) and on the laser diffraction phenomena in soil suspensions (the Low Angle Laser Light Scattering (LALLS) method). The first method assumes the determination of size distribution of soil particles according to their masses, whereas the second method takes into account the volumes of the particles. Thus, direct recalculation of the results from one method into another method is impossible, because the pipette method uses an assumption that the solid phase density (ρs, g/cm3) is the same for all the particles. If this is so, particle-size distributions based on the masses and volumes of particles should be the same. The differences between the two methods have been discussed in detail in a number of studies [51, 64, 71, 90, 135]. It was demonstrated that the major difference consists of a significantly lower content of clay particles determined by the laser diffractometry in comparison with that determined by the pipette method. For correct comparison of the results obtained with the use of different methods, their principles and major assumptions should be taken into account. The adaptation of the existing classifications of particle-size distribution to the results obtained by the laser diffraction method is necessary [126]. If the results obtained by the pipette method and by the method of laser diffraction are closely correlated, it is possible to build a regression model and to recalculate the data on its basis. After this, the soil texture can be determined from the results obtained by the laser diffraction method. However, if the correlation is absent or is weak, the data obtained by the laser diffraction method cannot be used for determination of the soil texture in agreement with the existing classifications (as the latter were developed for particle-size distribution data obtained by the classical pipette method [186]. Certain calibration of the equations is necessary for the groups of soils with close mineralogical and genetic characteristics [213].
Criteria for the choice of soil pretreatment method. The choice of appropriate pretreatment depends on the goal of the study: analysis of agrophysical soil properties, determination of soil genesis, development of soil reclamation, etc. The existing standards of particle-size distribution analyses depend on the method of this analysis and somewhat differ in different countries. They may include various chemical pretreatments, or some combination of chemical and physical pretreatment, or only physical methods of soil dispersion. A comparison of the results of particle-size distribution analyses should be performed with due account for the applied pretreatment of the samples and their dispersion [100].
To determine the classification position of soils according to particle-size distribution data, the pretreatment methods should be relatively simple and ensure the high efficiency of the analyses [31].
The efficiency of pretreatment procedures can be estimated from data on the release of clay fraction [31], or from data on the mean diameter of the soil particles [119, 172]. It is also possible to estimate it from data on the specific surface area [196]. One of the criteria of the quality of soil dispersion is the absence of microaggregates of larger sizes than the size of the finest determined particle-size fraction [128]. Another criterion is the cation exchange capacity of the samples [78, 153]. To control the quality of soil pretreatment and dispersion, microscopic methods can be recommended [75, 196].
Soil neoformations (pedofeatures). The methods based on sedimentation phenomena require the removal of soluble salts before the analysis, because they lead to coagulation of the particles in the analyzed suspension; in the case of high concentrations, they may lead to decomposition of hydrogen peroxide applied to remove soil organic matter [100]. The removal of soluble salts can be achieved with the help of dialysis or with the help of multiple soil washing with decantation [100, 165]. The method of laser diffraction does not necessitate the preliminary removal of soluble salts because of the high dilution of the analyzed soil water suspension, in which the concentration of salts is very low and does not induce coagulation of the soil particles [53].
To estimate the size and form of soluble salts, carbonates, and gypsum in soils, some liquids that do not cause dissolution of these substances in the course of the analysis can be applied. Gypsum-containing and gypsiferous soils represent one of the important and widespread soil groups. Their total world area is estimated at more than 100 million ha [70]. The water solution of ethanol (ethanol : water = 7 : 3) can be applied for the particle-size distribution analysis of these soils, because gypsum is not dissolved in this solution [157]. To estimate the particular forms of gypsum in the soil, the pretreatment of the samples with the water solution of barium chloride (BaCl2) can also be used. As a result, an insoluble film of barium sulfate is formed on the surface of gypsum particles, so that their further analysis in distilled water becomes possible [108, 138]. A drawback of this method is that it somewhat modifies particle-size distribution of gypsum owing to its recrystallization, partial cohesion, and dissolution [15]. It was also shown that this method cannot be applied for gypsum-rich soils with the gypsum content above 10% [60]. However, there is a method to determine particle-size distribution of gypsiferous soils containing more than 40% of gypsum; it is based on the assumption that this gypsum is mainly concentrated in the coarse soil fractions [157].
The behavior of some concretionary pedofeatures in soils is analogous to the behavior of rock debris. However, their dispersion leads to the additional release of clay into the suspension, which is not always justified [153]. Iron and manganic concretions are often brittle and can be crushed upon the soil rubbing with the formation of pseudosand particles. However, ultrasound dispersion does not lead to disintegration of these concretions.
Methods of soil dispersion. Physical methods of soil dispersion—ultrasound pretreatment, rubbing in the paste state, or shaking of suspensions—cause minimal transformation of the soil solid phase. The use of cation-exchange resins is also feasible [83]. For this purpose, resins saturated with monovalent cations are applied. The saturation with Na+ and Li+ cations proved to be more efficient than the saturation with K+ or \({\text{NH}}_{{\text{4}}}^{ + }.\)
The ultrasonic treatment of soil water suspensions is one of the most efficient methods of physical dispersion [4, 82, 85, 87]. A great number of studies have been devoted to the comparison of ultrasonic pretreatment with various chemical pretreatments. It is important that chemically tightly bound particles are not destroyed by ultrasonic pretreatment. For acid soils containing kaolinite, hematite, and gibbsite, as well as for gypsiferous and calcareous soils, ultrasonic dispersion is insufficient to reach the amount of clay analogous to that determined in these soils after their chemical pretreatment [87]. Soils with the low content of organic matter and the high content of carbonates are susceptible to the ultrasonic dispersion [82]. While studying the lower horizons, application of dispersing substance is required in order to avoid potential flocculation of the particles [87, 101]. Laser analyzers are equipped with a source of ultrasonic waves that prevent flocculation of the particles in the course of the analysis. In soils with the high content of allophanes, chemical reagents are necessary [123]. Rubbing of soil pastes and active shaking of soil suspensions are comparable with ultrasonic dispersion according to these efficiency [87, 153]. A study of 14 soils differing in their pH values and in the contents of organic carbon, carbonates, and clay demonstrated that their ultrasonic pretreatment coupled with application of cation-exchange resins is more efficient that the preliminary oxidation of organic matter with hydrogen peroxide followed by the soil dispersion with sodium hexametaphosphate ((NaPO3)6). At the same time, if the preliminary oxidation is not performed, the dispersing action of (NaPO3)6 is close to that of the ultrasonic pretreatment and ion-exchange resins [82].
The range of energies used for ultrasonic dispersion is wide: from 7 to 5350 J/mL [146]. For most soils, the energy level of 450–500 J/mL is sufficient [57, 171]. Minerals of the sand fraction of soils developed from volcanic ashes may be subjected to destruction at the energy of ultrasonic dispersion above 400–1500 J/mL [112]. Soil shaking and ultrasonic dispersion are not recommended for the soils developed from limestone, because brittle and porous limestone fragments can be destroyed [126]. In the weakly developed and young soils, the destruction of their primary particles may take place at the energy of ultrasonic dispersion above 1500 J/mL [122]. Ultrasonic dispersion may lead to the destruction of quartz grains, which was diagnosed from the appearance of quartz grains with sharp edges with an increase in the duration of ultrasonic treatment [75]. Arable soils require less energy for their dispersion in comparison with virgin soils [89]. A detailed review of the action of ultrasonic dispersion on soils can be found in [121].
Special methods are used to calibrate the energy of soil dispersion [154, 140]. To compare the results obtained by different researchers, it is necessary to know the applied energy of ultrasonic dispersion. Indication of the duration of the dispersion is insufficient, because the impact on the soil also depends of the type of ultrasonic source, its working frequency, and the volume of the suspension.
The efficiency of soil dispersion with the use of chemical agents depends on the individual properties of analyzed samples (their mineralogical composition, cation exchange capacity, and the quality of quantity of organic matter) and on the external factors (temperature, pH, duration of action). The major problems in the application of chemical reagents are related to the possibility of formation of some transitional products of the reaction that might be sorbed in the surface of soil particles [137] and to the synthesis of more active reagents destroying the mineral soil matrix in the course of the reaction [120, 212]. Other problems are related to the duration of the reaction and its “completeness” that depend on the type of analyzed soils. To obtain adequate results of the particle-size distribution analysis, the achieved level of soil dispersion should be maintained for all the samples. To preserve the dispersing action of sodium hexametaphosphate (Na6O6P18), the pH in the analyzed samples should be no less than 8.0 [194]. The level of pH after different pretreatments depends on the properties of the analyzed soil, including the composition of its exchange complex [164].
The application of standard pretreatments [16, 113] for the samples of some tropical and subtropical soils containing high amounts of iron, aluminum, organic matter, and clay does not ensure the dispersion of the samples to the size of primary elements [21]. For the soils enriched in allophanes, the acid dispersing medium is required; for the soils enriched in kaolinite and sesquioxides, the dispersing medium should have the alkaline reaction [123].
Most of the chemical methods of soil dispersion are based on the action of monovalent cations (Na+, Li+). The dispersion is achieved due to the increase in the thickness of the diffuse part of the electrical double layer. Sodium pyrophosphate (Na4P2O7) [18], sodium hexametaphosphate (NaPO3)6 [128, 194], sodium chloride (NaCl), sodium carbonate (Na2CO3) [21, 116], sodium oxalate (Na2C2O4) [7], lithium hydroxide (LiOH), and lithium carbonate (Li2CO3) [187] are usually applied as dispersing agents. Monovalent cation exert the dispersing action via substitution for bivalent cations in the exchange complex. The anions of dispersing agents should bind polyvalent metal ions into soluble complexes (Ме + anion) [82]. For examples, phosphates may form insoluble compounds with calcium, which may result in the incomplete dispersion of the soil or even in the formation of artifacts [7, 31]. Iron may also form insoluble compounds and precipitate in the course of soil dispersion in the presence of Na2CO3 [21].
Though Li+ ions have a smaller radius and are more hydrated than Na+ ions, the dispersing action of LiOH and Li2CO3 on some Oregon soils was less active than that of Na2CO3 and Calgon. The most efficient dispersion was reached after the soil boiling with Na2CO3; it was more efficient than the dispersion with Calgon [187]. At the same time, saturation of the samples with Li+ proved to be the most efficient pretreatment for the soils enriched in montmorillonite and developed from basalts [153].
For gray forest soils of the Volga–Kama forest-steppe, the dispersion with 4% Na4P2O7 did not lead to the complete destruction of microaggregates of the fine silt size (1–10 µm). After oxidation of organic matter, changes in the relative contents of the fine (physical clay) fractions were observed [8]. Rubbing of the soil paste with 4% Na4P2O7 did not ensure the maximum release of the clay fraction in chernozems [31]. At the same time, this pretreatment resulted in the same amount of physical clay (<10 µm) as in the case of the acid–base pretreatment suggested by Kachinskii (0.2 M or 0.05 M HCl for calcareous and noncalcareous soils, respectively (to remove CaCO3 and Ca2+) followed by shaking of the suspension and its boiling with 1 M NaOH), which ensured the most complete soil dispersion [23].
The most considerable differences in the results of particle-size distribution analysis with different pretreatments are observed for the soils containing carbonates [31]. For these soils, a preliminary treatment of the samples with diluted acid [166], or complete destruction of carbonates [7, 124] can be recommended. For this purpose, the soil is treated for 1 min at Т = 98°С by the concentrated formic acid (HCOOH) [133, 144], or by hydrochloric acid of different concentrations [7, 149, 162]. According to the international standard [113], 1 М HCl is recommended. In Russia, the soil is consecutively treated with 0.2 and 0.5 М HCl [7]. Carbonates may be also removed with 5–7% solution of acetic acid [27]. Kornilova with coauthors [27] recommended the use of 1 М CH3COOH, as this treatment does not destroy the silicate part of the soils. The application of sodium acetate (1 M C2H3NaO2 at рН 5) is also recommended because of the same reason [100]. The dispersing actions of CH3COOH and HCl are almost equivalent, but the concentration of CH3COOH should be two times higher; the reaction with this reagent proceeds slower than that with HCl [172]. The use of Na2C2O4 for base-saturated soils does not allow the preliminary treatment of the soil with HCl [156].
The application of diluted acids may lead to some midification of the clay fraction of soils [120]. In the acidified medium, the primary structure of aluminosilicates may be destroyed; in the case of the prolonged action, degradation or iron-containing clay minerals may take place [27]. To avoid these negative phenomena in the acid medium, the treatment with diluted acid to pH 5.5 is recommended; after 30-min reaction, soil organic matter is oxidized with hydrogen peroxide (the pH 5.5–H2O2 method) [164]. Schulte with coauthors [172] analyzed the influence of HCl pretreatment on the loess–paleosol sequences. They concluded that it is feasible to exclude the stage of the removal of carbonates in the particle-size distribution analysis with the laser diffraction method. According to these authors, the pretreatment with HCl is highly selective and its overall results are hardly predictable. The application of sodium hexametaphosphate (NaPO3)6 [194] or sodium hypobromite (NaBrO) [190] as dispersing agents makes it possible to exclude the preliminary stage of the removal of carbonates. The dispersing action of NaBrO on soils may be stronger than that of different combinations of soil treatment with H2O2, HCl, and (NaPO3)6 [164].
In Russia, the state standard suggests soil dispersion with the use of ammonium cations [16]. However, this method does not ensure complete release of the colloidal fraction from the microaggregates [2]. An advantage of this method is the removal of ammonium from the soil in the course of drying of the samples [128, 165].
A review of publications devoted to the removal of organic matter from soils with commonly used chemical reagents was made by Mikutta with coauthors [148]. Multiple oxidation of organic matter with 10% H2O2 is the most commonly used method [167], though it does not ensure complete removal of organic matter [148, 172, 196]. Some aliphatic components of the SOM are resistant to oxidation with H2O2 [93]. For coarse-textured forest soils (Haplic Podzols and Dystric Cambisols), the amount of organic carbon remaining in the soil after its long treatment with H2O2 may reach 3.4–58% of the initial carbon content [92]. The use of the acetate buffer (to exclude strongly acid reaction in the suspension) does not ensure complete oxidation and may lead to the sorption of acetate the surface of minerals [95, 158].
The most efficient method is the organic matter oxidation with sodium hypochlorite (NaClO) [59, 147] and sodium persulfate (Na2S2O8) [144]. In the alkaline medium (рН 9.5), NaClO may lead to partial release of Al from clay minerals [148]. The application of Na2S2O8 together with the acetic (NaHCO3) buffer maintains pH values within the range from 7 to 8.5 [127, 145]; however, \(-{\text{SO}}_{{\text{4}}}^{{{\text{2}} - }}\) and \( - {\text{HCO}}_{{\text{3}}}^{ - }\) anions may be sorbed on the surface of minerals (on the surface of Fe and Al oxides), if the latter are present in the soil in high amounts [148]. In comparison with H2O2, the action of NaClO and Na2S2O8 on layered silicates seems to be less pronounced, but this problem requires further studied [148]. Oxidation with Na2S2O8 has a selective action on the “young” organic carbon fraction and does not affect no less labile fraction of the organic carbon, which may vary from 1 to 30% of Corg in sandy Haplic Podzols and Dystric Cambisols in the upper and middle-profile horizons and reach up to 80% in the lower horizons [92]. Oxidation with Na2S2O8 is more efficient for the upper horizons, whereas H2O2 is more efficient for the lower horizons of forest soils [93]. Oxidation with NaClO and Na2S2O8 is possible without the preliminary removal of carbonates, sesquioxides, or amorphous silica. Sodium hypobromite (NaBrO) in 1 M or 2 M concentrations can also be used for this purpose [69, 190].
The only reagent efficient at root temperature is NaClO [148]. In general, the use of wet oxidation methods (with H2O2, NaClO, or Na2S2O8) inevitably leads to changes in the mineral phase of soils [148].
For the soils rich in iron oxides responsible for the formation of dispersion-resistant microaggregates, a procedure analogous to the soil pretreatment for mineralogical analysis can be used [106, 107, 131, 185]. The removal of iron often results in the decreasing relative contents of silt and clay fractions with a corresponding increase in the relative content of sand. The forming sand-size particles are tolerant to the subsequent stages of dispersion. Therefore, some authors recommend to exclude the stage of iron removal in the soil pretreatment for the particle-size distribution analysis [187]. If the soil contains manganese oxide (Mn2O) that can be dissolved in hydrogen peroxide, the preliminary soil treatment with sodium bisulfite (NaHSO3) is required [115].
MICROAGGREGATE-SIZE DISTRIBUTION ANALYSIS
The microaggregate-size distribution in soils can be determined by the methods based on sedimentation, laser diffraction, of soil sifting on a set of screens.
According to Russian State Standard [16], the boiling of soil water suspension (from 1 : 25 for clay to 1 : 12.5 for loamy sand) during 1 h is recommended as the pretreatment for this analysis. The method by Kachinskii suggests intense mechanical shaking (200 shakes/min) of the water suspension (1 : 20) for 2 j after the preliminary slaking of the samples in water for 24 h [23].
Several methods suggest chemical pretreatment. According to Shein and Pochatkova [50], 0.4% sodium pyrophosphate solution (Na4P2O7) is applied. In the international method B, the boiling or rubbing for 10 min followed by shaking of the suspension in the diluted ammonia solution (NH4OH) is suggested [23, 156]. The intensity of this action is comparable with that recommended by the Russian State Standard [16] for the particle-size distribution analysis.
Some researchers using the method of laser diffractometry add the sample in the dry state into the dispersion unit upon the switched off ultrasound. In this case, the dynamics of changes in particle-size distribution until stabilization of the diameters of the particles in the circulating suspension are analyzed [58, 161]. To characterize microaggregates from the upper soil horizons (0–20 cm, 36 soils in the middle reaches of the Ebro River, Spain) by the laser diffraction method, it was sufficient to stir the suspensions for 90 s [58]. At the full rotation velocity and circulation of the suspension for 450 s, the increase in the content of particles <5 µm was only about 2–4%, i.e., not very significant. To exclude the differences in results related to differences in pretreatment procedures, a preliminary shaking of the soil suspension (20 min, 43 min–1) can be recommended [161]. The soils with the high sand content are better dispersed in distilled water than heavy-textured soils [82].
Many studies are devoted to changes in the contents and sizes of microaggregates in dependence on the applied energy of ultrasonic treatment of soil water suspensions [82, 98, 105, 121]. Such studies make it possible to give direct quantitative estimates of the energy applied to the soil aggregates (in J/mL or J/kg) [154] and to trace the dynamics of changes in the particle-size distribution upon disintegration of microaggregates to elementary soil particles.
Special studies are devoted to the mechanisms of the formation of soil microaggregates. Their review is given in [189].
INTERPRETATION AND USE OF THE RESULTS OF PARTICLE-SIZE AND MICROAGGREGATE-SIZE DISTRIBUTION ANALYSES
To obtain maximum information about the studied object, the results of particle-size and microaggregate-size distribution analyses should be analyzed simultaneously. In fact, there are common approaches to their interpretation; a number of indices have been suggested to integrate the results of these analyses.
The particular indices used to describe soil macro- and microstructures are discussed in detain in [44]. An original approach to the interpretation of data from the macro- and microaggregate-size and particle-size distribution analyses suggested in this study is based on calculation of generalized indices: mean-weighted diameter D, entropy of particle distribution Н, deviations in size distributions for elementary particles and microaggregates (D+, D–, Ds, H+, H–, Hs), and the index of water stability (R).
The most widespread approach to interpretation of particle-size distribution analysis is the grouping of soil separates (elementary particles) into fractions; their contents is taken into account in the determination of soil textural classes [23, 48, 180]. There are various nonparametric methods for the analysis of particle-size distribution with the use of quantiles. The coefficients based on them are often applied to estimate the uniformity of soil textures, e.g., the coefficient of uniformity Cu = d60/d30; the coefficient of gradation Cg = d30/(d60/d10); etc. [183]. Some researchers suggest the use of the mean weighted diameter [197], whereas other researchers prefer using the weighted geometric mean diameter [141]. For a polymodal particle-size distribution, some parametric indices, such as the mean radius of all the particles, various deviations from it, asymmetry of distribution, and the degree of sorting of the particles cannot be calculated. An analogous problem appears upon calculation of the mean weighted diameter in the case of the asymmetric particle-size (or aggregate-size) distribution [181].
Several studies are devoted to approximation of particle-size distribution data by some mathematical functions. There are methods that allow us to obtain continuous distribution curves (laser diffraction and X-ray sedimentation methods). The results obtained by other methods may be presented as cumulative curves, for which fitting can be applied. The reviews of most commonly used models of particle-size distribution can be found in [71, 91, 152, 205]. As suggested in [99], particle-size distribution data can be approximated by a lognormal function with two parameters—the mean weighted diameter Mg and standard deviation σg). Microaggregate-size distribution is often described by a monomodal curve, whereas size distribution of primary elements can be presented as a sum of two or three simple functions.
Often, the analysis and interpretation of particle-size distribution curves are based on the assumption that they represent a sum of some normal distributions. However, this may lead to errors of different kinds [203]. Particle-size distribution data can be described by fragmentation models consisting of several domains, each of which is described by the power function [65]. Some statistical models can separate and describe certain subpopulations in the continuous particle-size distribution curves. In particular, these are end-member-modeling algorithms [206] and models based on the Weibull distribution [184].
A separate direction in the analysis of particle-size distribution data is the use of the theory of fractals to describe the geometry of particles and their size distribution [159, 160, 192, 193]. However, it is open to argument [63].
Soil texture has a great diagnostic meaning. In the Russian soil classification system, the order of texture-differentiated soils is based on the presence of the diagnostic BT horizon in the soil profile. The identification of this horizon implies calculation of the coefficient of textural differentiation 52]. The subspecies level in the Russian system is determined from data on the soil texture and content of coarse skeletal elements (gravels and rock fragments) [52]. In the American system, diagnostic argic, candic, and natric horizons should have a higher clay content in comparison with the overlying horizons [180]. In the WRB system [114], data on texture is used to diagnose argic and natric horizons. The ratio of the silt content to the clay content is also used as a diagnostic parameter is several classification systems; this ratio is considered to be indicative of the degree of alteration of the soil material [73, 94].
Particle-size distribution data are very important in order to estimate the lithological homogeneity of parent materials [33, 41, 133] and to diagnose eolian sediments [149, 199]. Particle size is the major criterion in the assessment of particle transport [199]. As shown for high-mountain soils, original pedogenetic interpretation of particle-size distribution data is possible [209].
To judge soil microaggregation, microaggregate-size and particle-size distribution data are to be analyzed. The stability of microaggregates can be assessed according to data on the contents of clay faction [23, 198], fractions <5 and/or 20 µm [55], and fraction <125 µm [134]. In some cases, only particle-size distribution data are used. Thus, according to Vadyunina [23], all ESPs are subdivided into active (<5 µm) and passive (>5 µm) particles in terms of their participation in the development of soil structure. The considered indices of the microaggregate-size distribution are unsuitable for coarse-textured soils, because the water-physical properties of these soils remain fairly good even in the case of the absence of the agronomically valuable structure in the soil material [23].
All the indices considered above were developed on the basis of particle-size distribution data obtained by sedimentation (pipette) methods. These indices require certain adaptation in order to be applicable for particle-size distribution data obtained by laser diffraction. Thus, it was shown that the coefficient of soil dispersity suggested by Kachinskii applied to humus-accumulative horizons becomes less informative for the data obtained by laser diffraction because of the absence (or close to zero value) of the particles <1 µm in the microaggregate-size distribution analysis by the laser diffraction method [54]. At the same time, the degree of aggregation (according to Baver) and the factor of structuring (according to Vadyunina) preserve their informativity [54].
Targulian [36] noted that the importance of the concept of elementary pedogenic processes for the development of pedology. Elementary pedogenic process (EPP) is a process constituting some part of pedogenesis and obligatorily forming a solid-phase feature (or a set of features) in the soil system. This feature should be stable in time and should be diagnostically meaningful in order to reveal spatial and temporal differences between soils [35, p. 1415]. Pedogenic changes in the soil solid phase result in the development of certain assemblages of pedogenic features and properties. In fact, EPPs can be diagnosed not only from changes in the substantive composition of the solid phase but also by its physical rearrangement in soil [36]. It is probable that, in dependence on the composition and properties of parent material subjected to continuous alteration by other factors, soil ESPs should be differentiated into some groups differing in their stability. Such groups may be considered “imprints” of the combinations of different EPPs. This means that the EPP concept may be applied to develop new diagnostic criteria based on particle-size distribution data.
Thus, for chernozems, the accumulation of organic ESPs in the silt fractions is typical [48]. Predominant destruction of the clay fraction is considered the diagnostic criterion of selective podzolization (selective mineral weathering in certain size fractions [39]), whereas the Al–Fe-humus process is diagnosed by the destruction of unstable minerals in all the fractions [40].
A comparison of particle-size distribution data obtained with the use of laser diffraction method with and without HCl pretreatment makes it possible to diagnose stable microaggregates and organomineral compounds and determine the range of particle sizes, where they are localized [172]. For correct determination of the size distribution of carbonates, particle-size distribution data obtained with different pretreatments (without the removal of carbonates and after their removal) should be analyzed [126].
DISCUSSION AND CONCLUSIONS
We argue that it is reasonable to distinguish between two different approaches to the objects of particle-size distribution analysis: primary soil particles should not be confused with elementary soil particles. The former represent individual mineral grains of soils. Each of these grains is characterized by its own chemical composition, morphology, and physical properties. Primary soil particles represent the carcass of the parent material, in which pedogenesis is developed. Elementary soil particles are solid-phase products of pedogenesis and are represented by the debris of rocks and minerals and by the organomineral and organic particles, all the components of which participate in chemical and physicochemical interactions. We further argue that indication of the method of peptization should be excluded from the definition of ESPs, as it does not add clarity to this concept. Elementary soil particles may be subdivided into the following types:
(a) Mineral particles, the surface of which may be covered by the films of organomineral compounds and hydroxides of Fe, Al, and Mn, though the presence of such films is not an obligatory condition;
(b) Stable organomineral complexes (primary organo-mineral complexes according to Chenu and Plante [76], or composite building units according to Totsche et al. [189]); and
(c) Organic particles, or, more precisely, particulate organic matter.
The concept of ESPs is of fundamental importance. Indeed, this is the level, from which the formation and development of soil as a separate natural body take place. At this level, unique soil properties are manifested and make it possible to distinguish between different soils. The term elementary is used to convey the meaning of the initial pedogenetic level. To differentiate between ESPs and microaggregates, the particular mechanisms of aggregation should be known. It is necessary to quantitatively determine the strength of the bonds inherent in ESPs and in microaggregates. We suppose that some additional sublevels may be separated within and between the levels of ESPs and microaggregates, because there are many mechanisms of aggregation, and they depend on the particular types of soils and soil horizons. In fact, we deal with a very complex system of structural elements having different stabilities and different mean residence times.
The choice of the particular pretreatment for particle-size distribution analyses depends on the goal of the study [96, 139]. It is unfeasible to speak about the only one “correct” determination of the clay content [153]. The use of chemical agents at room temperature increases the time required for complete oxidation, whereas the rise in temperature may involve changes in the properties of mineral soil components. In fact, the pretreated samples represent “the black box” rather than some “ultimate state” for different soils. The one-stage application of different oxidizing agents is impossible, which is also a serious drawback of the chemical pretreatment for the particle-size distribution analysis. The existing standards suggest several stages in dependence on the presence or absence of soluble salts, carbonates, iron compounds, and the quality and quantity of organic matter. Thus, we may conclude about the impossibility of finding some universal pretreatment procedure with the use of the methods of chemical dispersion of the samples. The same procedure might have different impacts depending on the nature of analyzed soils. Moreover, careful analysis of these differences may be indicative of the specificity of these soils [1, 122, 142, 143, 167, 172]. It should be stressed that new methods of particle-size distribution analysis making it possible to obtain continuous particle-size distribution curves and to notice minor changes yield much promise for future studies at the new quantitative level. The methods of soil dispersions that do not modify the solid phase of soils should be used for this purpose.
Our review of publications gives us ground to outline future challenges of soil physics and pedology. The concept of the hierarchy of soil structural levels should be quantitatively characterized. Further study of interactions lying in the basis of the formation and functioning of ESPs and soil microaagregates with the use of modern methods is necessary. Such a study will make it possible to specify the types of ESPs and soil microaggregates. Qualitative and quantitative descriptions of the structural organization of soils should help researchers to develop new diagnostic criteria for different kinds of pedogenic transformation of the soil solid phase. In this context, special attention should be addressed to various pedofeatures. Their role in the structural organization of soils should be specified with due account for not only their morphology but also their functioning in the soil system.
It seems feasible to shift the upper boundary of clay fraction in the Russian classification developed by N.A. Kachinskii from 1 to 2 µm. In fact, this will not change the principles of separation of soil textural classes, whereas the comparison of data obtained by researchers from different national schools of pedology will become easier. Moreover, this will reduce the time necessary for sampling the clay fraction in the pipette method by three times (from 24 to 8 h). At the same time, the idea to take into account the type of pedogenesis in the textural classification of soils suggested by Kachinskii seems to be promising. Its further development is required for the soils that were not included by Kachinskii into his system.
The method of laser diffraction has found wide application in recent years. It has a number of important advantages over the classical pipette method based on sedimentation: it is time-efficient, requires small sample size (mg), does not require additional knowledge of the solid phase density (ρs), and yields a continuous particle-size distribution curve. The existing classifications should be adapted to this method, and new classifications should be developed for it. We suppose that this method will find wide application for solving practical tasks of soil science.
The features of microstructural organization of soils may play the key role in the choice of particular indices to assess the physical state of soils and develop physical and other models. For example, the microaggregate-size distribution is of key importance for chernozems and ferrallitic soils, whereas particle-size distribution is more important for sandy soils. We argue that the microaggregate-size distribution analysis deserves more attention in terms of its practical use. This analysis is more sensitive to changes in the composition and properties of soil organic matter and might become an indispensable tool in the solution of problems related to the carbon pool in soils and other natural disperse organomineral systems. It is also promising for monitoring the agricultural and ecological state of the soil cover.
REFERENCES
T. V. Alekseeva, “Soil microstructure and factors of its formation,” Eurasian Soil Sci. 40, 649–659 (2007).
I. N. Antipov-Karataev, “The concept about soil as a polydisperse system and its development in the Soviet Union in 1917–1942,” Pochvovedenie, No. 6, 3–26 (1943).
Z. S. Artem’eva, Organic Matter and Granulometric System of Soil (GEOS, Moscow, 2010) [in Russian].
P. N. Berezin, “Specificity of particle-size distribution in soils and parent materials,” Pochvovedenie, No. 2, 64–72 (1983).
P. N. Berezin, Doctoral Dissertation in Biology (Moscow, 1995).
M. A. Bronnikova and V. O. Targulian, Assemblage of Cutans in Texturally Differentiated Soils (Akademkniga, Moscow, 2005) [in Russian].
A. F. Vadyunina and Z. A. Korchagina, Physical Analysis of Soils (Agropromizdat, Moscow, 1986) [in Russian].
A. A. Valeeva and G. S. Koposov, “Influence of soil preparation on the interpretation of soil particle-size distribution data,” Uch. Zap. Kazan. Univ., Ser. Estestv. Nauki 155 (2), 172–178 (2013).
A. Ya. Vanyushina and L. S. Travnikova, “Organic-mineral interactions in soils: a review,” Eurasian Soil Sci. 36, 379–387 (2003).
A. M. Vasil’ev, Analysis of Physical Properties of Soils (Gos. Izd. Mold., Chisinau, 1952) [in Russian].
A. M. Vasil’ev, “Physical constants of clay soils,” in Hydrogeology and Engineering Geology (Gosgeolizdat, Moscow, 1937), No. 4, pp. 37–40.
A. D. Voronin, Structural-Functional Hydrophysics of Soils (Moscow State Univ., Moscow, 1984) [in Russian].
A. D. Voronin, “Active surface of the fractions of mechanical elements in soil complexes of the light chestnut soil subzone,” Nauchn. Dokl. Vyssh. Shk., Biol. Nauki, No. 3, (1959).
K. K. Gedroits, Soil as a Cultural Environment for Agricultural Plants. Soil Colloids and Salinity of Soils According to the Data of Agrochemical Department of Nosovskaya Agricultural Experimental Station: A Review (Kiev-Pechat’, Kiev, 1926) [in Russian].
M. I. Gerasimova, S. V. Gubin, and S. A. Shoba, Micromorphology of Soils of the Natural Zones of the Soviet Union (Pushchino Scientific Center, Russian Academy of Sciences, Pushchino, 1992) [in Russian].
GOST (State Standard) 12536-2014: Soils. Methods of Laboratory Granulometric (Grain-Size) and Microaggregate Distribution (Standartinform, Moscow, 2015) [in Russian].
B. P. Gradusov, “Evolutionare stages of soddy-podzolic loamy soils,” Byull. Pochv. Inst. im. V.V. Dokuchaeva, No. 59, 14–22 (2007).
S. I. Dolgov, Agrophysical Analysis of Soils (Nauka, Moscow, 1966) [in Russian].
F. R. Zaidel’man, Theory of the Development of Light Acid Eluvial Soil Horizons and Its Applied Aspects (Krasand, Moscow, 2010) [in Russian].
F. R. Zaidel’man and A. S. Nikiforova, “Ferromanganese concretionary neoformations: a review,” Eurasian Soil Sci. 43, 248–258 (2010).
S. V. Zonn, Pedogenesis and Soil of the Tropics and Subtropics (Nauka, Moscow, 1974) [in Russian].
I. V. Ivanov, “The structure of soil systems,” in Soils, Biogeochemical Cycles, and the Biosphere (KMK, Moscow, 2004), pp. 50–69.
N. A. Kachinskii, Mechanical and Microaggregate Composition of Soil and Methods for Its Study (Academy of Sciences of Soviet Union, Moscow, 1958) [in Russian].
N. A. Kachinskii, “The nature of soil structuring,” in Physics, Chemistry, Biology, and Mineralogy of Soils of the Soviet Union, Ed. by I. P. Gerasimov (Nauka, Moscow, 1964) [in Russian].
D. S. Kashik, Methods of Mineralogical Studies: A Handbook (Nedra, Moscow, 1985), pp. 60–74.
A. V. Kinsht, “Chemical analysis of fine fractions of two types of soils with an eluvial–illuvial profile,” in Analysis of Siberian Soils (Novosibirsk, 1977) [in Russian].
A. G. Kornilova, A. A. Shinkarev, T. Z. Lygina, K. G. Giniyatullin, and R. R. Gil’mutdinov, “Optimization of sample preparation for the bulk elemental analysis of the mineral part of forest-steppe soils,” Uch. Zap. Kazan. Univ., Ser. Estestv. Nauki 153 (3), (2011).
A. O. Makeev and O. V. Makeev, Soils with Texture-Differentiated Profiles in the Main Cryogenic Areas of the North of the Russian Plain (Scientific Center of Biological Studies, Academy of Sciences of the Soviet Union, Pushchino, 1989) [in Russian].
A. A. Rode, Chemical Composition of Mechanical Fractions of Some Soils of Podzolic and Bog-Podzolic Types, Tr. Pochv. Inst. im. V.V. Dokuchaeva vol. 8 (Academy of Sciences of the Soviet Union, Leningrad, 1933) [in Russian].
B. G. Rozanov, Soil Morphology (Akademicheskii Proekt, Moscow, 2004) [in Russian]. ISBN 5-8291-0451-2.
S. V. Romanov, “Comparative characteristics of several methods of soil preparation for mechanical analysis,” Pochvovedenie, No. 4, 150–154 (1974).
E. M. Sergeev, Engineering Geology (Moscow State Univ., Moscow, 1982) [in Russian].
I. A. Sokolov, Pedogenesis and Exogenesis (Dokuchaev Soil Science Inst., Moscow, 1997) [in Russian].
T. A. Sokolova, “Transformation of clay material in some acid texture-differentiated soils with a bleached horizon,” in Problems in Soil Science (Nauka, Moscow, 1982) [in Russian].
V. O. Targulian, “Elementary pedogenic processes,” Eurasian Soil Sci. 38, 1255–1264 (2005).
V. O. Targulian and S. V. Goryachkin, Soil Memory: Soil as the Memory of the Biosphere–Geosphere–Anthroposphere Interactions (LKI, Moscow, 2008) [in Russian].
N. A. Titova, L. S. Travnikova, and M. Sh. Shaimukhametov, “Development of the studies on interaction between organic and mineral components of soils,” Pochvovedenie, No. 5, 639–646 (1995).
N. A. Titova, L. S. Travnikova, and Yu. V. Kuvaeva, “The composition of the components of fine particles in arable soddy-podzolic soil,” Pochvovedenie, No. 6, 89–97 (1989).
V. D. Tonkonogov, Clay-Differentiated Soils of the European Part of Russia (Dokuchaev Soil Science Inst., Moscow, 1999) [in Russian].
V. D. Tonkonogov, I. I. Lebedeva, and M. I. Gerasimova, “General horizon- and profile-forming processes in Russian soils,” in Pedogenic Processes (Dokuchaev Soil Science Inst., Moscow, 2006) [in Russian].
T. V. Tursina, “Approaches to the study of the lithological homogeneity of soil profiles and soil polygenesis,” Eurasian Soil Sci. 45, 472–487 (2012).
A. F. Tyulin, “Methods of peptization analysis in relation to general regularities in the chemical and physical properties of soils,” Pochvovedenie, Nos. 4–5, 3–15 (1943).
Chemical Encyclopedia, Vol. 1: Ablative Materials–Darzens Reaction (Sovetskaya Entsiklopediya, Moscow, 1988) [in Russian].
N. B. Khitrov and O. A. Chechueva, “Interpretation of data on macro- and microstructure of soils,” Pochvovedenie, No. 2, 84–92 (1994).
N. P. Chizhikova and P. G. Panin, “Informativness of fine-dispersed part of paleosols and loesses of the Late and Middle Pleistocene in the center of the East European Plain,” Byull. Pochv. Inst. im. V.V. Dokuchaeva, No. 59, (2007).
M. Sh. Shaimukhametov, N. A. Titova, L. S. Travnikova, and E. M. Labenets, “Physical fractionation methods for characterization of soil organic matter,” Pochvovedenie, No. 8, 131–141 (1984).
E. V. Shein, “The particle-size distribution in soils: problems of the methods of study, interpretation of the results, and classification,” Eurasian Soil Sci. 42, 284–291 (2009).
E. V. Shein, Soil Physics (Moscow State Univ., Moscow, 2005) [in Russian].
E. V. Shein, T. A. Arkhangel’skaya, V. M. Goncharov, A. K. Guber, T. N. Pochatkova, M. A. Sidorova, A. V. Smagin, and A. B. Umarova, Field and Laboratory Analysis of Physical Properties and Regimes of Soils: Methodological Manual (Moscow State Univ., Moscow, 2001) [in Russian].
E. V. Shein and T. N. Pochatkova, “Microaggregate analysis of soils,” in Theories and Methods of Soil Physics (Grif i K°, Moscow, 2007) [in Russian].
A. A. Shinkarev, A. G. Kornilova, F. A. Trofimova, A. S. Gordeev, K. G. Giniyatullin, and T. Z. Lygina, “Comparison of sedimentation and laser diffraction methods in the analysis of the granulometric composition of the clay fraction of soils,” Uch. Zap. Kazan. Univ., Ser. Estestv. Nauki 152 (2), (2010).
L. L. Shishov, Classification of Russian Soils (Dokuchaev Soil Science Inst., Moscow, 1997) [in Russian].
A. V. Yudina, “Granulometric composition and lithological heterogeneity of genetic horizons of soils of the Baer hills and associated landscapes,” Mater. Izuch. Russ. Pochv., No. 7 (34), 40–42 (2013).
A. V. Yudina and E. Yu. Milanovskii, “Microaggregate analysis of soils by laser diffraction: specificity of sample pretreatment and interpretation of the results,” Byull. Pochv. Inst. im. V.V. Dokuchaeva, No. 89, 3–20 (2017).
T. M. Abu-Sharar, F. T. Bingham, and J. D. Rhoades, “Stability of soil aggregates as affected by electrolyte concentration and composition,” Soil Sci. Soc. Am. J. 51, 309–314 (1987).
E. B. Alexander, Soils in Natural Landscapes (CRC Press, Boca Ration, Fl., 2013).
W. Amelung and W. Zech, “Minimization of organic matter disruption during particle-size fractionation of grassland epipedons,” Geoderma 92 (1–2), 73–85 (1999).
E. Amézketa, R. Aragüés, R. Carranza, and B. Urgel, “Macro- and micro-aggregate stability of soils determined by a combination of wet-sieving and laser-ray diffraction,” Span. J. Agric. Res. 1 (4), 83–94 (2003).
J. U. Anderson, “An improved pretreatment for mineralogical analysis of samples containing organic matter,” Clays Clay Miner. 10 (3), 380–388 (1963).
M. P. Arnett, PhD Thesis (Texas A&M Univ., College Station, TX, 2009).
L. M. Arya and J. F. Paris, “A physicoempirical model to predict the soil moisture characteristic from particle-size distribution and bulk density data,” Soil Sci. Soc. Am. J. 45 (6), 1023–1030 (1981).
A. Atterberg, “Die mechanische Bodenanalyse und die Klassifikation der Mineralböden Schwedens,” Int. Mitt. Bodenkd. 2, 312–342 (1912).
P. Barak, K. McSweeney, and C. A. Seybold, “Self-similitude and fractal dimension of sand grains,” Soil Sci. Soc. Am. J. 60 (1), 72–76 (1996).
L. Beuselinck, “Grain-size analysis by laser diffractometry: comparison with the sieve-pipette method,” Catena 32 (3), 193–208 (1998).
M. Bittelli, G. S. Campbell, and M. Flury, “Characterization of particle-size distribution in soils with a fragmentation model,” Soil Sci. Soc. Am. J. 63 (4), 782–788 (1999).
S. J. Blott and K. Pye, “Particle size scales and classification of sediment types based on particle size distributions: review and recommended procedures,” Sedimentology 59 (7), 2071–2096 (2012).
J. Bouma, “Using soil survey data for quantitative land evaluation,” Adv. Soil Sci. 9, 177–213 (1989).
J. Bouma and H. A. J. van Lanen, “Transfer functions and threshold values: from soil characteristics to land qualities,” in Proceedings of the International Workshop on Quantified Land Evaluation Procedures, April 27–May 2, 1986 (Washington, DC, 1986), pp. 106–110.
S. J. Bourget and C. B. Tanner, “Removal of organic matter with sodium hypobromite for particle-size analysis of soils,” Can. J. Agric. Sci. 33, 579–585 (1953).
T. G. Boyadgiev and W. H. Verheye, “Contribution to a utilitarian classification of gypsiferous soil,” Geoderma 74 (3–4), 321–338 (1996).
G. D. Buchan, K. S. Grewal, and A. B. Robson, “Improved models of particle-size distribution: an illustration of model comparison techniques,” Soil Sci. Soc. Am. J. 57 (4), 901–908 (1993).
P. Buurman, T. Pape, and C. C. Muggler, “Laser grain-size determination in soil genetic studies 1. Practical problems,” Soil Sci. 162 (3), 211–218 (1997).
M. N. Camargo, E. Klamt, and J. H. Kauffman, Soil Classification as Used in Brazilian Soil Surveys, Annual Report of ISRIC (International Soil Reference and Information Centre, Wageningen, 1986), pp. 7–42.
C. Cerli, L. Celi, K. Kalbitz, G. Guggenberger, and K. Kaiser, “Separation of light and heavy organic matter fractions in soil—testing for proper density cut-off and dispersion level,” Geoderma 170, 403–416 (2012).
A. Chappell, “Dispersing sandy soil for the measurement of particle size distributions using optical laser diffraction,” Catena 31 (4), 271–281 (1998).
C. Chenu and A. T. Plante, “Clay-sized organo-mineral complexes in a cultivation chronosequence: revisiting the concept of the 'primary organo-mineral complex,” Eur. J. Soil Sci. 57 (4), 596–607 (2006).
C. Chenu, “Influence of a fungal polysaccharide, scleroglucan, on clay microstructures,” Soil Biol. Biochem. 21 (2), 299–305 (1989).
D. J. Chittleborough, “Effect of the method of dispersion on the yield of clay and fine clay,” Aust. J. Soil Res. 20 (4), 339–346 (1982).
B. T. Christensen, “Physical fractionation of soil and organic matter in primary particle size and density separates,” in Advances in Soil Science (Springer, New York, 1992), pp. 1–90.
B. T. Christensen, “Physical fractionation of soil and structural and functional complexity in organic matter turnover,” Eur. J. Soil Sci. 52 (3), 345–353 (2001).
A. R. Dexter, “Advances in characterization of soil structure,” Soil Tillage Res. 11, 199–238 (1988).
A. P. Edwards and J. M. Bremner, “Dispersion of soil particles by sonic vibration,” J. Soil Sci. 18, 47–63 (1967).
A. P. Edwards and J. M. Bremner, “Dispersion of mineral colloids in soils using cation exchange resins,” Nature 205 (4967), 208–209 (1965).
A. P. Edwards and J. M. Bremner, “Microaggregates in soils,” J. Soil Sci. 18 (1), 64–73 (1967).
A. P. Edwards and J. M. Bremner, “Use of sonic vibration for separation of soil particles,” Can. J. Soil Sci. 44, 366 (1964).
E. T. Elliott and C. A. Cambardella, “Physical separation of soil organic matter,” Agric., Ecosyst. Environ. 34 (1–4), 407–419 (1991).
W. W. Emerson, “Determination of the contents of clay-sized particles in soils,” J. Soil Sci. 22 (1), 50–59 (1971).
EN ISO 14688-1:2002: Geotechnical Investigation and Testing—Identification and Classification of Soil. Part 1. Identification and Description (Comité Européen de Normalization, Brussels, 2002).
J. Eriksen, R. D. B. Lefroy, and G. J. Blair, “Physical protection of soil organic S studied using acetylacetone extraction at various intensities of ultrasonic dispersion,” Soil Biol. Biochem. 27 (8), 1005–1010 (1995).
G. Eshel, “Critical evaluation of the use of laser diffraction for particle-size distribution analysis,” Soil Sci. Soc. Am. J. 68 (3), 736–743 (2004).
L. Esmaeelnejad, F. Siavashi, J. Seyedmohammadi, and M. Shabanpour, “The best mathematical models describing particle size distribution of soils,” Model. Earth Syst. Environ. 2 (4), 166 (2016).
K. Eusterhues, C. Rumpel, M. Kleber, and I. Kögel-Knabner, “Stabilization of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation,” Org. Geochem. 34 (12), 1591–1600 (2003).
K. Eusterhues, C. Rumpel, and I. Kögel-Knabner, “Stabilization of soil organic matter isolated via oxidative degradation,” Org. Geochem. 36 (11), 1567–1575 (2005).
FAO/UNESCO Soil Map of the World, Revised Legend (Food and Agriculture Organization, Rome, 1988).
V. C. Farmer and B. D. Mitchell, “Occurrence of oxalates in soil clays following hydrogen peroxide treatment,” Soil Sci. 94 (4), 221–229 (1963).
R. E. Francis and R. Aguilar, “Calcium carbonate effects on soil textural class in semiarid wild land soils,” Arid Land Res. Manage. 9 (2), 155–165 (1995).
M. D. Fredlund, G. W. Wilson, and D. G. Fredlund, “Use of the grain-size distribution for estimation of the soil-water characteristic curve,” Can. Geotech. J. 39 (5), 1103–1117 (2002).
A. J. Fristensky and M. E. Grismer, “Evaluation of ultrasonic aggregate stability and rainfall erosion resistance of disturbed and amended soils in the Lake Tahoe Basin, USA,” Catena 79 (1), 93–102 (2009).
W. R. Gardner, “Representation of soil aggregate-size distribution by a logarithmic-normal distribution,” Soil Sci. Soc. Am. J. 20 (2), 151–153 (1956).
G. W. Gee and D. Or, “Particle-size analysis,” in Methods of Soil Analysis: Part 4 Physical Methods, Chap. 2: The Solid Phase, SSSA Book Series 5.4 (Soil Science Society of America, Madison, WI, 2002), No. 2.4.
D. A. Genrich and J. M. Bremner, “A reevaluation of the ultrasonic-vibration method of dispersing soils,” Soil Sci. Soc. Am. J. 36 (6), 944–947 (1972).
T. A. Ghezzehei, “Soil structure,” in Handbook of Soil Sciences: Properties and Processes, Ed. by P. Huang, Y. Li, and M. Sumner (CRC Press, Boca Raton, 2012), Vol. 2.
A. Golchin, J. M. Oades, J. O. Skjemstad, and P. Clarke, “Study of free and occluded particulate organic matter in soils by solid state 13C CP/MAS NMR spectroscopy and scanning electron microscopy,” Soil Res. 32 (2), 285–309 (1994).
D. J. Greenland, “Interactions between humic and fulvic acids and clays,” Soil Sci. 111 (1), 34–41 (1971).
E. G. Gregorich, M. H. Beare, U. F. McKim, and J. O. Skjemstad, “Chemical and biological characteristics of physically uncomplexed organic matter,” Soil Sci. Soc. Am. J. 70 (3), 975–985 (2006).
J. E. Guedez and R. Langohr, “Some characteristics of pseudo-silts in a soil-toposequence of the Llanos Orientals (Venezuela),” Pédologie 28, 118–131 (1978).
N. Q. Hai and K. Egashira, “Clay mineralogy of ferralitic soils derived from igneous rocks in Vietnam,” Clay Sci. 13 (6), 189–197 (2008).
P. R. Hesse, “Particle size distribution in gypsic soils,” Plant Soil 44 (1), 241–247 (1976).
D. Hillel, Introduction to Environmental Soil Physics (Elsevier, Amsterdam, 2004).
C. G. Hopkins, “A plea for the scientific basis for the division of soil particles in mechanical analysis,” US Dep. Agric., Div. Chem. Bull. 56, 64–66 (1899).
F. Hu, C. Xu, H. Li, S. Li, Z. Yu, Y. Li, and X. He, “Particles interaction forces and their effects on soil aggregates breakdown Feinan,” Soil Tillage Res. 147, 1–9 (2015).
C. R. Hunter and A. J. Busacca, “Dispersion of three andic soils by ultrasonic vibration,” Soil Sci. Soc. Am. J. 53 (4), 1299–1302 (1989).
ISO 11277:2009: Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation (International Organization for Standardization, Geneva, 2009).
IUSS Working Group WRB, World Reference Base for Soil Resources 2014, Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, World Soil Resources Reports No. 106 (Food and Agriculture Organization, Rome, 2015).
M. L. Jackson, Soil Chemical Analysis-Advanced Course (M.L. Jackson Publ., Madison, WI, 1969).
M. L. Jackson, L. D. Whittig, and R. P. Pennington, “Segregation procedure for the mineralogical analysis of soils,” Soil Sci. Soc. Am. Proc. 14, 77–81 (1950).
P. M. Jardine, J. F. McCarthy, and N. L. Weber, “Mechanisms of dissolved organic carbon adsorption on soil,” Soil Sci. Soc. Am. J. 53 (5), 1378–1385 (1989).
J. D. Jastrow and R. M. Miller, “Soil aggregate stabilization and carbon sequestration: feedbacks through organomineral associations,” in Soil Processes and the Carbon Cycle, Ed. by R. Lal, J. M. Kimble, R. F. Follett, and B. A. Stewart (CRC Press, Boca Raton, Fl., 1997), Vol. 11, pp. 207–223.
Z. Jiang and L. Liu, “A pretreatment method for grain size analysis of red mudstones,” Sediment. Geol. 241 (1–4), 13–21 (2011).
A. F. Joseph and F. J. Martin, “The determination of clay in heavy soils,” J. Agric. Sci. 11 (3), 293–303 (1921).
M. Kaiser and A. Asefaw Berhe, “How does sonication affect the mineral and organic constituents of soil aggregates?—A review,” J. Plant Nutr. Soil Sci. 177 (4), 479–495 (2014).
M. Kaiser, A. A. Berhe, M. Sommer, and M. Kleber, “Application of ultrasound to disperse soil aggregates of high mechanical stability,” J. Plant Nutr. Soil Sci. 175 (4), 521–526 (2012).
I. Kanno and S. Arimura, “Dispersion of humic allophane soils with supersonic vibration,” Soil Sci. Plant Nutr. 13 (6), 165–170 (1967).
B. A. Keen, “Mechanical analysis: national and international,” Soil Res. 1 (1), 43–49 (1928).
W. D. Kemper and W. S. Chepil, “Size distribution of aggregates,” in Methods of Soil Analysis, Part 1: Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling, Agronomy Monograph 9.1 (Soil Science Society of America, Madison, WI, 1965), Ch. 39.
R. Kerry, B. G. Rawlins, M. A. Oliver, and A. M. Lacinska, “Problems with determining the particle size distribution of chalk soil and some of their implications,” Geoderma 152 (3–4), 324–337 (2009).
R. Kiem and I. Kögel-Knabner, “Refractory organic carbon in particle-size fractions of arable soils II: organic carbon in relation to mineral surface area and iron oxides in fractions <6 μm,” Org. Geochem. 33 (12), 1699–1713 (2002).
V. J. Kilmer and L. T. Alexander, “Methods of making mechanical analyses of soils,” Soil Sci. 68 (1), 15–24 (1949).
M. Kleber, K. Eusterhues, M. Keiluweit, C. Mikutta, R. Mikutta, and P. S. Nico, “Mineral–organic associations: formation, properties, and relevance in soil environments,” Adv. Agron. 130 (1), 1–140 (2015).
M. Kleber, P. Sollins, and R. Sutton, “A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces,” Biogeochemistry 85 (1), 9–24 (2007).
G. W. Kunze and J. Dixon, “Pretreatment for mineralogical analysis,” in Methods of Soil Analysis, Part 1: Physical and Mineralogical Methods, SSSA Book Series 5.1, Ed. by A. Klute (Soil Science Society of America, Madison, WI, 1986).
J. Lehmann, J. Kinyangi, and D. Solomon, “Organic matter stabilization in soil microaggregates: implications from spatial heterogeneity of organic carbon contents and carbon forms,” Biogeochemistry 85 (1), 45–57 (2007).
M. Litaor, “The influence of eolian dust on the genesis of alpine soils in the Front Range, Colorado,” Soil Sci. Soc. Am. J. 51 (1), 142–147 (1987).
R. J. Loch and J. L. Foley, “Measurement of aggregate breakdown at the rain: comparison with tests of water stability and relationship with field measurements of infiltration,” Aust. J. Soil. Res. 32, 701–720 (1994).
J. L. Loizeau, D. Arbouille, S. Santiago, and J. P. Vernet, “Evaluation of wide-range laser diffraction grain size analyzer for use with sediments,” Sedimentology 41, 353–361 (1994).
P. J. Loveland and W. R. Whalley, “Particle size analysis,” in Soil and Environmental Analysis: Physical Methods, Revised, and Expanded, Ed. by K. A. Smith and C. E. Mullins (CRC Pres, Boca Raton, Fl., 2001).
R.T. Martin, “Calcium oxalate formation in soils from hydrogen perozide treatment,” Soil Sci. 77 (2), 143–146 (1954).
A. E. Matar and T. Doubleimy, Note on Proposed Method for the Mechanical Analysis of Gypsiferous Soils (Arab Center for the Studies of Arid Zones and Dry Lands, Damascus, 1978).
M. D. Matthews, “The effect of pretreatment on size analysis,” in Principles, Methods and Application of Particle Size Analysis (Cambridge University Press, Cambridge, 2007).
H. Mayer, A. Mentler, M. Papakyriacou, N. Rampazzo, Y. Marxer, and W. E. H. Blum, “Influence of vibration amplitude on the ultrasonic dispersion of soils,” Int. Agrophys. 16 (1), 53–60 (2002).
A. P. Mazurak, “Effect of gaseous phase on water-stable synthetic aggregates,” Soil Sci. 69 (2), 135–148 (1950).
W. McLean, “Effect of hydrogen peroxide on soil organic matter,” J. Agric. Sci. 21 (2), 251–261 (1931).
W. McLean, “The nature of soil organic matter as shown by the attack of hydrogen peroxide,” J. Agric. Sci. 21 (4), 595–611 (1931).
L. P. Meier and A. P. Menegatti, “A new, efficient, one-step method for the removal of organic matter from clay-containing sediments,” Clay Miner. 32, 557–563 (1997).
A. P. Menegatti, G. L. Frueh-Green, and P. Stille, “Removal of organic matter by disodium peroxodisulphate; effects on mineral structure, chemical composition and physicochemical properties of some clay minerals,” Clay Miner. 4 (2), 247–257 (1999).
A. Mentler, J. Schomakers, S. Kloss, S. Zechmeister-Boltenstern, R. Schuller, and H. Mayer, “Calibration of ultrasonic power output in water, ethanol and sodium polytungstate,” Int. Agrophys. 31 (4), 582–588 (2017).
E. H. Mikhail and G. P. Briner, “Routine particle size analysis of soils using sodium hypochlorite and ultrasonic dispersion,” Soil Res. 16 (2), 241–244 (1978).
R. Mikutta, M. Kleber, K. Kaiser, and R. Jahn, “Review: organic matter removal from soils using hydrogen peroxide, sodium hypochlorite, and disodium peroxodisulfate,” Soil Sci. Soc. Am. J. 69 (1), 120–135 (2005).
F. A. Mileti, G. Langella, M. A. Prins, S. Vingiani, and F. Terribile, “The hidden nature of parent material in soils of Italian mountain ecosystems,” Geoderma 207, 291–309 (2013).
C. Moni, D. Derrien, P. J. Hatton, B. Zeller, and M. Kleber, “Density fractions versus size separates: does physical fractionation isolate functional soil compartments?” Biogeosciences 9, 5181–5197 (2012).
M. Murray, “Is laser particle size determination possible for carbonate rick lake sediments?” J. Paleolimnol. 27, 173–183 (2002).
A. Nemes and W. J. Rawls, “Soil texture and particle-size distribution as input to estimate soil hydraulic properties,” Dev. Soil Sci. 30, 47–70 (2004).
K. Norrish and K. G. Tiller, “Subplasticity in Australian soils. V. Factors involved and techniques of dispersion,” Aust. J. Soil Res. 14 (3), 273–289 (1976).
P. F. North, “Towards an absolute measurement of soil structural stability using ultrasound,” J. Soil Sci. 27 (4), 451–459 (1976).
J. M. Oades and A. G. Waters, “Aggregate hierarchy in soils,” Aust. J. Soil Res. 29 (6), 815–825 (1991).
A. Olmstead, L. T. Alexander, and H. E. Middleton, “A pipette method of mechanical analysis of soils, based on improved dispersion procedure,” US Dep. Agric. Tech. Bull. 170, (1930).
M. J. Pearson, S. E. Monteith, R. R. Ferguson, C. T. Hallmark, W. H. Hudnall, H. C. Monger, T. G. Reinsch, and L. T. West, “A method to determine particle size distribution in soils with gypsum,” Geoderma 237, 318–324 (2015).
K. Pennell, L. M. Abriola, and S. A. Boyd, “Surface area of soil organic matter reexamined,” Soil Sci. Soc. Am. J. 59 (4), 1012–1018 (1995).
E. Perfect and B. D. Kay, “Applications of fractals in soil and tillage research: a review,” Soil Tillage Res. 36 (1–2), 1–20 (1995).
E. Perfect and B. D. Kay, “Fractal theory applied to soil aggregation,” Soil Sci. Soc. Am. J. 55 (6), 1552–1558 (1991).
R. Pini and G. Guidi, “Determination of soil microaggregates with laser light scattering,” Commun. Soil Sci. Plant Anal. 20 (1–2), 47–59 (1989).
C. A. Piper, Soil and Plant Analysis (Wiley, New York, 1950).
I. Plaza, A. Ontiveros-Ortega, J. Calero, and V. Aranda, “Implication of zeta potential and surface free energy in the description of agricultural soil quality: effect of different cations and humic acids on degraded soils,” Soil Tillage Res. 146, 148–158 (2015).
R. Protz and J. St. Arnaud, “The evaluation of four pretreatments used in particle-size distribution analyses,” Can. J. Soil Sci. 44, 345–351 (1964).
E. D. Rivers, C. T. Hallmark, L. T. West, and L. R. Drees, “A technique for rapid removal of gypsum from soil samples,” Soil Sci. Soc. Am. J. 46, 1338–1340 (1982).
G. W. Robinson, “Note on mechanical analysis of humus soils,” J. Agric. Sci. 12, 287–291 (1922).
G. W. Robinson and J. O. Jones, “A method for determining the degree of humification of soil organic matter,” J. Agric. Sci. 15 (1), 26–29 (1925).
S. S. Rousseva, “Data transformations between soil texture schemes,” Eur. J. Soil Sci. 48, 749–758 (1997).
D. Sarkar and A. Haldar, Physical and Chemical Methods in Soils Analysis. Fundamental Concepts of Analytical Chemistry and Instrumental Techniques (New Age International, New Delhi, 2005).
K. E. Saxton and W. J. Rawls, “Soil water characteristic estimates by texture and organic matter for hydrologic solutions,” Soil Sci. Soc. Am. J. 70 (5), 1569–1578 (2006).
M. W. I. Schmidt, C. Rumpel, and I. Kögel-Knabner, “Evaluation of an ultrasonic dispersion procedure to isolate primary organomineral complexes from soils,” Eur. J. Soil Sci. 50 (1), 87–94 (1999).
P. Schulte, F. Lehmkuhl, F. Steininger, D. Loibl, G. Lockot, J. Protze, P. Fischer, and G. Stauch, “Influence of HCl pretreatment and organo-mineral complexes on laser diffraction measurement of loess-paleosol sequences,” Catena 137, 392–405 (2016).
H. R. Schulten and P. Leinweber, “New insights into organic-mineral particles: composition, properties and models of molecular structure,” Biol. Fertil. Soils 30 (5–6), 399–432 (2000).
H. R. Schulten, P. Leinweber, and C. Sorge, “Composition of organic matter in particle-size fractions of an agricultural soil,” J. Soil Sci. 44 (4), 677–691 (1993).
S. M. Shevchenko and G. W. Bailey, “Non-bonded organo-mineral interactions and sorption of organic compounds on soil surfaces: a model approach,” J. Mol. Struct.: THEOCHEM 422 (1), 259–270 (1998).
L. G. Shield and M. W. Meyer, “Carbonate clay: measurement and relationship to clay distribution and cation-exchange capacity,” Soil Sci. Soc. Am. J. 28 (3), 416–419 (1964).
R. W. Simonson, “Sources of particle-size limits for soil separates,” Soil Horiz. 40 (2), 50–58 (1999).
J. M. Skopp, “Physical properties of primary particles,” in Soil Physics Companion, Ed. by A. W. Warrick (CRC Press, Boca Raton, Fl., 2002).
M. R. Soares, L. R. Alleoni, P. Vidal-Torrado, and M. Cooper, “Mineralogy and ion exchange properties of the particle size fractions of some Brazilian soils in tropical humid areas,” Geoderma 125 (3), 355–367 (2005).
Staff Soil Survey Keys to Soil Taxonomy (USDA National Resources Conservation Service, National Soil Survey Center, Lincoln, 2010).
G. B. Stirk, “Expression of soil aggregate distributions,” Soil Sci. 86 (3), 133–135 (1958).
Stuff Soil Survey Division, Soil Survey Manual, USDA Handbook 18 (U.S. Government Publishing Office, Washington, DC, 1993).
M. E. Sumner, Handbook of Soil Science (CRC Press, Boca Raton, Fl., 1999).
D. Sun, J. Bloemendal, D. K. Rea, J. Vandenberghe, F. Jiang, Z. An, and R. Su, “Grain-size distribution function of polymodal sediments in hydraulic and aeolian environments, and numerical partitioning of sedimentary components,” Sediment. Geol. 152, 262–267 (2002).
O. Tamm, “Determination of the inorganic components of the gel-complex in soils,” Medd. Stat. Skogförsöd. 19, 387–404 (1922).
H. Taubner, B. Roth, and R. Tippkötter, “Determination of soil texture: comparison of the sedimentation method and the laser-diffraction analysis,” J. Plant Nutr. Soil Sci. 172 (2), 161–171 (2009).
A. A. Theisen, D. D. Evans, and M. E. Harward, “Effect of dispersion techniques on mechanical analysis of Oregon soils,” Agric. Exp. Stat. Oregon State Univ. Tech. Bull. 104, 1–18 (1968).
J. M. Tisdall and J. M. Oades, “Organic matter and water-stable aggregates in soils,” J. Soil Sci. 33, 141–163 (1982).
K. U. Totsche, W. Amelung, M. H. Gerzabek, G. Guggenberger, E. Klumpp, C. Knief, L. Lehndorff, R. Mikutta, S. Peth, A. Prechtel, N. Ray, and I. Kögel-Knaber, “Microaggregates in soils,” J. Plant Nutr. Soil Sci. 18 (1), 104–136 (2018).
E. Troell, “The use of sodium hypobromite for the oxidation of organic matter in the mechanical analysis of soils,” J. Agric. Sci. 21 (3), 476–483 (1931).
E. Truog, J. R. Taylor, R. W. Pearson, M. E. Weeks, and R. W. Simonson, “Procedure for special type of mechanical and mineralogical soil analysis,” Soil Sci. Soc. Am. J. 1, 101–112 (1937).
D. L. Turcotte, “Fractals and fragmentation,” J. Geophys. Res.: Solid Earth 91 (2), 1921–1926 (1986).
S. W. Tyler and S. W. Wheatcraft, “Fractal scaling of soil particle-size distributions: analysis and limitations,” Soil Sci. Soc. Am. J. 56, 362–369 (1992).
E. H. Tyner, “The use of sodium metaphosphate for dispersion of soils for mechanical analysis,” Soil Sci. Soc. Am., Proc. 4, 106–113 (1940).
S. Uziak, “The mineral composition of clay fractions from a fossil loess soil,” Pol. J. Soil Sci. 10 (2), 157–164 (1977).
T. Vaasma, “Grain-size analysis of lacustrine sediments: a comparison of pre-treatment methods,” Est. J. Ecol. 57 (4), 231–243 (2008).
C. H. M. van Bavel, “Mean-weight diameter of soil aggregates as a statistical index of aggregation,” Soil Sci. Soc. Am. J. 14, 20–23 (1950.
H. van Olphen, An Introduction to Clay Colloid Chemistry: For Clay Technologists, Geologists, and Soil Scientists (Wiley, New York, 1977).
J. Vandenberghe, “Grain size of fine-grained windblown sediment: a powerful proxy for process identification,” Earth-Sci. Rev. 121, 18–30 (2013).
J. Vieillefon, “Contribution to the improvement of analysis of gypsiferous soils,” in Management of Gypsiferous Soils (Food and Agriculture Organization, Rome, 1997).
M. von Lützow, I. Kögel-Knabner, K. Ekschmitt, H. Flessa, G. Guggenberger, E. Matzner, and B. Marschner, “SOM fractionation methods: relevance to functional pools and to stabilization mechanisms,” Soil Biol. Biochem. 39 (9), 2183–2207 (2007).
M. von Lützow, I. Kögel-Knabner, K. Ekschmitt, E. Matzner, G. Guggenberger, B. Marschner, and H. Flessa, “Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review,” Eur. J. Soil Sci. 57 (4), 426–445 (2006).
E. K. Walton, W. E. Stephens, and M. S. Shawa, “Reading segmented grain-size curves,” Geol. Mag. 117 (6), 517–524 (1980).
Y. J. Wang, C. B. Li, W. Wang, D. M. Zhou, R. K. Xu, and S. P. Friedman, “Wien effect determination of adsorption energies between heavy metal ions and soil particles,” Soil Sci. Soc. Am. J. 72 (1), 56–62 (2008).
W. Weipeng, L. Jianli, Z. Bingzi, Z. Jiabao, L. Xiaopeng, and Y. Yifan, “Critical evaluation of particle size distribution models using soil data obtained with a laser diffraction method,” PloS One 10 (4), 1–18 (2015).
G. J. Weltje and M. A. Prins, “Genetically meaningful decomposition of grain-size distributions,” Sediment. Geol. 202 (3), 409–424 (2007).
R. Westerhof, P. Buurman, C. van Griethuysen, M. Ayarza, L. Vilela, and W. Zech, “Aggregation studied by laser diffraction in relation to plowing and liming in the Cerrado region in Brazil,” Geoderma 90 (3–4), 277–290 (1999).
J. H. M. Wösten, Y. A. Pachepsky, and W. J. Rawls, “Pedotransfer functions: bridging the gap between available basic soil data and missing soil hydraulic characteristics,” J. Hydrol. 251 (3), 123–150 (2001).
F. Yang, G. L. Zhang, F. Yang, and R. M. Yang, “Pedogenetic interpretations of particle-size distribution curves for an alpine environment,” Geoderma 282, 9–15 (2016).
G. Yuan, M. Soma, H. Seyama, B. K. G. Theng, L. M. Lavkulich, and T. Takamatsu, “Assessing the surface composition of soil particles from some podzolic soils by X-ray photoelectron spectroscopy,” Geoderma 86 (3), 169–181 (1998).
Z. Yutong, X. Qing, and L. Shenggao, “Distribution, bioavailability, and leachability of heavy metals in soil particle size fractions of urban soils (northeastern China),” Environ. Sci. Pollut. Res. 23 (14), 14600–14607 (2016).
H. Zhang and P. R. Bloom, “Dissolution kinetics of hornblende in organic acid solutions,” Soil Sci. Soc. Am. J. 63 (4), 815–822 (1999).
T. M. Zobeck, “Rapid soil particle size analyses using laser diffraction,” Appl. Eng. Agric. 20 (5), 633–639 (2004).
ACKNOWLEDGMENTS
This study was supported by the Russian Foundation for Basic Research, project no. 18-34-00825. The authors are grateful to Dr. N.B. Khitrov and Dr. E.B. Skvortsova for fruitful discussions and valuable comments and questions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Konyushkov
Rights and permissions
About this article
Cite this article
Yudina, A.V., Fomin, D.S., Kotelnikova, A.D. et al. From the Notion of Elementary Soil Particle to the Particle-Size and Microaggregate-Size Distribution Analyses: A Review. Eurasian Soil Sc. 51, 1326–1347 (2018). https://doi.org/10.1134/S1064229318110091
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229318110091