Abstract
Electrical resistivity and magnetic susceptibility of Co48Fe25Si4B19Nb4 + REM (REM = Nd, Sm, Tb, Yb) alloys in crystalline and liquid states are studied. Experimental data were used to calculate the electronic characteristics (effective magnetic moment, density of states at the Fermi level, and paramagnetic Curie temperature) of melts. It is shown that all the rare-earth metals considered must increase the glass-forming ability of alloys, but neodymium and samarium can have the maximum effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cobalt- and iron-based alloys, especially Co–Fe–Si–B—Nb compositions, are being actively studied due to the electrical and magnetic properties exhibited in their amorphous state [1]. These alloys are used as highly sensitive detectors and sensors and are soft magnetic materials with a high coercive force not exceeding 2 A/m [2]. One disadvantage of these materials that hinders their widespread use is a relatively low glass-forming ability (GFA) [3]. Methods of calculating GFA have been addressed in many works [4–8]. Unfortunately, the current criteria of the GFA do not have a predictive ability. First, it is required to obtain high-quality amorphous samples (rods or ribbons) and to determine their characteristic temperatures (solidus, liquidus, glass transition point, etc.) with high accuracy, and only after that is it possible to calculate the GFA.
On the other hand, it was shown in [9, 10] that the study of the electrical and magnetic properties of the initial alloys in the liquid state and the determination of their electronic characteristics may be a promising method for a priori determination of the glass-forming ability even before the preparation of amorphous samples.
One way to increase the GFA of alloys is by microalloying them. In this work, we present the results of analysis of electrical resistivity and magnetic susceptibility of Co48Fe25Si4B19Nb4 + REM alloys (REM = Nd, Sm, Tb, Yb) in crystalline and liquid states. The concentration of rare-earth metals was 1 and 2 at %. Based on the experimental results, the electronic characteristics—the density of states at the Fermi level N(EF); the effective magnetic moment per atom in the alloy μeff; and paramagnetic Curie temperature θ—were calculated.
EXPERIMENTAL
Alloys of the composition Co48Fe25Si4B19Nb4 (master alloy, MA) with small additions of rare-earth metals (REM = Nd, Sm, Tb, Yb) with concentrations of 1 and 2 at % were obtained by remelting the initial components in an induction furnace at a temperature of 1700°C for half an hour in an argon atmosphere.
Electrical resistivity ρ of the alloys was measured on an automated experimental setup that implements a contactless method in a rotating magnetic field [11]. This method is relative; therefore, a zone-purified molybdenum single crystal was used as a reference sample. The experiments were carried out in the mode of stepwise heating and subsequent cooling with a temperature step of 25°C and isothermal holdings for 15 min. The experiments were carried out in the range 800–1600°C in a high-purity helium atmosphere. The choice of the temperature range is due to the fact that, below 800°C, the alloys are ferromagnetic and, as a consequence, their resistivity cannot be studied by this method. The samples were preliminarily remelted in a resistance furnace in beryllium oxide crucibles to give them a strict cylindrical shape. According to this method, the electrical resistivity is directly proportional to the ratio of the densities of the test and reference samples; therefore, the accuracy in determining the density of alloys makes a significant contribution to the ρ measuring error. The density was measured on an automated setup that implements the absolute version of the method of penetrating gamma radiation, a detailed description of which is given in [12]. As a result, the total relative error in determining the electrical resistivity was ±3%.
Magnetic susceptibility χ was studied using a setup implementing the Faraday method. The experiments were carried out in the mode of stepwise heating and subsequent cooling with a step of 15–20°C and isothermal holdings for 5 min (the samples for measuring the magnetic susceptibility were much smaller than those for measuring the resistivity). The experiments were carried out in a high-purity helium atmosphere in the temperature range of 800–1500°C. A more detailed description of the setup and the measurement technique is given in [13]. The relative error in determining the magnetic susceptibility did not exceed ±2%. When measuring both resistivity and magnetic susceptibility, BeO crucibles were used.
RESULTS AND DISCUSSION
The typical temperature dependences of electrical resistivity in crystalline and liquid states are shown in Fig. 1.
It was found that, in crystalline state, electrical resistivity of the basic composition (MA) increases upon heating and reaches a maximum at solidus temperature. In this case, the resistivity curve cannot be approximated by a linear function. The process of the onset of melting of the alloy is characterized by the absence of changes in the absolute values of electrical resistivity. An upward jump of ρ is observed closer to the liquidus, but does not begin at solidus, as in the case of density. This is most likely due to the fact that, at such absolute values of resistivity, the mean free path of conduction electrons becomes comparable with the interatomic distance and, therefore, electrical resistivity is mainly determined by the short-range order. This order is not violated in the case of solidus; therefore, no significant anomalies are observed in dependence ρ(T). For all samples, a significant (up to 105°C) overcooling before crystallization was detected, which indicates the “unwillingness” of the melts to form a crystal lattice.
Figure 2 shows the temperature dependences of electrical resistivity of alloys with REM additives in liquid state.
It can be seen from Fig. 2 that, in the liquid state, the resistivity polytherms can be described by an equation of the form
where ρ0 is the value of the electrical resistivity at liquidus temperature TL and β is the temperature coefficient of resistivity (TCR). The coefficients of linear approximation of the electrical resistivity of the alloys in the liquid state are presented in Table 1.
The effect of rare earth metals on the absolute values of electrical resistivity of MA in crystalline (870°C) and liquid (1400°C) states is shown in Fig. 3. The REM additives are arranged in order of increasing atomic number in the lanthanide series.
It was found that the REM additives monotonically increase electrical resistivity of MA in crystalline state and have a complex effect on this property in liquid state: neodymium and samarium decrease ρ, while terbium and ytterbium increase it. In this case, the lowest resistivity at a temperature of 1400°C is observed for the alloys with samarium. Moreover, the addition of samarium to the MA results in the highest TCR among the composition under study.
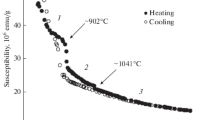
Typical polytherms of magnetic susceptibility are shown in Fig. 4. It was found that χ decreases monotonically with increasing temperature. Small anomalous changes in the properties are observed only at the solidus and liquidus temperatures. This fact indicates that the susceptibility, as well as the electrical resistivity, is mainly determined by the short-range order in the alloys. The electrical resistivity, apparently, remains the same in both solid and liquid states. The heating of the melt to 1300°C is accompanied by the appearance of a hysteresis of the property: the cooling curve lies below the heating curve for all compositions in both liquid and solid states. In addition, at a temperature of about 905°C, the magnetic susceptibility curves exhibit a distinct downward jump χ, after which the temperature dependence of the property becomes flatter. Since this temperature is approximately 100°C lower than the solidus temperature, it is most likely that a bcc–fcc polymorphic transformation occurs here, which is typical of both pure iron and Fe–Co alloys.
REM additives strongly affect the absolute values of the property, and the maximum is attained for samarium (Fig. 5). The REM additives are arranged in the order of increasing their atomic numbers in the lanthanide series.
The temperature dependences of the susceptibility in the liquid state were approximated by the generalized Curie–Weiss law,
and the electronic characteristics of the alloys—the density of states at the Fermi level, N(EF); the effective magnetic moment per atom in the alloy, μeff; and the paramagnetic Curie temperature θ—were calculated. The results are presented in Table 2.
It is worth noting our previously stated hypothesized that the glass-forming ability of the alloy is increased by an additive that increases the paramagnetic Curie temperature in the liquid state [9, 10, 14]. Thus, the results of our studies of the electrical resistivity and magnetic susceptibility suggest that the most effective additives increasing the GFA of the Co48Fe25Si4B19Nb4 alloys will be 2% neodymium and/or samarium.
CONCLUSIONS
Our experimental studies of the electrical resistivity and magnetic susceptibility suggest that the Co48Fe25Si4B19Nb4 + REM melts remain essentially microheterogeneous systems up to 1300°C, which should be taken into account when preparing the melt for quenching. All the rare-earth metals used must increase the glass-forming ability of the alloys, and the greatest effect can be exerted by the addition of neodymium and samarium.
REFERENCES
C. Suryanarayana and A. Inoue, Bulk Metallic Glasses (CRC Press, Boca Raton, 2011).
Q. Man, H. Sun, Y. Dong, B. Shen, H. Kimura, A. Makino, and A. Inoue, Intermetallics 18, 1876 (2010). https://doi.org/10.1016/j.intermet.2010.02.047
M. Aykol, M. V. Akdeniz, and A. O. Mekhrabov, Intermetallics 19 (9), 1330 (2011). https://doi.org/10.1016/j.intermet.2011.05.004
Z. L. Long, W. Liu, M. Zhong, Y. Zhang, M. Zhao, G. Liao, and Z. Chen, J. Therm. Anal. Calorim. 132 (3), 1645 (2018). https://doi.org/10.1007/s10973-018-7050-0
R. Deng, Z. Long, L. Peng, D. Kuang, and B. Ren, J. Non-Cryst. Solids 533, 119829 (2020). https://doi.org/10.1016/j.jnoncrysol.2019.119829
S. Guo and C. T. Liu, Intermetallics 18 (11), 2065 (2010). https://doi.org/10.1016/j.intermet.2010.06.012
Z. L. Long, G. Q. Xie, H. Q. Wei, X. Su, J. Peng, P. Zhang, and A. Inoue, Mater. Sci. Eng., A 509 (1), 23 (2009). https://doi.org/10.1016/j.msea.2009.01.063
G. H. Zhang and K. C. Chou, J. Appl. Phys. 106 (9), 094902 (2009). https://doi.org/10.1063/1.3255952
V. Sidorov, J. Hosko, V. Mikhailov, I. Rozkov, N. Uporova, P. Svec, D. Janickovic, I. Matko, P. Svec Sr, and L. Malyshev, J. Magn. Magn. Mater. 354, 35 (2014). https://doi.org/10.1016/j.jmmm.2013.10.038
V. A. Mikhailov, V. E. Sidorov, and A. A. Sabirzyanov, Russ. Metall. (Metally) 2019, 159 (2019). https://doi.org/10.1134/S0036029519020162
I. G. Brodova, P. S. Popel’, N. M. Bardin, and N. A. Vatolin, Initial Melts as the Basis of Formation of the Structure and the Properties of Aluminum Alloys (Ural. Otd. Ross. Akad. Nauk, Ekaterinburg, 2005) [in Russian].
B. A. Rusanov, E. S. Baglasova, P. S. Popel, V. E. Sidorov, and A. A. Sabirzyanov, High Temp. 56 (3), 439 (2018). https://doi.org/10.1134/S0018151X18020190
N. S. Uporova, S. A. Uporov, and V. E. Sidorov, J. Rare Earths 29 (8), 768 (2011). https://doi.org/10.1016/S1002-0721(10)60539-X
V. E. Sidorov, V. A. Mikhailov, and A. A. Sabirzyanov, Russ. Metall. (Metally) 2016, 109 (2016). https://doi.org/10.1134/S0036029516020166
Funding
This study was supported by the Russian Foundation for Basic Research, project no. 18-03-00433. The work of P. Svec Sr. and D. Janickovic was carried out as a part of projects VEGA 2/0144/21 and APVV-19-0369.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Chernokozhin
Rights and permissions
About this article
Cite this article
Rusanov, B.A., Sidorov, V.E., Mikhailov, V.A. et al. Electrical Resistivity and Magnetic Susceptibility of CoFeSiBNb + REM Alloys at High Temperatures. Tech. Phys. 66, 1348–1352 (2021). https://doi.org/10.1134/S1063784221080132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063784221080132