Abstract
The results of the growth of bulk crystals of aluminum and gallium nitrides on foreign seeds by the sublimation sandwich method are reviewed. The kinetics and mechanism of sublimation and condensation are analyzed depending on the growth conditions, vapor phase composition, crystal orientation, and the distance between the source and the seed. It is experimentally found that during joint annealing of aluminum nitride and silicon carbide, the sublimation rate of aluminum nitride substantially increases due to the formation of a liquid phase on the crystal surface. The inhomogeneous distribution of the liquid phase, localized mainly near structural and morphological defects, leads to the selective nature of surface etching and causes a deterioration in the quality of the growing crystal. A process of growth of bulk AlN crystals with simultaneous seed evaporation was implemented, which yields crystals without cracks and with improved parameters. Bulk crystals of aluminum nitride and gallium nitride up to 2 inches in diameter were grown on SiC seeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Group III metal nitrides (AlN, GaN, InN and their solid solutions) have a unique combination of physical properties that guarantee their relevance in modern micro- and optoelectronics [1]. These properties include a large bandgap, a high saturation drift velocity, high breakdown voltage, high thermal conductivity, excellent chemical and thermal resistance, etc. Due to these characteristics, nitrides are currently considered the most promising materials for creating powerful high-frequency transistor structures capable of functioning at high temperatures and in aggressive environments. Devices of this type can be used in wireless communication systems, in switching systems in the energy sector, for quick detection of chemical or biological contamination, etc. However, the highest expectations in connection with the development of nitride technologies relate to solid-state light sources, including short-wavelength LEDs and lasers of the visible and UV ranges, as well as basic UV elements of bright, white LEDs. Their use is also relevant for the manufacture of high-power high-frequency transistors and UV photodetectors that can operate at elevated temperatures and in aggressive environments. To benefit from these undoubted advantages of nitrides, it is necessary to ensure the growth of bulk crystals of high quality, with a diameter of 50 mm or more, for use as substrates.
Several publications are devoted to the growth of bulk crystals of nitrides, which are discussed in excellent reviews (see, for example, [2]). The disadvantages of most of these methods are due to the relatively low growth temperatures at which there is the probability of formation of a large number of individual crystallization centers on the surface of the substrate. In addition, growth is carried out in the presence of chemically active additives that create an impurity chemosorbate, which usually impedes the lateral movement of atoms over the surface. The high concentration of impurities also leads to their partial capture by the crystal with the formation of inclusions of the second phase. The sublimation method of growth (physical vapor transport, PVT), in which transporters are not used, is from these shortcomings. Therefore, PVT growing ensures the growth of single crystals at higher rates than other methods [2].
The main problem in creating a new generation of nitride-based devices is the lack of high-quality GaN and AlN seeds of the required size.
In this paper, we report the PVT growth of bulk crystals of aluminum and gallium nitrides by the sublimation sandwich method (SSM) [3]. We analyze the kinetics and mechanism of sublimation and condensation of nitrides in the sandwich cell depending on the state of the source and process conditions in order to optimize the crystallization conditions. It is shown that the formation of a liquid layer on the surface of the source and the seed enables one to increase the mass transfer efficiency and improve the quality of the growing crystal.
2 EXPERIMENTAL
The apparatus for growing AlN and GaN crystals has been described previously [4, 5]. Due to the low decomposition temperature of gallium nitride, the growth of GaN crystals is carried out in an open system under ammonia at a temperature of 1100–1200°C.
Bulk AlN crystals are grown in closed containers made of carbidized tantalum under nitrogen at a temperature of 1950–2100°C. The nitrogen pressure ranged from 10–2 to 10 atm.
Monocrystalline seeds made of silicon carbide of polytypes 4H or 6H in the form of disks 55–60 mm in diameter are commonly used for growing ingots.
As a sublimation source, gallium nitride or aluminum nitride powder is used. However, in this case, the condensation rate is not high enough and decreases sharply with time. An increase in the growth rate can be achieved by adding metallic gallium or aluminum to the source [5]. Moreover, the sources of metallic gallium turned out to be the most promising for the growth of gallium nitride.
The dependence of the growth rate on the distance to the seed (Δx) for such a source is shown in Fig. 1. It is seen that sufficiently high growth rates are achieved only at relatively small Δx, of the order of 2–4 mm. Theoretical analysis showed that the experimentally observed dependence of V(Δx) is not explained in terms of the model of mass transfer in the diffusion mode. In this case, the experimentally observed growth rate is almost an order of magnitude higher than the calculated rate of gallium evaporation. The results were explained by the fact that when the gallium melt is heated under ammonia, the liquid surface loses stability, and gallium is sprayed and falls on the seed surface in the form of individual drops. Thus, mass transfer of gallium is ensured, which then binds to nitrogen, forming a gallium nitride layer. It is essential that the presence of liquid gallium on a growing surface promotes the qualitative growth of a GaN crystal with extremely high rates (up to 1 mm/h) [5]. The high quality of the crystals is confirmed by the results of X-ray diffraction measurements, as well as by the analysis of the luminescence spectra and electron-paramagnetic resonance (EPR) [5].
A sharp increase in the growth rate is also observed during the sublimation growth of aluminum nitride, when aluminum melt is used as a source [6]. However, such a process is difficult to carry out for obtaining bulk crystals, due to the high aggressiveness of liquid aluminum with respect to the material of the container.
It was further shown [5] that the effect of increasing the growth rate also occurs when using an aluminum nitride source into which silicon or silicon carbide is added. In particular, when silicon carbide is added to the source, the sublimation rate of aluminum nitride increases by 5–10 times, which makes it possible to decrease the sublimation growth temperature of aluminum nitride by 150–200°C in comparison with its growth under pure conditions [2].
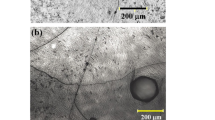
An additional study revealed the existence of a liquid phase on the surface of both the source and the seed. For example, under rapid cooling, droplet-like formations were found on the surface (Fig. 2), which were located in recesses (pits). The sizes of the recesses increased with the cooling time; often, they transformed into very long grooves or holes. A study of the Raman spectra revealed that free silicon is included in the droplets.
Nitrogen pressure has a significant effect on the transfer of matter from the source to the seed in the sandwich system (Fig. 3). It is seen that the most complete transfer, at which Kt = 1 (where Kt is the AlN transfer coefficient) is achieved only at a relatively high nitrogen pressure (above 0.2 atm). The decrease in transfer efficiency is obviously due to the low coefficient of sticking of molecular nitrogen to the seed surface [7]. The transfer coefficient also decreases with decreasing temperature difference between the source and the seed (Fig. 4).
3 RESULTS AND DISCUSSION
It is known that the sublimation of nitrides is a rather complicated process, and the correlation of experimentally observed evaporation rates with calculated data requires the introduction of kinetic constraints in the form of evaporation coefficients. Evaporation coefficients for nitrides are abnormally low and are in the range 10–3 to 10–5 [8]. However, the real system contains impurities, primarily oxygen and carbon, which can significantly affect the mass transfer of aluminum nitride.
Gallium nitrides and, especially, aluminum nitrides are easily oxidized in air. Therefore, commercial nitride powders used as a source always contain a large amount of oxygen (up to 10%).
We studied the sublimation rate of polycrystalline aluminum nitride of various origins. The evaporated powder was placed in TaC containers intended for the growth of AlN crystals. The annealing temperature (Ta) ranged from 1800 to 2300°C; the annealing duration ranged from 1 to 10 h. At a temperature of 1800°C, annealing was carried out in vacuum, and at higher temperatures, the crystals were annealed under nitrogen. The evaporation rate was controlled by changes in the weight and thickness of the initial crystal.
It is assumed that the equilibrium vapor above gallium nitride and aluminum nitride consists only of metal atoms and nitrogen molecules (N2). In the case of aluminum nitride, at growth temperatures of 1900–2300°C, the aluminum vapor pressure is quite high, and it is assumed that the aluminum capture factor is close to unity. In contrast, the capture factor of molecular nitrogen is rather small, of the order of 10–5 to 10‒6, at the aluminum nitride growth temperatures [9].
It is known that nitrogen molecules are extremely strong; their lifetime on the surface of a growing crystal is extremely short, which leads to low condensation coefficients of N2 molecules during sublimation growth. Namely the low efficiency of nitrogen capture is the reason that the sublimation growth of nitrides (in contrast to the growth of SiC) is difficult to achieve in a vacuum or under an inert gas. Usually, nitride crystals are grown under nitrogen (N2 or NH3), at relatively high partial pressures of nitrogen (significantly higher than the equilibrium pressure); therefore, in the case of gallium nitride, sublimation growth is possible only with the introduction of active nitrogen (NH3).
Low nitrogen capture factors lead to the expansion of the range of nitrogen pressures at which the growth rate is limited by surface reactions rather than by diffusion transport through the vapor phase. For a process limited by diffusion, the relation of V = AP–1 is fulfilled, where V is the growth rate, P is the nitrogen pressure, and A is a constant depending on the growth conditions and the surface state. Under molecular kinetic growth conditions, either the dependence on pressure is weaker or the rate is independent of pr-essure.
When analyzing the kinetics of mass transfer, the effect of various factors should be taken into account. First, theoretical and experimental data indicate that aluminum and gallium nitrides with a high oxygen content have the highest evaporation rate. During annealing, the oxygen concentration decreases markedly, which leads to a change in the sublimation rate.
The resublimated AlN source, as the purest in oxygen, was characterized by a minimum evaporation rate. Thus, the conclusions of theoretical studies are confirmed; namely, oxygen accelerates mass transfer, being an AlN transporter. Further studies of the growth of aluminum nitride using a source with different oxygen contents showed that the quality of the growing crystal deteriorates with increasing oxygen content in the source. Therefore, it is desirable to use the purest source for growing.
Second, the sublimation rate of aluminum and gallium nitrides is affected by the introduction of metals, that is, aluminum or gallium, as well as silicon, which are in the liquid state at the growth temperature.
The studies also revealed a significant increase in the sublimation rate of aluminum nitride upon introduction of silicon carbide into the system.
The presence of a liquid phase on the surface seems the most correct explanation for accelerated sublimation and, hence, the growth of nitrides. The liquid phase, obviously, is formed both on the evaporated and on the growth surfaces. It can exist on the surface either in the form of a thin layer or in the form of individual drops. The formation of a uniform thin layer is generally favorable for crystal growth, since the coalescence of emerging nuclei is facilitated and, consequently, their density is decreased, which contributes to the layer-by-layer growth mechanism (Fig. 5b). However, the appearance of droplets on the growth surface, observed when the temperature decreases during the cooling stage, apparently leads to the formation of pores and grooves due to the selective etching of the growing and evaporated surfaces (Fig. 2).
4 CONCLUSIONS
The growth conditions, composition of the vapor phase, and the required gap between the source and substrate are considered. In the presence of silicon carbide, the sublimation rate of aluminum nitride significantly increases due to the formation of a liquid phase on the surface. The process of growth of bulk AlN crystals with simultaneous evaporation of the substrate was implemented, which made it possible to obtain crystals of better quality without cracks. Bulk AlN crystals with a diameter of up to 2 inches were grown on SiC substrates. The results of obtaining bulk crystals of aluminum and gallium nitrides on foreign substrates using the sublimation sandwich method are reviewed. The kinetics and mechanism of sublimation–condensation of these nitrides are considered.
REFERENCES
H. Markoc, S. Strite, G. B. Gao, M. E. Lin, B. Sverdlov, and M. Burns, J. Appl. Phys. 76, 1363 (1994).
C. Hartmann, A. Dittmar, J. Wollweber, and M. Bickermann, Sci. Technol. 29, 084002 (2014).
Yu. A. Vodakov and E. N. Mokhov, UK Patent No. 1458445 (1977).
O. V. Avdeev, T. Yu. Chemekova, H. Helava, M. G. Ramm, Yu. N. Makarov, E. N. Mokhov, S. S. Nagalyuk, and A. S. Segal, in Comprehensive Semiconductor Science and Technology (Elsevier, Amsterdam, 2011), p. 282.
E. N. Mokhov and A. A. Wolfson, in Single Crystals of Electronic Materials: Growth and Properties, Ed. by R. Fornari (Woodhead, Cambridge, MA, 2018), p. 401.
P. T. Wu, M. Funato, and Y. Kawakami, Sci. Rep. 5, 17405 (2015).
S. Yu. Karpov, A. V. Kulik, M. S. Ramm, E. N. Mokhov, A. D. Roenkov, Yu. A. Vodakov, and Yu. N. Makarov, Mater. Sci. Forum. 353–356, 779 (2001).
Z. Y. Fan and N. Newman, Mater. Sci. Eng. B 87, 244 (2001).
S. G. Mueller, R. T. Bondokov, K. E. Morgan, G. A. Slack, S. B. Schujman, J. Grandusky, J. A. Smart, and L. J. Schowalter, Phys. Status Solidi A 206, 1153 (2009).
Funding
The study was supported by the Russian Foundation for Basic Research, project no. 19-02-00649-a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Mokhov, E.N., Wol’fson, A.A. & Kazarova, O.P. Growing Bulk Aluminum Nitride and Gallium Nitride Crystals by the Sublimation Sandwich Method. Phys. Solid State 61, 2286–2290 (2019). https://doi.org/10.1134/S1063783419120321
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783419120321