Abstract
Using high-performance differential scanning calorimetry, crystallization processes in pure metals and some binary alloys on their basis have been investigated. Heat flow jumps are detected at crystallization upon reaching the liquidus lines. It is assumed that these phenomena are associated with the formation of large amounts of local microvolumes (concentration fluctuations) enriched with a component causing crystallization in the liquid. Their appearance precedes the spontaneous processes of the formation and growth of the crystals of a new phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The theoretical analysis of crystallization processes goes back to Kurdyumov, Meyl, and Mirkin [1, 2], who described the kinetics of this process. Further, the analysis was based on the ideas of Gibbs, which made it possible to write equations for the rate of formation of crystallization nuclei and their further growth [1–3]. Typically, the crystallization of single-component liquids was studied. For two-component liquids, the crystallization of alloys of noneutectic composition has not been sufficiently developed in theoretical studies, except for specific cases, which are described in various reviews (see, for example, [4, 5]). Experimental studies are mainly represented by the classical works of Tamman [3, 4] and the Davidenkov’s group [1], which were carried out for the crystallization of organic liquids, and Tarnball studied the crystallization of metals [3, 4].
Undoubtedly, great achievements are associated with the experiments devoted to the construction of phase diagrams of metal alloys, which made it possible to determine the area of existence of separate phases or structural components. Starting from Kurnakov [6], physical methods are widely used for monitoring the processes of crystallization or collapse of supersaturated solid solutions upon studying phase transitions. The most commonly used methods among them are the methods of differential thermal analysis (DTA), which allow one to determine, in some cases precisely enough, the beginning and completion of structural and phase transformations. However, such studies do not provide information about the processes taking place in the intercritical temperature range.
Theoretical approaches to the description of the processes of solidification, nucleation, and growth of crystals of a new phase are based on perceptions about the change of the system’s free energy upon the transition from the liquid to the crystalline state. However, the number of experimental studies devoted to the study of calorimetric effects upon crystallization of binary alloys is relatively small.
In the last decade, experimental techniques and instruments for high-performance differential scanning calorimetry (DSC) have been developed, which allowed one to revisit experimental studies in this field. Nevertheless, the studies were focused on crystallization of amorphous metallic alloys that represent, in most cases, multicomponent systems [7–9]. A substantially less number of DSC studies were devoted to crystallization processes in melts. But even in this case, the crystallization of multicomponent alloys for purely industrial applications was considered, and the technology issues were the priority (see, for example, [10, 11]). The study [12], in which the DSC analysis of crystallization of hypoeutectic, eutectic, and hypereutectic alloys of the Al–Si system was performed, can be considered as one exception. But even in this study, the primary emphasis was made on the influence of modifying additives on the thermal effects during crystallization and grinding the structure of alloys.
In the present study, the application of these techniques for such simple systems, a common type of which can be represented by the second-order phase diagram, for example, the phase diagram of the Bi–Sn system, are considered for the first time. For such systems, differential scanning calorimetry of alloys with a relatively low content of one component and not prone to a eutectic reaction upon cooling (type I) is carried out. Types II, III, and IV refer to alloys of hypoeutectic, eutectic, and hypereutectic compositions.
EXPERIMENTAL
Pure metals (Sn, Bi, Pb, Zn, Al, Ag, Au, and Cu) and binary alloys of the Pb–Sn, Pb–Bi, Bi–Sn, and Al–Cu systems represented by duralumin alloys D1 (4.1% Cu, 0.7% Mg, and 0.8% Mn) and D19 (4.0% Cu, 1.7% Mg, and 0.5% Mn) were chosen as objects of the study.
The differential scanning calorimetry data were obtained using a Jupiter 449 STA instrument from Netzsch. The heating and cooling cycles were carried out at rates of 5 and 10 K/min in the argon atmosphere (99.999% Ar). The gas flow rate was 25–30 mL/min. The data obtained in DSC experiments were processed using the Proteus Analyses and Fityk software packages.
To avoid the risk of emerging artifacts caused by the peculiarities of the experiment in the study of alloys, the experimental data were smoothed using an eighth-order polynomial. The same technique was used to analyze the first (DDSC) and second (DDDSC) derivatives of the DSC signal DSC with respect to temperature. As shown in the experiments with pure aluminum (99.999 wt % of Al), the temperature ranges and thermal effects of transitions determined before smoothing the experimental curves and after their approximation are almost equal. The discrepancy was about 1%. The results provided in this communication refer to a cooling rate of 10 K/min.
RESULTS AND DISCUSSION
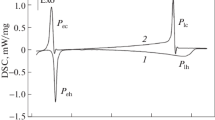
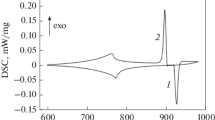
The DSC curve obtained at cooling of an alloy of the eutectic composition and its second derivative with respect to temperature are shown in Fig. 1. On the exterior, the two dependences are quite similar to the behavior of DSC and DDDSC signals upon crystallization of a single-component alloy (see Fig. 2). This is not surprising, since a eutectic reaction is characterized by an invariant equilibrium in the phase equilibrium diagrams of binary alloys.
The situation is totally different for crystallization of binary alloys of the first type (see Figs. 3 and 4). Upon reaching the liquidus line, a heat release jump is observed. The shape of the first derivative of the DSC signal in this area indicates the same. If the lever rule is used to determine the amount of a new phase formed directly upon crossing the liquidus line, then the amount of a solid phase is relatively low. From this point of view, the appearance of the latter should not be accompanied by such a notable heat release jump, since the heat flow is determined by the heat of transition and the volume fraction of a formed new phase (see, for example, [5]).
Moreover, if the dependence of the volume fraction of a crystallizing phase on the cooling temperature is constructed using the lever rule in the intercritical range of temperatures (the cooling rate is constant), then the reduced volumetric rate of transition should pass through a maximum in the temperature range between the liquidus and solidus lines. The DSC data do not confirm that.
In principle, the same pattern is observed also upon crystallization of alloys of the hypoeutectic composition, which undergo a eutectic reaction upon cooling from the liquid state (type II). An example of the DSC dependence for this case is given in Fig. 5.
The character of changing the DSC signal during a eutectic reaction (peak Pe) in this alloy is different from the data given in Fig. 1. There is noticeable asymmetry of the peak in the DSC dependence and the trend towards accelerated heat release is recorded upon reaching the line of a eutectic reaction. It can be assumed that the presence of one of the phases precipitated in the liquid when crossing the liquidus line creates favorable conditions for the crystallization of another phase also involved in a eutectic reaction.
Largely similar behavior of the DSC signal is observed also upon cooling an alloy of the hypereutectic composition (see Fig. 6). In this case, a heat release jump and some deformation of the DSC signal in the course of a eutectic reaction are also recorded upon reaching the liquidus line. Moreover, it might be assumed again that such a feature is associated with the active precipitation of another phase involved in the formation of the eutectic.
The described patterns are observed also upon crystallization of other binary alloys, such as the Pb–Sn, Pb–Bi, Bi–Sn, and Al–Cu alloys (see [12–14]). It should be noted that the same trends in changing the DSC and DDSC signals upon crystallization of alloys of the Al–Si system were observed in [12], but the above patterns were not discussed at all there.
The following explanation can be proposed for the determined trends. To initiate the precipitation of crystals, the composition of which markedly differs from the average composition of components in the liquid state, the layering into regions with relatively low and relatively high, close to the composition of emerging crystals, concentrations of one of the components should occur in this liquid upon approaching the liquidus line. Something close to the spinodal decay of supersaturated solid solutions takes place [15].
Upon crossing the liquidus line, a large number of crystallization sites are spontaneously formed in such volume regions already “prepared” to the emergence of a new phase. A significant part of the crystallizing liquid is involved in this process. Altogether, this presumably leads to intense heat release upon crossing the liquidus line.
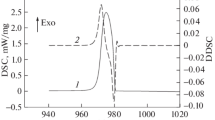
The PL peak on the cooling DSC curves of alloys of type I can be represented as a superposition of two overlapping subpeaks (see Fig. 7). The high-temperature subpeak having the abrupt front on the right is apparently associated with the emergence of new crystals, while the second subpeak, low-temperature and more symmetrical, is driven by the growth of crystals. This fact can be considered some sort of confirmation of the perceptions developed in the present work about the peculiarities of crystallization processes in binary alloys obeying the phase equilibrium diagrams of types I−III.
Structure of the exothermic peak given in Fig. 4: dots show the experimental data, Papp is the result of approximation, and P1 and P2 are subpeaks.
The following considerations can be provided to confirm the above assumptions.
According to the widely accepted perceptions, the maximum rate of nucleation of crystallization sites and the maximum crystal growth rate are observed at temperatures significantly lower than the temperature of the start of crystallization [3–5]. This is considered to be reasonable for single-component liquids.
In the context of the above, it seems appropriate to stay on trying to theoretically determine the crystal nucleation rates.
One of the attempts is connected with the studies by Frenkel (see [1, 2]), according to which the rate of nucleation of crystallization sites can be expressed as a product of two exponential functions that depend on the degree of supercooling, ΔT. In this case, the maximum rate of nucleation of crystallization sites lies in the range 1/2Ts < ΔT < 2/3Ts, where Ts is the melting temperature in K.
Another approach was proposed by Chalmers [3, 4]. It is anticipated that the number of nuclei comprised of ni atoms is determined by the following expression:
where n is the total number of atoms per unit volume, and ΔG is the free energy change upon the formation of a crystal nucleus.
If we assume that each nucleus of the critical size grows into a crystal, then it can be excluded from the statistical size distribution of clusters. Therefore, the corresponding rate of formation of nuclei is determined by the value, starting from which smaller clusters reach the critical size.
Chalmers has proposed the following expression for the nucleation rate:
where \(n_{s}^{*}\) ≈ 100 is the number of atoms of a liquid that are in contact with the surface of the critical nucleus, ε ~ 1/6 is the probability of hopping an atom in the specified direction, \({{{v}}_{L}}\) ~ 1013 is the frequency of atomic vibrations, and \(\Delta G_{d}^{*}\) ~ kT is the free energy of activation of diffusion processes in the melt.
If this equation is shown graphically, then one can see a rapid increase in the rate of formation of crystal nuclei at a supercooling degree of about 0.2Ts, which is so sensitive to the value of the exponential term in this equation that a change in the preexponential factor by several orders of magnitude does not have a significant effect on the degree of supercooling.
The results of applying the method of dispersing samples of a crystallizing liquid into microscopic droplets were generalized by Tarnball [3], and it was found that ΔTmax > 0.15Ts for most liquids and ΔTmax ~ 0.18Ts for metals with FCC and BCC lattices. This is close enough to the theoretical analysis of the matter. The reviews devoted to solidification of liquids, which have been published recently (see [4, 5]), barely changed the perceptions about the nucleation rate of crystallization sites.
The conceptual feature of studies devoted to determination of the rate of formation of crystallization sites and the rate of their subsequent growth is that they involve the performing of isothermal experiments with a specified degree of supercooling, ΔT. Though even in these monographs, the processes taking place upon crystallization of binary alloys are practically not considered. As for the provided data on crystallization of eutectic melts, they concern the growth rate of cells with alternating precipitations of two simultaneously crystallizing phases rather than the rate of nucleation of new crystals (see, for example, [1–5]).
A completely different situation arises when the melt is cooled continuously. Upon reaching the temperature of crystallization of a single-component alloy, an invariant equilibrium is achieved in accordance with the phase rule. An idle point is observed in the curves describing the variation of temperature in time when cooling the liquid phase. According to our data, the maximum crystallization rate upon cooling single-component liquids of pure metals is characterized at a cooling rate of 10 K/min by the following sequence of values: 0.021\(T_{s}^{*}\) for Sn, 0.015\(T_{s}^{*}\) for Bi, 0.021\(T_{s}^{*}\) for Pb, 0.026\(T_{s}^{*}\) for Zn, 0.015\(T_{s}^{*}\) for Al, 0.012\(T_{s}^{*}\) for Ag, 0.011\(T_{s}^{*}\) for Au, and 0.013\(T_{s}^{*}\) for Cu. Herein, \(T_{s}^{*}\) is the melting temperature determined from the DSC data. In [5], it is given as equal to 0.15Ts.
Such a small degree of supercooling allows one to imply that the rate of nucleation of crystallization sites is not a limiting factor in crystallization of single-component liquids. If this assumption is correct, then it follows that numerous clusters with the arrangement of atoms, which is close to the spatial arrangement of atoms in the crystal, emerge in the liquid phase when approaching the temperature of crystallization.
According to Chalmers [3–5], such a cluster should consist of about 100 atoms. Frenkel’s theory [1, 2] gives the critical nucleus size, which is larger by several orders of magnitude (104 atoms). However, not only theoretical calculations, but also some experiments suggest (see [1–5]) that the short-range order in the arrangement of atoms in the liquid agrees with their arrangement in the crystalline phase. Here, it also can be mentioned the idea of the existence of cybotactic groups of atoms that are close to their arrangement in the crystal. These perceptions are integrated to a certain extent in [5].
Once, Poisson drew attention to the impediment of the crystallization process in the cases when the composition of the crystals is different from the composition of the liquid phase [1, 16]. The above-discussed crystallization theory adequately covers only those processes, in which the composition of the solid phase coincides with the composition of the crystallizing liquid, namely, the crystallization of melts of pure metal and homogeneous chemical compounds. Only in these cases, the probability of formation of an equilibrium crystal nucleus is determined solely by a need to create an interface between the solid and liquid phases, and equals the probability of an energy fluctuation.
If the average number of molecules or atoms of a crystallizing substance in the volume of a liquid, which equals the volume of an equilibrium nucleus, is denoted by ε and the number of the same molecules (atoms) in the crystallized nucleus by j, then the probability of the existence of a corresponding fluctuation is expressed by the Poisson equation [16]:
Thus, the liquid phase is fragmented into a huge number of clusters, in which the structure and the ratio of components are close to the counterparts for the emergent crystalline phase, not only in the case of crystallization of a single-component liquid, but primarily upon crystallization of binary melts near the crystallization temperature (liquidus line). As a result, numerous crystals appear in a significant volume of the liquid phase upon crossing the liquidus line. The spontaneous emergence of a large number of such crystals gives rise to the observed calorimetric effects. The lever rule indicates that a quite insignificant amount of a new phase appears immediately after crossing the liquidus line. Therefore, its application does not explain the patterns established in this study.
For alloys of the eutectic composition, the positions of liquidus and solidus lines can be determined from the data of differential thermal analysis (DTA), and this is implemented in most cases when constructing the phase equilibrium diagrams of binary alloys. But the DTA methods do not give any information on the processes taking place in the temperature range between the liquidus and solidus lines. At the same time, it is rather problematic to carry out in-situ structural studies at elevated and, not to mention, very high temperatures.
In this respect, the high-performance DSC methods allow one, as shown in this paper, to supplement the existing perceptions about the crystallization patterns of binary alloys of different compositions in the phase equilibrium diagrams of types I−III.
It should be deemed that the patterns observed in this study, i.e., a heat release jump, can take place also upon decay of supersaturated solid solutions. Some experimental evidences of that are obtained in [13].
CONCLUSIONS
The degrees of supercooling that correspond to the maximum rate of releasing the transformation heat. The degree of supercooling is less than 0.02\(T_{s}^{*}\). This may indicate that the maximum rates of nucleation and growth of crystals are observed at low supercooling degrees near the temperature of crystallization.
It is shown that the rate of releasing the crystallization heat dramatically increases near the liquidus line upon crystallization of alloys based on the Pb–Sn, Pb–Bi, Bi–Sn, and Al–Cu systems and then fades away upon depletion of the liquid phase.
It is suggested that the start of crystallization of binary alloys upon approaching the liquidus line is connected with the formation of local microvolumes and clusters, whose appearance precedes the processes of formation and growth of crystals of a new phase, and which are enriched with a component that causes crystallization in the liquid. Rapid development of the crystallization process is accompanied by an active evolution of heat immediately after crossing the liquidus line.
REFERENCES
Ya. S. Umanskii, B. I. Finkel’shtein, and M. E. Blanter, Physical Metallurgy (Metallurgizdat, Moscow, 1958) [in Russian].
Ya. S. Umanskii and Yu. A. Skakov, Physics of Metals (Atomizdat, Moscow, 1978) [in Russian].
H. Biloni and W. J. Boetinger, in Physical Metallurgy, 4th ed., Ed. by R. W. Cahn and P. Haasen (North-Holland, Amsterdam, 1996), p. 669.
M. E. Glicksman, Principles of Solidification: An Introduction to Modern Casting and Crystal Growth Concepts (Springer, 2011).
W. Kurz and D. J. Fisher, Fundamentals of Solidification, 4th ed. (Trans. Tech. Publ., Switzerland, 1998).
N. S. Kurnakov, Introduction to Physicochemical Analysis, 4th ed. (Akad. Nauk SSSR, Moscow, 1940) [in Russian].
K. Suzuki, H. Fujimori, and K. Hashimoto, Amorphous Metals (Butterworths, London, 1982; Metallurgiya, Moscow, 1987).
B. Cantor, J. Therm. Anal. 42, 647 (1994).
L. V. Spivak and A. V. Shelyakov, Bull. Russ. Acad. Sci.: Phys. 73, 1266 (2009).
L. Heusler and W. Schneider, J. Light Met. 2, 17 (2002).
J. Pitkowski, V. Przeliorz, and V. Szymszal, Arch. Foundry Eng. 17, 207 (2017).
J. Pitkowski and B. Gajdzik, MetaBK 52, 469 (2013).
L. V. Spivak and N. E. Shchepina, Fundam. Probl. Sovrem. Materialoved. 11, 376 (2014).
L. V. Spivak, Vestn. Perms. Univ., Ser. Fiz. 1 (26), 9 (2014).
A. V. Skripov and V. P. Skripov, Sov. Phys. Usp. 22, 389 (1979).
E. S. Venttsel’ and L. A. Ovcharov, Theory of Probability and its Engineering Applications, 2nd ed. (Vysshaya Shkola, Moscow, 2000) [in Russian].
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by O. Kadkin
Rights and permissions
About this article
Cite this article
Spivak, L.V., Shchepina, N.E. Calorimetry of Crystallization Processes in Binary Alloys. Phys. Solid State 60, 2598–2603 (2018). https://doi.org/10.1134/S1063783418120272
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063783418120272