Abstract
The interaction between molecules of different types through the thermal radiation emitted by them due to the overlap of spectral lines is considered. This emission results from transitions between vibrational and rotational states of molecules. It is shown that the interaction is governed by Kirchhoff’s law, according to which each molecule is both an emitter and an absorber. Kirchhoff’s law, together with data from the HITRAN bank, which contains information on the parameters of radiative transitions for many molecules, is fundamental for the analysis of the greenhouse effect in the atmosphere. The computer program underlying this analysis uses about two thousand spectral lines taken from the HITRAN databank. The greenhouse effect in the Earth’s atmosphere is associated with a change in the radiative flux to the Earth’s surface as a result of a change in the concentration of one of the greenhouse components. When analyzing this problem within universal climatological models, which are complex computer programs taking into account the primary and secondary atmospheric factors, one neglects the interaction between greenhouse components through the radiation they emit, that is, one neglects Kirchhoff’s law. It is shown that when the variable component is carbon dioxide, this leads to a fivefold error. If this component is atmospheric water molecules, this leads to a threefold error. When the variable parameter is the concentration of nitrogen dioxide, this gives an overestimation of the radiative flux by a factor of 2, while, in the case of ozone, the change in the radiative flux emitted by ozone molecules coincides with the change in the total radiative flux. As follows from the analysis performed, the effect under consideration is associated with the location of the spectra of molecules and the rate of radiative transitions between molecular states. This emphasizes the important role of the HITRAN databank in solving the problems under consideration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
According to Kirchhoff’s law [1], each emitting particle is simultaneously an absorber. In fact, this law reflects the principle of detailed balance for the processes of radiation and absorption [2, 3], which establishes a relationship between the rates of radiation and absorption. Below, as an absorbing medium, we consider a mixture of molecular gases, i.e., a gas consisting of molecules of different types. The nature of the absorption of this medium at a given point is described by the absorption coefficient kω for emission at a given frequency ω, according to the definition of which the ratio 1/kω is the photon mean free path at a given frequency in the medium. Based on Kirchhoff’s law or the detailed balance principle, when analyzing emission processes, we use the absorption coefficient kω as a parameter describing the emission of a molecular gas.
When analyzing emission and absorption processes in a mixture of different atoms or molecules, i.e., particles with different absorption spectra, Kirchhoff’s law is also responsible for the interaction of different components in radiative processes. For the gas system containing molecules of different types, it is possible to determine the thermal radiative fluxes emitted by each component of this gas at a given frequency. Now let us change the concentration of a certain type of molecules. This will lead to a change in the radiative flux emitted by the molecules of this type. One may be tempted to take this change as a change in the total radiative flux. However, this is true only for an optically transparent gas, i.e., for the situation when the photon free path at a given frequency due to absorption by other components is large compared to the size of the gas system. If this condition is not met, then one should take into account Kirchhoff’s law, according to which other components become absorbers in this case. This means that, under the described conditions, along with an increase in the radiative flux at a given frequency due to the considered component, there is a decrease in the radiative flux emitted by other components, since the radiation is partially absorbed by new molecules introduced into the system.
Unfortunately, Kirchhoff’s law in this form is neglected in climatological models when analyzing the greenhouse effect and its variations in the Earth’s atmosphere [4–10]. Climatological models are complex computer programs aimed at describing the behavior of all parameters of the atmosphere at every point in space. One of the blocks of such a program is the calculation of the radiation parameters of the atmosphere. Due to the complexity of the system, Kirchhoff’s law is neglected in this block; that is, the interaction between individual types of molecules through the radiation field is neglected. This leads to incorrect conclusions about the evolution of the thermal state of our planet. These conclusions form the basis of the Paris agreement on climate, which creates a misconception about climate change and the factors influencing it. Incidentally, the same situation occurs in other cases when one neglects physical principles. Below, we will not touch upon this aspect of the problem and will focus on finding out what error can be caused by the neglect of Kirchhoff’s law when analyzing the emission of a gas consisting of molecules of different types.
Let us concentrate on the algorithm for calculating the radiative flux from a plane-parallel layer of a weakly inhomogeneous gas containing molecules of different types. Our goal is to determine the radiative flux emitted by a gas containing emitting and absorbing molecules of different types. For definiteness, we will focus on the Earth’s atmosphere, simulating it by a plane-parallel weakly inhomogeneous layer of gas. Since the air pressure in this case is on the order of atmospheric pressure, the width of an individual spectral line due to certain vibrational–rotational or rotational transitions of molecules is small compared with the frequency difference between adjacent spectral lines. This means that the emission spectrum of the molecular gas layer consists of a large number of peaks in the infrared region, and, to analyze the radiative flux from the molecular gas, one should apply the line-by-line model [11, 12], which requires an analysis of the radiation parameters at each frequency separately.
The interaction of molecules of different types, which is based on Kirchhoff’s law, uses the fact that the radiation emitted by molecules of one type can be absorbed by molecules of another type and vice versa. This means that the interaction is determined by the overlap of the spectra of different molecules, and its analysis requires detailed information on both the behavior of the spectra of the molecules and the parameters of radiative transitions for these molecules. Therefore, the HITRAN databank, due to which such information is available, is of great importance for the processes under consideration [13, 14]. Note that the existence of the HITRAN databank reflects the level of the modern quantum theory of radiation and allows solving problems that could not be solved previously.
2 RADIATIVE FLUX FROM A PLANE-PARALLEL LAYER OF A MOLECULAR GAS
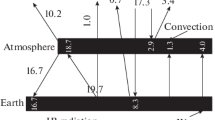
Our goal is to find radiative fluxes from a molecular gas layer simulating the atmosphere and to determine the role of Kirchhoff’s law in this problem. Before analyzing the radiation parameters of the gas layer, we present examples where this physical situation is realized. First, consider a warehouse fire or a forest fire occurring over large areas. In this case, radiation emitted by hot air containing optically active components and combustion products plays a significant role in the thermal balance of air above the hot surface. Another example is the greenhouse effect in the Earth’s atmosphere whose thermal radiation is produced by impurities including mainly water molecules and water microdroplets that make up clouds, as well as carbon dioxide molecules, while the bulk of the air (nitrogen and oxygen molecules) is a buffer medium. The last example will demonstrate the role of Kirchhoff’s law in the emission of a gas layer containing different molecules.
Next, we present an expression for the radiative flux from a plane-parallel layer containing a mixture of molecular gases [15]. The nature of the emission from a plane-parallel layer of molecular gas is determined by the optical thickness uω of the layer at a given radiative frequency ω:
where h is the height measured from the boundary and the integral is taken over the entire height of the layer. We will assume first that the temperature T of the layer does not change with height. Then the radiative flux Jω from the gas layer at a given radiative frequency ω is [16, 17]
where Iω(T) is the equilibrium radiative flux from a plane-parallel layer, which is determined by the Planck formula [18, 19]
The opaque factor g(uω) of the layer appearing in formula (2.2) is given by the expression [16, 17]
and represents the probability that a photon of a given frequency ω arising at one boundary and directed to the other will not reach the latter, being absorbed on the way. In this case, the act of emission of a photon by a molecule is isotropic.
Now, consider the emission of the Earth’s atmosphere by simulating it with a weakly inhomogeneous layer of gas. The weak inhomogeneity of the layer means that a relative temperature change in the layer is small, i.e., there is a small parameter, and the expression for the radiative flux Jω emitted by the Earth’s atmosphere can be represented as an expansion in this small parameter. As a result, the expression for the radiative flux is described by the modified formula (2.2) represented as [20, 21]
where Tω is the effective radiation temperature of the layer at a given frequency, and the value of the radiative flux can be found as a result of expansion in the small parameter. This operation is described in [20, 21].
When analyzing the radiation emitted by the Earth’s atmosphere, we will use the standard atmosphere model [22], within which the atmospheric parameters are averaged both over the Earth’s surface and over time. In this case, the main greenhouse components of the atmosphere are water molecules and carbon dioxide, as well as microdroplets of water that form clouds. The density distribution of water and carbon dioxide molecules over height is obtained from the standard atmosphere model [22], as well as from subsequent measurements. The radiation parameters of molecules, including transition rates between vibrational or rotational states of the molecules, as well as the broadening parameters of the spectral lines, are taken from the HITRAN databank [13, 14], which summarizes the results of measurements and calculations.
It should be noted that this bank is important for spectroscopic calculations. The quantum theory of radiation, which could be used to analyze the processes of emission of molecules, applies only to symmetric molecules and allows one to present the results in an analytical form (for example, the regular model or the Elsasser model [23]). The HITRAN bank has changed our ability to perform spectroscopic calculations. In short, based on the data of the HITRAN bank [13, 14], one can determine the absorption cross section of a photon at a given frequency, and, on the basis of the spatial distribution of molecules, one can determine the radiative flux emitted by a plane-parallel layer of gas containing these molecules by formula (2.2). Thus, the HITRAN bank, which contains data on the radiation parameters of molecules, including the broadening of spectral lines, is an important tool for modern molecular spectroscopy, which enabled one to make a significant step in the analysis of the radiation parameters of a molecular gas. Naturally, this required the development of a special mathematical apparatus to present the data of the HITRAN bank [24, 25].
3 CLOUDS IN ATMOSPHERIC EMISSION
Our goal is to determine the error caused by neglecting Kirchhoff’s law, as is done in climatological models. To this end, we have to formulate a model of an emitting atmosphere. This model was presented in the monograph [26], where it was used to calculate the radiation parameters of the atmosphere in the spectral region up to 1200 cm–1. This made it possible to analyze the nature of atmospheric emission associated with changes in the concentration of carbon dioxide molecules. In this work, we have extended the spectrum under consideration to 2600 cm–1. About 0.1% of the radiative flux emitted by an absolutely black body at the temperature of the Earth’s surface remains outside this spectrum. The inclusion of more than 2000 spectral lines from the HITRAN databank into consideration makes it possible to analyze changes in the radiation associated not only with the greenhouse components, but also with the participation of the so-called trace components, the total contribution of which to the atmospheric radiative flux is about 1%.
Note that the three main greenhouse components of the atmosphere, namely, water and carbon dioxide molecules and microdroplets of water that form clouds, together provide more than 99% of the atmospheric radiative flux. As for the greenhouse molecular components, as mentioned above, for given temperature and density profiles of molecules, based on the data from the HITRAN bank, one can determine the infrared radiative fluxes of the atmosphere for each component and at each frequency. In this case, the layer of emitting gas is assumed to be plane-parallel, which corresponds to the assumption that the characteristic dimensions in the horizontal direction on which the spatial density and temperature distributions of molecules change noticeably significantly exceed the size of the vertical region, which is responsible for the formation of emission. In reality, this condition is satisfied.
However, in the case of emission of clouds, we do not have the information necessary to determine the associated atmospheric radiative fluxes with the same accuracy as in the case of molecular components. The condensation of water in the atmosphere occurs when a stream of warm moist air from the lower layers of the atmosphere is mixed with cold air of the upper layer under the action of a vertical wind, i.e., as a result of a nonequilibrium process. This is a rare process; therefore, atmospheric water consists mainly of water molecules, and the condensed phase of water in the atmosphere constitutes a small fraction of atmospheric water. Moreover, the standard atmosphere model [22] excludes the condensed phase of water in the atmosphere.
Nevertheless, clouds make an appreciable contribution to the atmospheric emission, so that the total radiative flux from the atmosphere to the Earth’s surface, which follows from the energy balance of the Earth, includes the emission of clouds. Clouds divide the troposphere into upper and lower parts: the lower part is responsible for the atmospheric emission to the Earth’s surface, while, in the upper part of the troposphere, infrared radiation is emitted that goes beyond the atmosphere. In this case, the optical thickness of the clouds is sufficient for the upper and lower parts of the troposphere not to influence each other. Within the framework of the atmosphere model [26], which describes atmospheric emission to the Earth’s surface, the clouds are located at a certain height hcl, and their emission corresponds to an absolutely black body, whose temperature Tcl coincides with the air temperature at this height. In this case, the radiative flux emitted by clouds is the difference between the total radiative flux of the atmosphere, which follows from the Earth’s energy balance, and the radiative flux due to water and carbon dioxide molecules, which can be calculated based on the data of the HITRAN bank. Earlier [26], data from the NASA program obtained half a century ago [27] were used as the Earth’s energy balance. At present, one uses other versions of the energy balance of the Earth and its atmosphere.
Among additional sources for the energy balance of the Earth and its atmosphere, we use the version given in the book by Salby [28]. This version, along with data obtained by NASA, uses the results of additional measurements of atmospheric emission. In addition, it uses the data of [29–32] carried out for the International Meteorological Society, as well as the Earth’s energy balance constructed in [33, 34]. Below we use the parameters of the energy balance of the Earth and its atmosphere that are based on statistical averaging of the parameters for each variant of the Earth’s energy balance.
The atmospheric emission model used within the line-by-line model leads to the following expression for the atmospheric radiative flux Jω at a given radiative frequency ω:
The first term on the right-hand side of the equation is given by formula (2.5) and describes the emission of atmospheric molecules. The second term refers to the emission of clouds, which, within this model, emit as an absolutely black body with the temperature Tcl of air in the region of the atmosphere where the clouds are located. This radiation reaches the Earth’s surface with probability 1 – g(uω). Hence we obtain an integral relation for the total radiative flux J↓ from the atmosphere to the Earth’s surface:
This flux can be divided into two parts:
where
In this case, the radiative flux Jm to the Earth’s surface is emitted by optically active molecules of the atmosphere; i.e., it is mainly determined by atmospheric molecules of water and carbon dioxide, and the radiative flux Jcl results from the emission of water microdroplets of clouds. Just as in the case of atmospheric molecules, the emission of water microdroplets occurs under the condition of thermodynamic equilibrium between the radiation field and air molecules.
In fact, relation (3.3) is an equation for determining the height hcl of the clouds, as well as the temperature Tcl of the atmospheric region where the cloud boundary is located. The parameters of these formulas are presented in Table 1 and obtained on the basis of the data given above.
4 KIRCHHOF’S LAW FOR A STANDARD ATMOSPHERE
Next, we will demonstrate the role of Kirchhoff’s law for the greenhouse effect, i.e., for the emission of the Earth’s atmosphere. For this purpose, we developed a computer program based on the above algorithm, which uses radiative transition data from the HITRAN bank for greenhouse molecules H2O and CO2, as well as for trace-component molecules N2O, CH4, and O3. In total, more than two thousand spectral lines are taken into account. The computer program makes it possible to calculate, within the standard atmosphere model, both the radiative fluxes incident on the Earth’s surface and their dependence on the radiative frequency. Hence one can determine the changes in the partial and integral atmospheric radiative fluxes to the Earth’s surface due to a change in the composition of the atmosphere.
It is more convenient to demonstrate the effect of Kirchhoff’s law on the change in the radiative flux from the atmosphere to the Earth’s surface as a result of a change in the composition of the atmosphere due to the overlap of the spectra of these components by an example of trace components, since they act in a limited spectral region. Next, consider the interaction of radiation with methane molecules, whose absorption band is centered at 1306 cm–1, as well as with nitrogen dioxide molecules, for which two absorption bands are centered at 1285 cm–1 and 2224 cm–1.
Figures 1–3 show the values of the optical thickness of the atmosphere for individual optically active components in the region of absorption bands for the trace components of the atmosphere. The contemporary concentration of trace components in the atmosphere is 1.9 ppm [35] for methane molecules and 0.29 ppm [36, 37] for nitrogen dioxide molecules. These figures show that the spectral absorption lines of different components overlap in the spectral regions under consideration.
Optical thickness uω of the atmospheric layer located between the Earth’s surface and clouds for CH4, N2O, and H2O molecules near the absorption spectrum of methane molecules. The height of the clouds is taken to be hcl = 4.6 km. The straight line uω = 2/3 separates the regions of low and high optical thicknesses of the atmosphere for a given component.
According to Figs. 1 and 2, in the spectral region where the methane and nitrogen dioxide molecules absorb, there is an overlap of the spectra of different optically active molecules. It is this fact that determines the influence of different molecules on the change in the radiative flux from the atmosphere in these spectral regions. In particular, the neglect of the absorption of water molecules on the wing of the atmospheric emission spectrum in [26] led to an overestimated contribution of N2O molecules to the radiative flux, although this contribution did not exceed one percent of the total radiative flux from the atmosphere to the Earth’s surface even in this case. Note that the spectroscopic frequency unit, just as the photon energy, is cm–1, so that its inverse is the wavelength.
Optical thickness uω of the atmospheric layer located between the Earth’s surface and clouds for optically active molecules near the second absorption band of nitrogen dioxide molecules. The height of the clouds is taken to be hcl = 4.6 km. The straight line uω = 2/3 separates the regions of low and high optical thicknesses of the atmosphere for a given component.
Thus, there are two absorption bands for N2O molecules in the thermal emission spectrum of the Earth’s atmosphere. The lower band, which is associated with the bending vibrations of a molecule, overlaps with the absorption spectrum of methane and water molecules, while the second absorption band, which corresponds to antisymmetric vibrations of a molecule, overlaps with the absorption spectra of water and carbon dioxide molecules. Doubling the concentration of nitrogen dioxide molecules leads to a smaller increase in the total radiative flux of the atmosphere towards the Earth’s surface compared with an increase in the radiative flux emitted by nitrogen dioxide molecules in the absorption band of these molecules.
Table 2 demonstrates the general character of change in radiative fluxes due to the emission of molecules of each type when the concentration of molecules of a certain type is changed. Namely, an increase in the concentration of molecules of a given type leads to a relatively smaller increase in the radiative flux due to the molecules of this type and to an even smaller relative increase in the total radiative flux of the atmosphere to the Earth’s surface. This change leads to a decrease in the contribution of other components to the total radiative flux of the atmosphere.
Note that the example presented above is a demonstration of the optical interaction of different components; it does not play a role in the radiation of the atmosphere. Indeed, taking the global temperature, i.e., the average temperature of the Earth’s surface, equal to TE = 288 K, and assuming that the Earth radiates as an absolutely black body, we find that the radiative flux in this frequency range is 0.68 W/m2, while the total radiative flux from the Earth’s surface is equal to about 390 W/m2. This fact also demonstrates that the total flux of atmospheric radiation to the Earth’s surface emitted by trace molecules is relatively small and does not exceed 1%. The radiative temperature of the standard atmosphere in this frequency range is 272 K.
There is another trace component, ozone, whose contribution to the total radiative flux of the atmosphere to the Earth’s surface is comparable to the contribution of other trace components. The concentration of tropospheric ozone in the atmosphere is an order of magnitude lower than the concentration of nitrogen dioxide and amounts to 20–30 ppb [38]. However, the absorption band of an ozone molecule, centered at about 1042 cm–1, falls simultaneously into the region of maximum thermal emission and the transparency region of the atmosphere. Although the concentration of stratospheric ozone is significantly higher than that of tropospheric ozone, the emission of the stratosphere does not reach the Earth’s surface, being absorbed on the way by clouds.
Table 3 demonstrates the optical properties of the atmosphere in the region of the absorption band of ozone molecules; it is an analog of Table 2 for the absorption band of nitrogen dioxide molecules. The data in this table, just as in Fig. 3, refer to a concentration of 25 ppb for the standard atmosphere and 50 ppb for the atmosphere with a doubled concentration of ozone molecules. Since the optical thickness of the atmosphere due to ozone molecules is much less than unity, the contribution of ozone molecules to the total radiative flux of the atmosphere changes in proportion to the change in the concentration of ozone molecules, provided that ozone molecules play the main role in this spectral region.
Optical thickness uω of the atmospheric layer located between the Earth’s surface and clouds for optically active molecules near the absorption band of ozone molecules. The contribution of atmospheric water to the optical thickness of the atmosphere is marked in black, of carbon dioxide, in red, and of ozone, in blue and brown, respectively, for its contemporary and doubled concentrations in the atmosphere. The height of the clouds is hcl = 4.6 km. The straight line uω = 2/3 separates the regions of low and high optical thicknesses of the atmosphere for a given component.
Further, despite the relatively low concentration of ozone molecules in the atmosphere, the contribution of ozone to atmospheric emission is comparable to the contribution from other trace optically active molecular components of the atmosphere. In this case, the low concentration of ozone molecules in the troposphere is compensated by the spectral region favorable for emission, as well as by the transparency of the atmosphere in this spectral region.
Obviously, the main changes in the greenhouse effect are associated with the main molecular components, namely, with carbon dioxide and water molecules. In the case of carbon dioxide molecules, one usually compares the global temperatures for the contemporary and doubled concentrations of these molecules. Therefore, below we will use this change in the concentration of carbon dioxide molecules as a measure of its effect on global temperature. A change in the concentration of carbon dioxide molecules primarily manifests itself in the radiative temperature of the atmosphere. In this case, the change in the global temperature due to a change in the concentration of carbon dioxide molecules is small near the centers of the absorption bands associated with the corresponding vibrational transitions. In particular, this is the case for the strongest vibrational transition of a carbon dioxide molecule under normal conditions, which occurs between the ground and lower deformation (bending) vibrational states of a carbon dioxide molecule centered at 667 cm–1. The same situation is observed in the frequency range corresponding to the centers of the strongest vibrational transitions, where the radiative temperature is close to the temperature of the Earth’s surface.
Therefore, the main contribution to the change in the radiative temperature of the atmosphere and, accordingly, to the change in the radiative flux of the atmosphere to the Earth’s surface is made by the frequency ranges where the optical thickness of the atmosphere is on the order of unity. In addition, a certain contribution to the change in the radiative temperature and, accordingly, to the change in the radiative flux emitted by carbon dioxide molecules in the atmosphere, is made by the regions of laser transitions near the wavelengths of 9.4 and 10.6 μm, since the laser transitions are in the transparency region of the atmosphere. At the same time, the contribution of laser transitions to the radiative flux emitted by carbon dioxide molecules is about 2%.
Figure 4 demonstrates the frequency dependence of the radiative flux emitted by carbon dioxide molecules at frequencies below the indicated one, as well as the difference between the radiative fluxes emitted by these molecules for the doubled and contemporary concentrations of carbon dioxide molecules in the atmosphere. One can see that the radiative flux increases rather monotonically with frequency, while the difference between the fluxes for different concentrations of atmospheric carbon dioxide increases in jumps near the boundaries of the corresponding absorption bands. Figure 5 shows the comparison of the changes in the radiative flux of the atmosphere to the Earth’s surface emitted by carbon dioxide molecules and in the total radiative flux of the atmosphere.
Radiative flux \({{J}_{ \downarrow }}\)(CO2) from the atmosphere to the Earth’s surface emitted by CO2 molecules in the atmosphere for the contemporary concentration of CO2 molecules in the atmosphere (dark symbols) and its change Δc when the concentration of carbon dioxide molecules in the atmosphere is doubled in accordance with its definition according to formula (4.1) (open symbols). The circles correspond to the work [26], and the squares refer to the present work.
Changes in radiative fluxes from the atmosphere to the Earth’s surface determined by formula (4.1) when the concentration of carbon dioxide molecules in the atmosphere is doubled compared to its contemporary value. Here Δc is a change in the radiative flux emitted by carbon dioxide molecules (open symbols), and Δ is a change in the total infrared radiative flux from the atmosphere to the Earth’s surface (dark symbols). The circles correspond to the results of calculations from [26], and the squares correspond to the calculations performed in this paper.
Now, let us analyze the nature of the change in the radiative fluxes emitted by different greenhouse components from the viewpoint of Kirchhoff’s law. Consider three main greenhouse components, namely, water molecules, carbon dioxide molecules, and water microdroplets that form clouds. Denote by Δc, Δw, and Δd the changes in the radiative fluxes emitted by the molecules of carbon dioxide, water, and by water microdroplets, respectively, when the concentration of carbon dioxide molecules in the atmosphere changes. Thus, we introduce these changes by the relations
where Jω(CO2), Jω(H2O), and Jω(drop) are radiative fluxes from the atmosphere to the Earth’s surface that are emitted by the above components for the contemporary concentration of carbon dioxide molecules, and the radiative fluxes \(J_{\omega }^{'}\)(CO2), \(J_{\omega }^{'}\)(H2O), and \(J_{\omega }^{'}\)(drop) correspond to an increased concentration of carbon dioxide. The change Δ in the total radiative flux from the atmosphere to the Earth’s surface is
Note that the computer program used in [26] refers to the frequency range from 0 to 1200 cm–1. Although this frequency range includes only a part of the frequency range responsible for the atmospheric thermal emission, it is sufficient for analyzing the radiation associated with carbon dioxide molecules, since the thermal emission spectrum of carbon dioxide molecules is concentrated within this range. Therefore, the results of this work with a wider frequency range of investigations from 0 to 2600 cm–1 should be close to the results of calculations of [26]. Indeed, the comparison of these results, presented in Figs. 4 and 5, shows that the difference between the frequency-integrated radiative fluxes does not exceed 7%. The integral radiative fluxes from the atmosphere to the Earth’s surface, averaged over two groups of calculations, are
The calculations performed allow us to determine the error due to the neglect of Kirchhoff’s law. This error accompanies climatological models. Indeed, if we neglect the overlap of the spectrum of carbon dioxide molecules with the spectra of water molecules and water microdroplets, then the change Δc in the radiative flux emitted by carbon dioxide molecules coincides with the change Δ in the total radiative flux. For real spectra and the radiation parameters of greenhouse components, we have the following ratio for the changes in radiative fluxes:
In addition, using the linear dependence of the total radiative flux J↓ from the atmosphere to the Earth’s surface on the concentration of carbon dioxide c(CO2) molecules in the atmosphere, which follows from the calculations, we can conveniently represent formula (4.3) as
where c(CO2) is the contemporary concentration of carbon dioxide molecules in the atmosphere, c'(CO2) is the changed concentration of atmospheric carbon dioxide molecules, and Δ is the change in total infrared radiative flux from the atmosphere to the surface Earth for a given change in the concentration of atmospheric carbon dioxide molecules.
In this work, based on the computer program used for the thermal emission spectrum in the range from 0 to 2600 cm–1, we also determined the role of Kirchhoff’s law in changing the concentration of water molecules in the atmosphere. In this case, as before, we believe that the changes in the concentration of molecules in the atmosphere preserve the radiative temperature of clouds, that is, the temperature of the cloud boundary remains constant during this change. Therefore, the change in the radiative flux emitted by clouds to the Earth’s surface is associated only with the shielding of the flux by additional molecules introduced into the atmosphere. Applying the procedure previously used with atmospheric carbon dioxide to water molecules in the atmosphere, in the case of a change in the concentration of water molecules in the atmosphere, we obtain
where Δw is the change in the radiative flux from the atmosphere to the Earth’s surface emitted by water molecules and Δ is the change in the total infrared radiative flux. In addition, for the change in the radiative flux due to a change in the concentration of atmospheric water, we have
where c(H2O) is the contemporary concentration of atmospheric water molecules and c'(H2O) is the changed concentration of water molecules in the atmosphere. Hence it follows that carbon dioxide molecules are about an order of magnitude more effective than water molecules in terms of the formation of the total radiative flux to the Earth’s surface, since the concentration of carbon dioxide molecules in the standard atmosphere is almost 40 times lower than the concentration of atmospheric water molecules.
5 CONCLUSIONS
The analysis carried out demonstrates the importance of Kirchhoff’s law for the radiative processes in a mixture of molecular gases. This law manifests itself in the influence not only of carbon dioxide on the greenhouse effect [39, 40], but also of various components of molecular mixtures. At the same time, since Kirchhoff’s law occupies an important place in the physics of radiation of molecular gases, carbon dioxide was not ruled out from physical considerations. In particular, in [41, 42] the authors pointed out that, according to the information of that time (1956), the overlap of the spectra of carbon dioxide and water molecules leads to a decrease in the radiative flux emitted by atmospheric carbon dioxide molecules by about 20%. Subsequently, the study of the greenhouse effect of the Earth’s atmosphere became the subject of climatology, where the choice of the main factors was made intuitively, and Kirchhoff’s law was neglected.
At the same time, we stress the fundamental role of the HITRAN databank in this analysis. Indeed, without these data, the problem under consideration could not be solved, and the information required for the purpose is very extensive. In particular, the computer program used for the above analysis includes the parameters of about two thousand radiative transitions in molecules, and such information cannot be presented in a limited number of articles, as well as in a review or a monograph. Therefore, the conclusions of this article also demonstrate the importance of the modern molecular physics tool—the HITRAN databank.
In addition to what has been said, we note that the neglect of Kirchhoff’s law is possible only in the spectral region where the optical thickness of the layer for each molecular component is small, and then the influence of other components on the radiation yield of a given component is relatively small. This occurs in the spectral region containing the absorption band of ozone molecules, where the optical thickness of the atmosphere is small. Then the radiative flux emitted by ozone molecules changes proportionally to the ozone concentration in the atmosphere, and the changes in the radiative flux from the atmosphere to the Earth’s surface emitted by ozone molecules coincide with the respective changes in the total radiative flux.
REFERENCES
G. Kirchhoff and R. Bunsen, Ann. Phys. Chem. 109, 275 (1860).
L. D. Landau and E. M. Lifshitz, Course of Theoretical Physics, Vol. 3: Quantum Mechanics: Non-Relativistic Theory (Nauka, Moscow, 1964; Pergamon, New York, 1977).
V. P. Krainov and B. M. Smirnov, Atomic and Molecular Radiative Processes (Springer Nature, Switzerland, 2019).
N. Andronova and M. Schlesinger, J. Geophys. Res. 106, D22605 (2001).
M. A. Snyder, J. L. Bell, and L. C. Sloan, Geophys. Res. Lett. 29, 014431 (2002).
J. D. Annan and J. C. Hargreaves, Geophys. Res. Lett. 33, L06704 (2006).
A. Ganopolski and T. Schneider von Deimling, Geophys. Res. Lett. 35, L23703 (2008).
M. E. Walter, Not. Am. Mat. Soc. 57, 1278 (2010).
A. Schmittner, N. M. Urban, J. D. Shakun, et al., Science (Washington, DC, U. S.) 334, 1385 (2011).
J. T. Fasullo and K. E. Trenberth, Science (Washington, DC, U. S.) 338, 792 (2012).
R. M. Goody, Atmospheric Radiation: Theoretical Basis (Oxford Univ. Press, London, 1964).
R. M. Goody and Y. L. Yung, Principles of Atmospheric Physics and Chemistry (Oxford Univ. Press, New York, 1995).
https://www.cfa.harvard.edu/.
http://www.hitran.iao.ru/home.
B. M. Smirnov, J. Exp. Theor. Phys. 126, 446 (2018).
Ya. B. Zel’dovich and Yu. P. Raizer, Physics of Shock Waves and High-Temperature Hydrodynamic Phenomena (Academic, New York, 1966).
B. M. Smirnov, Physics of Weakly Ionized Gases (Nauka, Moscow, 1972; Mir, Moscow, 1980).
F. Reif, Statistical and Thermal Physics (McGraw-Hill, Boston, 1965).
L. D. Landau and E. M. Lifshits, Course of Theoretical Physics, Vol. 5: Statistical Physics (Nauka, Moscow, 1995; Pergamon, Oxford, 1980).
B. M. Smirnov, Physics of Weakly Ionized Gases (Mir, Moscow, 1980).
B. M. Smirnov, Physics of Ionized Gases (Wiley, New York, 2001).
U. S. Standard Atmosphere (U.S. Government Printing Office, Washington, 1976).
W. M. Elsasser, Phys. Rev. 54, 126 (1938).
http://www.hitran.org/links/docs/definitions-andunits/.
M. Simeckova, D. Jacquemart, L. S. Rothman, et al., J. Quant. Spectrosc. Radiat. Transfer 98, 130 (2006).
B. M. Smirnov, Transport of Infrared Atmospheric Radiation (de Gruyter, Berlin, 2020).
Understanding Climate Change (Nature Acad. Sci., Washington, 1975).
M. L. Salby, Physics of the Atmosphere and Climate (Cambridge Univ. Press, Cambridge, 2012).
J. T. Kiehl and K. E. Trenberth, Bull. Am. Meteorol. Soc. 78, 197 (1997).
K. E. Trenberth, J. T. Fasullo, and J. T. Kiehl, Bull. Am. Meteorol. Soc. 90, 311 (2009).
K. E. Trenberth and J. T. Fasullo, Surf. Geophys. 33, 413 (2012).
J. T. Fasullo and K. E. Trenberth, Science (Washington, DC, U. S.) 338, 792 (2012).
G. L. Stephens, J. Li, M. Wild, et al., Nat. Geosci. 5, 691 (2012).
M. Wild, D. Folini, Ch. Schär, et al., Clim. Dyn. 40, 3107 (2013).
https://en.wikipedia.org/wiki/Atmosphericmethane.
D. Pierotti and A. Rasmussen, J. Geophys. Res. 82, 5823 (1977).
B. D. Hall, G. S. Dutton, and J. W. Elkins, J. Geophys. Res. 112, D09305 (2007).
https://en.wikipedia.org/wiki/Tropospheric-ozone.
B. M. Smirnov, Int. Rev. At. Mol. Phys. 10, 39 (2019).
B. M. Smirnov, J. Atmos. Sci. Res. 2 (4), 21 (2019).
G. N. Plass, Tellus VIII, 141 (1956).
G. N. Plass and D. I. Fivel, Quant. J. R. Met. Soc. 81, 48 (1956).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. Nikitin
Rights and permissions
About this article
Cite this article
Zhilyaev, D.A., Smirnov, B.M. Kirchhoff’s Law in the Emission of a Mixture of Molecular Gases. J. Exp. Theor. Phys. 133, 687–695 (2021). https://doi.org/10.1134/S1063776121110066
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063776121110066