Abstract
A magnetic resonance method is applied to the investigation of a number of isostructural diamagnetic compounds ABO3 (A = Sc, In, and Ga) with small additions of Cr3+ ions (S = 3/2) sufficient to observe single-ion and pair spectra. It is shown that the resonance spectra for isolated Cr3+ ions can be described to a good accuracy by the ordinary axial spin Hamiltonian for 3d ions in octahedral oxygen environment. The parameters of the spin Hamiltonian are determined for single Cr3+ ion and Cr3+–Cr3+ pair. Lattice distorsions of the parent ABO3 crystals caused by the Cr3+ impurities is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Borates of transition metals with chemical formula ABO3 (A = Fe, V, Cr, Ti) have attracted attention because of the variety of their physical properties that are manifested in this isostructural series of compounds [1]. However, a number of borates of 3d metals ABO3, except for FeBO3, remain poorly studied. For example, from among the whole series of 3d borates, magnetic anisotropy has been experimentally investigated to date only in FeBO3 [2]. In the present study, we apply the electron paramagnetic resonance method to the experimental investigation of the anisotropic properties of Cr3+ ions in diamagnetic matrices of isostructural compounds of ABO3 (A = Sc, In, Ga) borates. In this case, a Cr3+ ion was chosen due to the unusual magnetic properties of the isostructural crystal CrBO3 [3]. For example, in [4] the authors showed that the magnetic properties of the CrBO3 crystal can be described on the basis of a simple model of a collinear two-sublattice antiferromagnet with magnetic moments along a [111] axis. The authors of [3] suggested, on the basis of static magnetic measurements, that it is more probable that, on the contrary, the antiferromagnetism vector of CrBO3 lies in a plane close to the base plane.
The present paper is the continuation of the investigations presented by us in [5]. In [5] only single ion spectra for A = In, Sc, and Lu were discussed and was pointed out the presence of a Cr3+–Cr3+ pair spectra. In this paper we present the new data of the Electron Spin Resonance (ESR) spectra of single Cr3+ ions in diamagnetic matrix GaBO3 and pair spectra of Cr3+–Cr3+ ions were treated and discussed for A = Sc, In, Ga compounds.
2 MATERIALS AND METHODS
ABO3 (A = Sc, In, Ga) crystals with small (about 5 at % of A) addition of Cr3+ ions were grown from the solution-melt with composition Cr2O3–M2O3–B2O3–(70PbO–30PbF2 wt %). The detailed synthesis technology is described in [6]. In this technology, a Cr3+ ions substitute the A ions. We obtained single crystals in the form of thin plates with a size of 2 × 2 mm and thickness of about 0.1 mm with a smooth shining surface of light gray color. ABO3 (A = Sc, In, Ga) isostructural crystals have a rhombohedral symmetry in space group R\(\bar {3}\)c, the point group symmetry of the A ion is (–3m). Parameters of the unit cell were determined by using X-ray Smart APEX II (Bruker) (Mo Kα radiation) installation at room temperature and are presented in Table 1 for Sc, In, Ga.
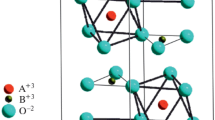
The C3-axis of the crystal is normal to sample plate (the c-axis in Fig. 1). The A ions are located in the octahedrons formed by oxygen ions connected with boron ions by a strong covalent bond.
In addition, there is a possibility for nearest Cr3+ ions to form a magnetically coupled pairs. The projection of the pair’s axes onto basal plane of the crystal is presented in Fig. 2. All pairs are magnetically equivalent. The Cr3+–Cr3+ distances and angles between C3 axis of the crystal and pair axes are shown in Table 1.
The electron paramagnetic resonance measurements were carried out on a Bruker Elexsys E-580 spectrometer operating at X-band at temperatures 300 and 77 K.
3 RESULTS
3.1 Single-Ion Spectra
In this chapter we present the experimental results for the single ion ESR spectra of Cr3+ ions obtained in ABO3 (Sc, In, Ga) compounds. So far this paper is the continuation of the investigations presented by us in [5], let us consider more detailed only experimental data for the GaBO3 crystal, that was not discussed previously.
The angular dependence of the resonance fields of the single Cr3+ ions transitions observed in the (ac) plane are shown in Fig. 3. The resonance spectra for isolated ions of Cr3+ can be described by the following axial spin Hamiltonian for 3d ions:
where D is the axial constant of the spin Hamiltonian, g|| and g⊥ are the values of the g-tensor for parallel and perpendicular orientations of the external magnetic field with respect to the C3 axis of the crystal, β is the Bohr magneton, Si and Hi are the projections of the spin of a Cr3+ ion and the external magnetic field to the C3 axis of the crystal, and S = 3/2 is the spin of the Cr3+ ion. The experimental and theoretical spectra were fitted with the use of the XSophe software [8]. The results are presented in Table 2.
(Color online) Angular dependence of resonance fields of the ESR signals observed in GaBO3 (ac) plane of the crystal. Dots represent experiment and solid curves—calculation (see Table 2).
The values of the g-tensor obtained are nearly isotropic and correspond to appropriate values for d3 ions in the octahedral environment [9]. The spin-Hamiltonian constant D correlates with those in the earlier investigated compounds Al2O3 [10] and ZnGa2O4 [11, 12], in which the Cr3+ ion is also in an octahedral coordination. The D sign for Cr3+ ion in GaBO3 was determined by comparison of the resonance lines intensities of MS = 1/2 ↔ 3/2 and MS = –1/2 ↔ –3/2 transitions at 300 and 77 K respectively as in [5].
As an example, Fig. 4 demonstrates the calculated schemes of energy levels of the Cr3+ ion in GaBO3.
3.2 Pair Spectra
In compounds with A = Sc, In, Ga the Cr3+–Cr3+ pair spectra were observed. The example of the pair spectra for ScBO3 at room temperature is presented in Fig. 5. The intense line in the right and left sides corresponds to the single ion transitions.
Cr3+–Cr3+ pair forms by the nearest neighbors with the distances r = 3.7424, 3.774, and 3.5459 Å for A = Sc, In, and Ga compounds respectively (see Table 1). The angle between pair axes and C3 axis of the crystals is equal approximately θ ≈ 47° for all compounds. When two Cr3+ ions interact to form a magnetically coupled pair, the spins (s) on each ion combine as vectors to produce a manifold of four spin states each characterized by a total spin quantum number S, which value varies from (s1 + s2), (s1 + s2 – 1) … to 0. Assuming that the energy intervals between these spin states are large compared with the other magnetic interactions, a separate spin Hamiltonian may be written for each spin state. In our case, it was possible to identify resonance spectra for pair multiplets with total spin S = 2 and 3 only. The angle dependencies of the resonance fields of the signals in basal plane of the crystals are shown in Figs. 6–8.
(Color online) Angle dependencies of the resonance fields in basal plane of the crystals for ScBO3 crystal at room temperature. Dots—experiment, solid lines—fitted curves by using of spin Hamiltonian (2) with parameters from Table 3.
Fitting of the experimental spectra were carried out with XSophe program [8] and the spin Hamiltonian (2).
where g|| and g⊥ are the values of the g-tensor for parallel and perpendicular orientations of the external magnetic field with respect to the C3 axis of the crystal, β is the Bohr magneton. Spin operators \(O_{4}^{0}\), \(O_{4}^{2}\), and \(O_{4}^{4}\) are mentioned in [13]. Third and fourth terms in (2) describe the value of the “fine” structure for the total spin S of pair’s multiplet and have the form [13]
where De, Ee are the dipole-dipole interaction constants (assuming isotropic exchange interaction) in point approximation, Dc, Ec—“single ion” multiplet terms and
The N ↔ J symbols in Figs. 6–8 denote transitions between corresponding MS numbers in pair multiplets. Note, that the real experimental spectrum for each crystal consists from three identical spectra from Figs. 6–8 corresponding to Cr3+–Cr3+ pairs rotated by 60 degrees in basal plane (see Fig. 2).
(Color online) Angle dependencies of the resonance fields in basal plane of the crystals for InBO3 crystal at room temperature. Dots—experiment, solid lines—fitted curves by using of spin Hamiltonian (2) with parameters from Table 3.
(Color online) Angle dependencies of the resonance fields in basal plane of the crystals for GaBO3 crystal at room temperature. Dots—experiment, solid lines—fitted curves by using of spin Hamiltonian (2) with parameters from Table 3.
The best fitting spin-Hamiltonian (2) values are presented in Table 3.
4 DISCUSSION
Let us, firstly, discuss the experimental results for Cr3+–Cr3+ pairs in ABO3 crystals. The De term can be obtained directly from DS = 2 value (Eq. (3)) since there is no lattice contribution (β = 0 for S = 2 (Eq. (4))). For a pair system which exhibits a nearly isotropic g-tensor the anisotropic zero-field term De is almost entirely due to dipole–dipole interaction. If the paramagnetic ions which form the pair are treated as point dipoles, the value of De can be calculated from the following expression. De = –g2β2/R3 (where R is the interionic separation). From the De (for S = 2) values observed for the Cr3+–Cr3+ pairs in ABO3 a distances can be calculated for separation between two Cr3+ ions. The results are presented in Table 4. Note, that Cr3+–Cr3+ distance in pure CrBO3 crystal is equal to 3.5535 Å [14].
In Table 4Rtheor is the interionic A3+–A3+ separation X-ray data from Table 1, \(D_{e}^{{{\text{theor}}}}\) = –g2β2/\(R_{{{\text{theor}}}}^{3}\), \(D_{{S = 2}}^{{{\text{theor}}}}\) = 3/2\(D_{e}^{{{\text{theor}}}}\), Rexp are the interionic Cr3+–Cr3+ distance values calculated from \(D_{{S = 2}}^{{{\text{exp}}}}\). From Table 4 one can see the lattice distortions caused by the impurity Cr3+–Cr3+ pairs in the parent ABO3 lattice. Figure 9 presents these distortions vs. interionic separation of the crystals.
One can notice a tendency: the smaller the interionic A3+–A3+ distances, the stronger the distortions. Moreover, the sign of distortion is changing at A3+–A3+ distance approximately equal to 3.7 Å.
From the Eq. (3) the Dc values can be found also (\(D_{{S = 2}}^{{{\text{exp}}}}\) value was used) (see Table 5).
It is seen, that Dc values are close enough to the axial constants D of the spin-Hamiltonian (1) for the single Cr3+ ion in ABO3 crystals except for the GaBO3. The reasonable origin of this difference for the GaBO3 crystal may be explained if we take into account the strong lattice distortion observed for Cr3+–Cr3+ pair in GaBO3.
Figure 10 shows the dependencies of the pair spin-Hamiltonian (2) term Ds on Cr3+–Cr3+ distance, calculated in Table 4.
DS = 2—closed circles and DS = 3—open circles vs. calculated Cr3+–Cr3+ pair distance for ABO3 (A = In, Sc, Ga) crystals. Rexp is the real Cr3+–Cr3+ distance, determined in Table 4.
Now, let us consider the axial constant D of the single ion spin-Hamiltonian (1). Assuming the model of the point dipole and considering only six nearest oxygen ions (forming octahedral environment of Cr3+ ion) it is possible roughly to estimate the electric field gradient (EFG) for Cr3+ site using the X-ray data (Table 1). Obviously, the EFG value substantially determines the D value. Figure 11 presents D and c/a dependencies on EFG for ABO3 (A = Ga, In, Sc, Lu) crystals.
The general trend is an increase in EFG as the c/a ratio increases. At the same way, the absolute value of the axial constant D increases with the EFG growing except for Cr3+ ion in GaBO3. This fact may be explained if we assume that the strong lattice distorsion observed for Cr3+–Cr3+ pair in GaBO3 (see Fig. 9 and Table 4) may be implemented for single Cr3+ ion in GaBO3 too. In this case, the EFG value calculated from X-ray data (Table 1) may sufficiently differs from the real one.
5 CONCLUSIONS
Single ion and pair spectra of Cr3+ ions in ABO3 (A = Sc, In, Ga) diamagnetic crystals were explored by using electron paramagnetic resonance technique. Spin-Hamiltonian values for single ions and Cr3+–Cr3+ pairs at room temperature were determined. The lattice distortions of the parent ABO3 (A = Sc, In, Ga), caused by Cr3+ impurity were discussed. This work will be prolonged by the pair’s resonance line intensities vs. temperature investigation in order to determine the signs and the values of the Cr3+–Cr3+ exchange integrals J.
REFERENCES
N. B. Ivanova, V. V. Rudenko, A. D. Balaev, N. V. Kazak, S. G. Ovchinnikov, I. S. Edel’man, A. S. Fedorov, and P. V. Avramov, J. Exp. Theor. Phys. 94, 299 (2002).
G. V. Bondarenko, S. G. Ovchinnikov, V. V. Rudenko, V. M. Sosnin, V. I. Tugarinov, and A. M. Vorotynov, J. Magn. Magn. Mater. 335, 90 (2013).
A. D. Balaev, N. B. Ivanova, N. V. Kazak, S. G. Ovchinnikov, V. V. Rudenko, and V. M. Sosnin, Phys. Solid State 45, 287 (2003).
T. A. Bither, C. G. Frederick, T. E. Gier, J. F. Weiher, and H. S. Young, Solid State Commun. 8, 109 (1970).
A. M. Vorotynov, S. G. Ovchinnikov, V. V. Rudenko, and O. V. Vorotynova, J. Exp. Theor. Phys. 122, 734 (2016).
V. V. Rudenko, Inorg. Mater. 34, 1253 (1998).
R. D. Shannon, Acta Crystallogr. A 32, 751 (1976).
M. Griffin, A. Muys, C. Noble, D. Wang, C. Eldershaw, K. E. Gates, K. Burrage, and G. R. Hanson, Mol. Phys. Rep. 26, 60 (1999).
S. A. Altshuler and B. M. Kozyrev, Electron Paramagnetic Resonance in Compounds of Transition Elements (Wiley, New York, 1974; Nauka, Moscow, 1972).
M. J. Berggren, G. F. Imbusch, and P. L. Scott, Phys. Rev. 188, 675 (1969).
J. C. M. Henning, J. H. den Boeff, and G. G. P. van Gorkom, Phys. Rev. B 7, 1825 (1973).
G. L. McPherson and Wai-ming Heung, Solid State Commun. 19, 53 (1976).
A. Abragam and B. Bleaney, Electron Paramagnetic Resonance of Transition Ions (Clarendon, Oxford, 1970).
T. Bither and H. S. Young, J. Solid State Chem. 6, 502 (1973).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Vorotynov, A.M., Rudenko, V.V., Ovchinnikov, S.G. et al. Electron Paramagnetic Resonance of Cr3+ Ions in ABO3 (A = Sc, In, Ga) Diamagnetic Crystals. J. Exp. Theor. Phys. 127, 1067–1073 (2018). https://doi.org/10.1134/S1063776118120245
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063776118120245