Abstract
The centimeter-sized naphthalene, anthracene, and tetracene crystals have been grown from the vapor phase. An isothermal thermogravimetric method for determining the sublimation enthalpy during crystal growth under conditions of classical physical vapor transport is proposed. The sublimation enthalpy has been calculated using the obtained approximate equation for the temperature dependence of the intensity of the flux of molecules sublimating from the solid surface in the quasi-steady-state mode. The sublimation enthalpies of the linear acenes under study have been determined in narrow temperature ranges to be 71 ± 2 kJ mol–1 (328–353 K), 96 ± 3 kJ mol–1 (423–458 K), and 124 ± 11 kJ mol–1 (513–573 K) for naphthalene, anthracene, and tetracene, respectively. The found values are in good agreement with the experimental data in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Physical vapor transport (PVT) is the most efficient method for growing centimeter-sized (0.2–2 cm) single crystals of poorly soluble conjugate semiconductor organic molecules. Due to the specific growth-medium conditions (temperature-field gradient, weak inert-gas flow), this method provides high-purity crystals with the highest structural quality for shorter times in comparison with the solution methods in the case of materials with low solubility [1–3]. In addition, the high consumption efficiency of material (which may be fairly expensive) is another important advantage of PVT as compared to the solution crystal-growth techniques.
In analysis of the processes of crystal formation and growth from vapor phase, the sublimation enthalpy ΔHS is a key thermodynamic value, which plays the role of measure of the interaction energy between the nearest neighbors [4]. To date, there exist some methods for determining its value: calorimetric method [5, 6], Knudsen effusion method [7, 8], manometric method [9, 10], quartz-cavity method [11], gas saturation method [12, 13], thermogravimetric method [14, 15], and inert-gas flow method [16]. Concerning the conditions and specific features of mass exchange, the latter method is the closest to PVT. Unfortunately, for most research groups, which focus on the growth and properties of crystals of novel, largely unstudied materials, these techniques are often not readily available. In this context, a question arises whether it is possible to determine the sublimation enthalpy of an organic one-component crystal according to a significantly simplified scheme, based on static thermogravimetric measurements with the use of PVT in a stationary weak flow of an inert gas, which conditions (jointly with the temperature gradient) the mass transfer of sublimating molecules in the growth tube from a material source to the crystal growth zone.
The purpose of this study was to analyze the model of mass transfer of sublimating molecules, located in equilibrium above the surface of related crystalline material in an inert atmosphere under close-to-normal conditions. The experimental results of studying the crystal growth, crystal structure, and temperature dependence of the intensity of crystalline-material weight loss at isothermal exposure under PVT conditions are presented by an example of well-known materials from the family of linear acenes (naphthalene, anthracene, tetracene).
EXPERIMENTAL
Materials
Naphthalene (99%), anthracene (99%), and tetracene (98%) (Sigma-Aldrich) were used. Nitrogen (grade 6; NII KM, Moscow) was used as an inert gas for growth-tube purging during crystal growth.
Crystal Growth and Analysis of the Material Weight Loss at Sublimation
The PVT system for growing crystals in a gradient temperature field was designed according to the classical scheme [1, 2]. The inner diameter of the growth quartz tube was 19 mm. In the experiments, the growth tube was purged using a weak nitrogen flow with a consumption of ~0.2 L/h. The inert-gas flow in the growth tube was controlled using a RRG-12 flow-rate indicator with external RRG-K interface (Eltochpribor, Zelenograd). The source temperature was monitored and controlled using a Termodat-16K6 temperature controller (Sistemy kontrolya, Perm). The error in automatic temperature setting near the source was δ < 0.5 K. The material source was located in a commercial 2-mL glass vial with an output hole 5.9 mm in diameter, into which a homogeneous layer of the crystalline material under study was poured (powder mass 50–100 mg). The total error in source-temperature setting did not exceed 1.5 K. The mass of the source with material before and after experiment was determined using an AUW-220D analytical balance (Shimadzu) with an allowable error of ±0.1 mg (accuracy class according to GOST (State Standard) 24104-I). The time of each growth experiment was fixed: 4 h for naphthalene and 5 h for anthracene and tetracene. Three experiments were performed for each temperature of the material source. The thicknesses of crystalline films and plates were determined using an Olympus LEXT OLS 3100 confocal microscope.
X-Ray Diffraction (XRD) Analysis
Structures of the largest single-crystal films and plates were investigated on a Miniflex 600 X-ray powder diffractometer (Rigaku, Japan). The XRD parameters were as follows: CuKα radiation, λ = 1.54178 Å, scan rate 2 K/min. The crystal samples were fixed on a quartz substrate using drops of water or alcohol, and XRD reflection from the most developed plane crystal surface was recorded.
DERIVATION OF THE EXPRESSION FOR THE TEMPERATURE DEPENDENCE OF THE MATERIAL-SUBLIMATION INTENSITY
Let us consider a solid, material of which at given pressure and temperature can be in equilibrium (not passing to the liquid state) with only its saturated vapor. Let this body be placed in a gas medium under close-to-normal conditions and the saturated vapor pressure of this material be much lower than the pressure of carrier inert gas. In this case, if the environment partial pressure of this material is smaller than the saturated vapor pressure, vapor sublimation from the body surface occurs. If the vapor pressure in the medium exceeds the saturated vapor pressure, solid sublimation from vapor occurs and the solid volume increases.
In the quasi-steady-state approximation, a current distribution of volume vapor concentration n(x, y, z) is described by the Laplace equation \({{\nabla }^{2}}\)n = 0, and the sublimation fluence is described by Fick’s law j = –D\(\nabla \)n, where D is the vapor diffusion coefficient in the gas medium. The Maxwell boundary conditions are as follows: the vapor concentrations on the body surface S and at large distance from it are equal to the saturated vapor concentration ns and some value n∞, respectively. The number of molecules sublimating from the surface per unit time is determined by the integral of fluence over the body surface
where F(S) is some function, determined by only the solid geometry. For example, if the solid is a hemisphere, whose flat side lies on a planar substrate, F = 2πR, where R is the hemisphere radius [17, p. 375].
According to the molecular-kinetic theory of gases, the expression for the diffusion coefficient for a minor gas impurity, consisting of molecules of the same type, is as follows [18, p. 145]:
where \({v} = {{(2{{k}_{{\text{B}}}}T{\text{/}{\mu }})}^{{1{\text{/}}2}}}\) is the average relative collisional rate of impurity and carrier gas (external atmosphere) molecules, μ is the reduced mass of colliding particles, kB is the Boltzmann constant, \(l = 1{\text{/}}({{n}_{0}}{{\sigma }})\) is the mean free path of vapor molecules in the carrier gas (it is assumed that vapor concentration is much lower than the carrier inert gas concentration, ns \( \ll \) n0), and σ is the collisional cross section for vapor and carrier-gas molecules. Since the constant carrier-gas pressure p = n0kBT, the temperature dependence of the mean free path is determined by the formula \(l = {{k}_{{\text{B}}}}T{\text{/}}p{{\sigma }}\). It is assumed that the collisional cross section barely depends on temperature. In this approximation,
The saturated vapor pressure above the material-source surface can be defined as a function of temperature according to the following formula ([19, p. 295]:
where A is a constant, cp is the isobaric molar specific heat of vapor, c is the specific heat of solid, and ΔHS,0 is the sublimation enthalpy at 0 K. The sublimation enthalpy at an arbitrary temperature T is described by the formula [19, p. 295]
Let us assume that ns \( \gg \) n∞ under experimental conditions, so that the vapor concentration at a large distance from the sublimating body in formula (1) can be neglected. Having substituted (3) and (4) into (1), we obtain the sublimation rate of a solid as the following function of temperature:
Having denoted the experimental constant as
one can write expression (6) in the form
Taking into account that the number of molecules sublimated per unit time is I = Δm/(Mτ) (Δm is the mass loss of the weight, τ is the experiment duration), an experimental measurement of I(T) makes it possible to find the sublimation enthalpy at zero temperature based on temperature dependence (8) and recalculate it with respect to the experimental temperature using formula (5).
For data processing, it is more convenient to transform (8) into
Let us now pass to the coordinate x = T–1 in Eq. (9). Then,
If measurements are performed in a narrow temperature range [T0, T0 + ΔT] (so that ΔT \( \ll \) T and, therefore, |Δx| \( \ll \) |x|), having restricted ourselves to the linear term over increment x, we obtain
Then,
Taking into account (5), one can describe the sublimation enthalpy at temperature T0 by the formula
from which we obtain
Expression (13) can be rewritten in the form
where
Thus, having calculated the dependence of lnI on T–1, which is almost linear in a narrow temperature range [T0, T0 + ΔT], one can find the sublimation enthalpy in the beginning of the temperature range ΔHS(T0) using formula (14).
RESULTS AND DISCUSSION
Crystal Growth
As an example, Fig. 1 shows the samples of crystals of the materials under study, grown from the vapor phase.
The naphthalene crystals presented in Fig. 1a were grown naturally on the walls of a plastic vessel with material during its storage at 0°C. One can observe somewhat faceted convex crystals with lengths up to 5 mm. Lamellar faceted naphthalene crystals with lengths up to 10 mm and thicknesses up to 1 mm can be grown on a thread under the lid of a sealed glass vial with fine-grained material at its bottom, being heated on a hot plate at 40–50°C for several hours (Fig. 1b).
Figure 1c shows the largest anthracene single crystal (under UV illumination), which was grown using PVT for 12 h at a material-source temperature of 150°C. This temperature was shown to be optimal for growing large crystalline anthracene samples. The crystal length was 17 mm, and its thickness was no more than 500 µm. Further crystal growth was limited by the growth-space sizes. It can be seen that the crystal is partially faceted on the left side; however, the lateral faces are mainly curved.
The samples of tetracene crystalline films (under UV illumination), grown for 72 h at a temperature of 215°C near the material source, are shown in Fig. 1d. The length and thickness of the largest crystalline samples reached 5 mm and 10–30 µm, respectively. Because of small thickness, the tetracene crystalline films are often curved.
Thus, the growth period of centimeter-sized crystals increases strongly, and their shape acquires a higher degree of anisotropy with an increase in the length of conjugate linear molecule.
XRD Analysis
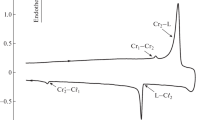
At room temperature, the structures of naphthalene and anthracene crystals are characterized by the monoclinic symmetry P21/a, and the tetracene structure is characterized by the triclinic symmetry P\(\bar {1}\) [20–22]. The XRD patterns of flat single crystals of naphthalene, anthracene and tetracene, obtained at reflection from the primary face, are shown in Fig. 2.
As one can see in Fig. 2, the X-ray diffraction pattern for the materials under study is a set of narrow peaks, the positions of which are approximately multiple of the position of the corresponding principal maximum (Table 1). According to [20–22], the observed diffraction pattern for the acene crystals under study corresponds to the reflection from the family of planes (00l) and characterizes high structural quality. The data on the crystal structure; the interplanar distances d00l, calculated according to the Bragg equation; and the corresponding values from the literature are listed in Table 1. The lengths of molecules ln are also given for comparison. It can be seen that the interplanar distances d00l, determined under our conditions, are in good agreement with the data for the materials under study from [20–22].
Isothermal Thermogravimetry and Determination of Sublimation Enthalpy
The dependences of the logarithm of the intensity of source-material weight loss on reciprocal temperature for the linear acenes under study are shown in Fig. 3. The melting temperatures of naphthalene and anthracene (353 and 491 K, respectively) are indicated by arrows.
It can be seen that the ln(I) = f(T–1) dependence for naphthalene is closest to linear (R2 = 0.996) in the range of 328–353 K, and the plot for the liquid phase above the melting temperature has a nonlinear, with a steeper slope.
For anthracene, the best convergence of points to a linear dependence in the investigated temperature range is obtained in the range of 423–458 K (R2 = 0.995). One can see that the ln(I) = f(T–1) curve flattens significantly at higher temperatures of the anthracene solid phase (up to the melting point) and grows radically at the transition to the molten state. The dependence of the flattening near the melting temperature may be related to sintering of the powder material, which decreases the specific surface area of the solid phase and, correspondingly, the flux of sublimating molecules.
The dependence for tetracene is closest to linear in the investigated temperature range at 513–573 K (R2 = 0.992).
The results of approximating the experimental data according to formula (14) and found values of sublimation enthalpy ΔHS,T for naphthalene, anthracene, and tetracene in the corresponding temperature ranges with the best convergence to a linear dependence are presented in Table 2. The sublimation enthalpy values were recalculated at 298 K using the data on the temperature dependences of the isobaric molar specific heat for naphthalene [23], anthracene [24], and tetracene [25]. The found ΔHS,298 values are given in Table 2. The experimental data on the sublimation enthalpies of the materials under study in similar temperature ranges are shown on the right for comparison. It can be seen that the results obtained are in good agreement with the data in the literature on the linear acenes under study.
CONCLUSIONS
The results obtained indicate that the method of physical vapor transport can be efficiently used to grow centimeter-sized organic semiconductor crystals (in particular, tetracene). It was shown that the growth rates and crystal sizes reach maximum at some optimal gradient temperature field. With an increase in the molecular length, the growth rate of crystals decreases strongly, and their shape anisotropy increases (crystals are generally shaped as plates (naphthalene, anthracene) or films (tetracene)). According to the XRD data, the grown centimeter-sized plane crystals are oriented in the (001) plane and characterized by high structural quality.
The analysis of the process of material sublimation from the surface of crystalline material in the quasi-steady-state approximation for narrow temperature ranges provided a linear dependence of logarithm of the sample weight-loss intensity on the reciprocal temperature (the sublimation-enthalpy value with a small correction is used as the proportionality factor). Under the PVT conditions, the obtained dependence is in good agreement with the experimental results for the linear acenes under study in the corresponding temperature ranges: 328–353, 423–458, and 513–573 K for naphthalene, anthracene, and tetracene, respectively. The calculated values of sublimation enthalpies of the investigated materials are in good agreement with the data in the literature. It should be noted that, in view of some model assumptions, the proposed thermogravimetric method cannot be considered as a high-precision one; however, it makes it possible to obtain relatively easily an experimental estimate of the sublimation enthalpy under the crystal-growth conditions using the PVT method (by determining the weight loss and fixing temperature near the material source and the process duration). Due to this, the method may be of great interest for the research groups investigating the crystallization of novel and understudied organic molecules.
REFERENCES
R. A. Laudise, C. Kloc, P. G. Simpkins, and T. Siegrist, J. Cryst. Growth 187, 449 (1998). https://doi.org/10.1016/S0022-0248(98)00034-7
V. A. Postnikov, N. I. Sorokina, M. S. Lyasnikova, et al., Crystals 10, 363 (2020). https://doi.org/10.3390/cryst10050363
V. A. Postnikov, A. A. Kulishov, O. V. Borshchev, et al., Poverkhn.: Rentgenovskie, Sinkhrotronnye Neitr. Issled., No. 1, 28 (2021). https://doi.org/10.31857/s1028096021010131
V. A. Postnikov, A. A. Kulishov, M. S. Lyasnikova, et al., Zh. Fiz. Khim. 95, 1101 (2021). https://doi.org/10.31857/S0044453721070220
J. P. Murray, K. J. Cavell, and J. O. Hill, Thermochim. Acta 36, 97 (1980). https://doi.org/10.1016/0040-6031(80)80114-6
L. A. Torres-Gómez, G. Barreiro-Rodríguez, and A. Galarza-Mondragón, Thermochim. Acta 124, 229 (1988). https://doi.org/10.1016/0040-6031(88)87025-4
M. Knudsen, Ann. Phys. 333, 999 (1909). https://doi.org/10.1002/andp.19093330505
M. A. V. Ribeiro da Silva, M. J. S. Monte, and L. M. N. B. F. Santos, J. Chem. Thermodyn. 38, 778 (2006). https://doi.org/10.1016/j.jct.2005.08.013
D. Ambrose, I. J. Lawrenson, and C. H. S. Sprake, J. Chem. Thermodyn. 7, 1173 (1975). https://doi.org/10.1016/0021-9614(75)90038-5
C. G. de Kruif, T. Kuipers, J. C. van Miltenburget, et al., J. Chem. Thermodyn. 13, 1081 (1981). https://doi.org/10.1016/0021-9614(81)90006-9
O. T. Glukhova, N. M. Arkhangelova, A. B. Teplitsky, et al., Thermochim. Acta 95, 133 (1985). https://doi.org/10.1016/0040-6031(85)80041-1
B. T. Grayson and L. A. Fosbraey, Pestic. Sci. 13, 269 (1982). https://doi.org/10.1002/ps.2780130308
K. Nass, D. Lenoir, and A. Kettrup, Angew. Chem. Int. Ed. Engl. 34, 1735 (1995). https://doi.org/10.1002/anie.199517351
O. Shalev and M. Shtein, Org. Electron. 14, 94 (2013). https://doi.org/10.1016/j.orgel.2012.09.033
R. V. Ralys, G. S. Yablonsky, and A. A. Slobodov, Sci. Tech. J. Inf. Technol. Mech. Opt. 15, 1072 (2015). https://doi.org/10.17586/2226-1494-2015-15-6-1072-1080
W. Zielenkiewicz, G. L. Perlovich, and M. Wszelaka-Rylik, J. Thermal Anal. Calorim. 57, 225 (1999). https://doi.org/10.1023/A:1010179814511
P. V. Lebedev-Stepanov, Introduction into Self-Organization and Self-Assembly of Nanoparticle Ensembles (NRNU MEPhI, Moscow, 2015) [in Russian].
I. S. Grigor’ev and E. Z. Meilikhov, Physical Values: A Handbook (Energoatomizdat, Moscow, 1991) [in Russian].
L. D. Landau and E. M. Lifshitz, Course of Theoretical Physics, Vol. 5: Statistical Physics, Part 1 (Fizmatlit, Moscow, 2002; Pergamon, Oxford, 1984).
D. W. J. Cruickshank, Acta Crystallogr. 10, 504 (1957). https://doi.org/10.1107/s0365110x57001826
C. P. Brock and J. D. Dunitz, Acta Crystallogr. B 46, 795 (1990). https://doi.org/10.1107/S0108768190008382
J. M. Robertson, V. C. Sinclair, and J. Trotter, Acta Crystallogr. 14, 697 (1961). https://doi.org/10.1107/s0365110x61002151
R. D. Chirico, S. E. Knipmeyer, and W. V. Steele, J. Chem. Thermodyn. 34, 1873 (2002). https://doi.org/10.1016/S0021-9614(02)00262-8
P. Goursot, H. L. Girdhar, and E. F. Westrum, J. Phys. Chem. 74, 2538 (1970). https://doi.org/10.1021/j100706a022
M. Fulem, V. Laštovka, M. Straka, et al., J. Chem. Eng. Data 53, 2175 (2008). https://doi.org/10.1021/je800382b
B. Stevens, J. Chem. Soc. 2973 (1953). https://doi.org/10.1039/jr9530002973
L. Malaspina, R. Gigli, and G. Bardi, J. Chem. Phys. 59, 387 (1973). https://doi.org/10.1063/1.1679817
R. Bender, V. Bieling, and G. Maurer, J. Chem. Thermodyn. 15, 585 (1983). https://doi.org/10.1016/0021-9614(83)90058-7
F. Emmenegger and M. Piccand, J. Therm. Anal. Calorim. 57, 235 (1999). https://doi.org/10.1023/A:1010100531350
M. A. Siddiqi, R. A. Siddiqui, and B. Atakan, J. Chem. Eng. Data 54, 2795 (2009). https://doi.org/10.1021/je9001653
V. Oja, X. Chen, M. R. Hajaligol, and W. G. Chan, J. Chem. Eng. Data 54, 730 (2009). https://doi.org/10.1021/je800395m
P. E. Fielding and A. G. Mackay, Aust. J. Chem. 17, 1288 (1964). https://doi.org/10.1071/CH9641288
G. C. Morris, J. Mol. Spectrosc. 18, 42 (1965). https://doi.org/10.1016/0022-2852(65)90059-7
N. Wakayama and H. Inokuchi, Bull. Chem. Soc. Jpn. 40, 2267 (1967). https://doi.org/10.1246/bcsj.40.2267
V. Oja and E. M. Suuberg, J. Chem. Eng. Data 43, 486 (1998). https://doi.org/10.1021/je970222l
ACKNOWLEDGMENTS
The experiments were carried out using equipment of the Center for Collective Use “Structural Diagnostics of Materials.”
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation within the State assignment for the Federal Scientific Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Sin’kov
Rights and permissions
About this article
Cite this article
Postnikov, V.A., Kulishov, A.A., Yurasik, G.A. et al. Growth of Linear Acene Crystals and Determination of Their Sublimation Enthalpy under Conditions of Physical Vapor Transport. Crystallogr. Rep. 67, 608–615 (2022). https://doi.org/10.1134/S1063774522040137
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774522040137