Abstract
The first representative of the eudialyte group with a primitive unit cell has been investigated by X-ray diffraction analysis, electron probe microanalysis, and IR spectroscopy. The mineral under study belongs to the system of sergevanite–raslakite–oneillite solid solutions; it is a low-symmetry variety of sergevanite, in which a high-valence zirconium cation compensates charge at the M2 site. The trigonal unit-cell parameters of the mineral are found to be a = 14.204(1) Å, c = 30.087(3) Å, and V = 5257.0(1) Å3; sp. gr. P3; the number of independent sites is 165. The crystal structure is refined to the final reliability factor R = 4.9% in the anisotropic approximation of atomic displacements using 5620 reflections with F > 3σ(F). The idealized formula of the mineral (Z = 3) is Na15Ca3(Mn,Fe)3Zr3[Na2Zr][Si26O72](O,OH)3Cl⋅Н2О.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The structures of the zirconosilicates belonging to the eudialyte group are based on a heteropolyhedral framework, characterized by a constant composition, which determines the constancy of the R symmetry of these minerals [1–4] (rascrystal.ru). Both the variable composition of extra-framework sites and the isomorphic substitutions in them obey the symmetry of this type and the mineral structures are described within three space groups: R3m, R\(\bar {3}\)m, and R3. In most cases, the absence of symmetry plane in the R3 group is due to the isomorphism in the СаО6 octahedra of six-membered ring at a significantly reduced calcium content and its replacement with manganese, iron, and some other elements. Ordering of these elements over the sites of six-membered ring leads to its division into two triads of octahedra with shared edges, but with nonequivalent compositions and sizes. In the case of zirconium and titanium ordering in isolated framework octahedra (alluaivite, dualite [1]), isomorphism is implemented with parameter doubling (c ~ 61 Å), but also with conservation of R symmetry. Ordering of other elements along the c axis may also lead to cell doubling within three above-mentioned space groups. Although dozens of structurally studied eudialyte-group minerals (EGMs) were described within the R lattice [3, 4], it is theoretically possible that the isomorphism in the eudialyte-structure sites may lead to further symmetry lowering with violation of R translation. However, the transition to a primitive cell, accompanied by a threefold increase in the number of independent sites, requires a corresponding increase in the number of experimental reflections, which should contain reflections that do not obey the R-lattice extinction laws. These reflections have often been experimentally observed; however, they were rare and barely exceeded the 3σ value. In addition, the number of unique reflections after averaging of equivalents was generally in the range from 2000 to 3000 (most often, ~2500) reflections, which is insufficient for analyzing a structure containing more than 150 independent sites. For example, an attempt to lower the symmetry of the structure of a calcium-poor eudialyte-like mineral with a transition from an R3 cell to a primitive cell using ~2000 unique reflections [5] was unsuccessful and did not make it possible to solve the structure. The same is valid for the structural refinement within sp. gr. R3m with one cation site in the octahedra of a six-membered ring: the R factor remained above 20%, the atomic displacement parameters were overestimated, and the sites could hardly be localized using difference maps.
The purpose of this study was to analyze for the first time the aforementioned mineral, which made it possible to lower its symmetry to P3 and to obtain close-to-real information about the eudialyte structure.
EXPERIMENTAL
The EGM under study, whose composition is similar to that of sergevanite [6] (however, with elevated zirconium content), was found in an ultra-agpaite pegmatite on the Alluaiv mountain (Lovozero alkaline complex, Kola peninsula). It forms bright red (to yellow-orange in thin cleavages) xenomorphic single-crystal individuals up to 3 cm in size in a giant-grained aggregate, composed mainly of nepheline, sodalite, microcline, and arfvedsonite with subordinate EGM, lamprophyllite, lorenzenite, and aegirine. The pegmatite also contains lomonosovite as an accessory component.
The chemical composition was investigated by electron probe X-ray microanalysis using a Tescan Vega-II XMU scanning electron microscope (EDS mode, accelerating voltage 20 kV, current 400 pA) and an INCA Energy 450 system for detecting X rays and calculating the sample composition. The chemical composition is given in Table 1; the composition calculated for 25.7 Si atoms (according to the X-ray diffraction data) is described by the following empirical formula (the ranges of the component contents are given taking into account the grain inhomogeneity, Z = 3): Na16–17K0.3–0.4Ln0.2–0.3Y0–0.2Ca2.75–2.9Mn1.5–1.6 Fe1.2–1.3Zr3.9–4.0Ti0.3–0.4Nb0.1–0.3Hf0.2Si25.6–25.9O72(OH,O,H2O)x Cl0.85–0.9.
The IR spectrum of the mineral, which was previously ground in an agate mortar and pelletized with KBr powder, was recorded on an ALPHA FTIR Fourier spectrometer (Bruker Optics, Germany) in the range of wavenumbers of 360–3800 cm–1 with a resolution of 4 cm–1; the number of scans was 16. A similar pure KBr pellet was used as a reference.
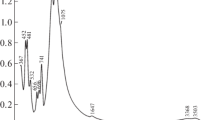
The IR spectrum of the EGM under study (Fig. 1) contains bands due to O–H stretching vibrations (in the range of 3400–3600 cm–1), bending vibrations of H2O molecules (at 1636 cm–1), Si–O stretching vibrations (in the range of 930–1100 cm–1), and various vibrations of the heteropolyhedral framework. The strong bands at 939 and 528 cm–1 indicate a high silicon content at the sites centering the Si9O27 ring and a high zirconium content in the planar-square coordination, respectively. Very weak bands at 1495 and 1425 cm–1 are due to carbonate anions, which are present in the mineral in trace amounts.
The diffraction data (МоKα radiation) were collected on a single-crystal Xcalibur CCD diffractometer (Oxford Diffraction). The main crystallographic characteristics and details of X-ray experiment are listed in Table 2.
Taking into account the low calcium content and possible ordering of the composition in the octahedra of six-membered ring, the structure was investigated within the sp. gr. R3. The atomic coordinates of the framework in the zirconium-poor species of sergevanite (whose composition is close to that of the sample under study) were used as a starting set [6]. The extra-framework sites were found from a series of difference electron-density maps. The compositions of cations at some sites and subsites were refined taking into account the mixed atomic-scattering curves. Despite a sufficiently low value of the R factor (4.9%) and the fact that satisfactory values of refined parameters were obtained at 70 independent sites, it was difficult to interpret the composition distribution because of the large number (50% of the total amount) of split and statistically occupied sites. In addition, the atomic displacements from the sites tending to splitting were overestimated.
At the same time, the diffraction dataset contained about 1% reflections that were not compatible with the R-lattice extinctions (–h + (k + l) = 3n). The intensities of most of 425 such reflections (with equivalents) exceeded 10 standard deviations (for 20 reflections, the intensities exceeded the 20σ value). According to the statistics of the set of normalized structure factors, the Еhkl values with indices –h + (k + l) = 3n are 0.875, 1.056, and 1.451 for E > 0, 0.5, and 1.0, respectively, whereas these values for Еhkl with indices –h + (k + l) = 3n + 1 and –h + (k + l) = 3n + 2 are 0.277, 0.717, and 1.014, respectively. Taking into account the fact that the total number of unique reflections exceeded common values by a factor of 2–2.5 and amounted to 5690 averaged reflections (with an averaging R factor of 2.8%), the symmetry was lowered, and a transition to a primitive lattice was performed. Based on the Wilson statistics for 1999 normalized structure factors, the experimental average value corresponds to E = 0.859, and the theoretical values are 0.886 and 0.798 for noncentrosymmetric and centrosymmetric structures, respectively, which indicates that the structure is likely acentric.
The structural model within the sp. gr. P3 was obtained using the “phase correction” procedure, which was developed with the AREN program [7]. After several iterations, 165 sites corresponding to the complete model of the P3 structure with an R factor of 28%, were obtained from the initial fragment, which contained 35 framework sites. Distribution of elements between the sites in correspondence with the chemical composition data and further refinement in the isotropic and anisotropic approximations of atomic displacements reduced the R factor to 4.9%, and the number of statistically occupied sites in the model decreased to 20%.
All calculations were performed within the AREN crystallographic software [7]. The refined structural parameters and characteristics of coordination polyhedra are given in Tables 3–5. For brevity, Table 3 contains the structural parameters of extra-framework atoms and only part of framework ones.
DESCRIPTION OF STRUCTURE AND DISCUSSION OF RESULTS
The main compositional and structural features of the EGM under study are reflected in its crystallochemical formula (Z = 1), which is in good agreement with the empirical one: N1–5[(Na47.85K1.2] M1.1[(Mn,Fe)6Ca3]M1.2[Ca5.7(Mn,Fe)2.4Ce0.9]Z[Zr8.4Hf0.6] M2[Na6.15Zr2.85]M3[Si1.4Nb0.5Ti10.4]M4[Si1.5Ti0.79Nb0.5] [Si24O72]3(OH)7.17Cl2.75⋅3Н2О. Here, the compositions of key structure sites are given in square brackets. The idealized formula is Na15Ca3(Mn,Fe)3Zr3[Na2Zr] [Si26O72](OH,O)3Cl⋅Н2О (Z = 3), which is close to that of sergevanite (Na15(Ca3Mn3)(Na2Fe)Zr3Si26O72(OH)3⋅ H2O) [8]. The difference is in the presence of chlorine and zirconium in the M2 site as high-valence charge-compensating cations.
The characteristics of the framework SiО4 tetrahedra are close to those of the tetrahedra in the R-centered lattice. The standard deviations of the z coordinate for cations are 0.0001, while those of the x and y coordinates are, on average, 0.0002–0.0005. For oxygen atoms, these deviations are in the ranges of 0.0002–0.0006 and 0.001–0.002, respectively. The deviations from R centering for cations and anions lie in the ranges of 0.0001–0.0005 and 0.001–0.005, respectively. The equivalent atomic displacement parameters are within 0.5–1.3 and 1.0–2.5 Å2 for cations and anions, respectively, which indicates the absence of correlations of the structural atomic characteristics at the framework sites bound by R-lattice pseudotranslations.
The mineral under study has some chemical and structural features, which were revealed at the key structural sites at symmetry lowering. The Z site, which is unified in the R cell, is split into three independent sites, occupied by Zr atoms with Hf impurity (Hf is fixed at one site rather than distributed between all three ones) with violation of the R translation: (1/3 2/3 2/3) and (2/3 1/3 1/3) (Tables 3, 4).
The chemical composition of the sample under study is characterized by low calcium content and approximately equal Fe and Mn contents, which supplement missing Ca amount in one out of two independent octahedra of the six-membered ring of R cell. These two sites in the P cell correspond to six independent M1 sites (Fig. 2), one of which is split into two subsites, spaced by 0.4 Å and occupied by (Mn, Fe) and Ca atoms, respectively, the (Mn,Fe) subsite being dominant (Table 4). Similar splitting was repeatedly observed previously at one out of two M1 sites of structurally investigated minerals of a group with the R3 symmetry [9, 10]. This splitting is also retained at symmetry lowering in this sample; however, it occurs only along one out of three axes with violation of the R centering. Other sites are occupied by either Ca or Mn(Fe) atoms; note that their distribution around three axes violates R centering because of the composition alternation: Са–(Mn,Fe)–(Ca+Ce) and (Mn,Fe,Ca)–Ca–(Mn,Fe). Additional violation is possible due to the distribution of Mn and Fe atoms.
Parallel edges of the octahedra belonging to neighboring six-membered rings form a polyhedron with a configuration close to a flat square, which coordinates the key site M2. In the sample analyzed, this site in the R cell is split into three statistically realized sites with the М2–М2а and М2–М2b distances of 0.48(1) and 0.64(1) Å, respectively. Two subsites are occupied with sodium and the third one is occupied with zirconium. The square center is occupied with zirconium cations (excessive with respect to three Zr atoms per formula unit at the Z1.1, Z1.2, and Z1.3 sites) with the average distance М2–О = 2.12 Å, and the Na subsites with the М2a–O and М2b–O distances of 2.15 and 2.16 Å, respectively, are located at both square sides in five-vertex polyhedra. At symmetry lowering, all three subsites exist independently near three axes at large distances from each other and with different occupancies, which make in total 100% (Fig. 3). The zirconium (with Na impurity) site is located near one axis in square coordination with the distance to the square vertices М2.3–О = 2.00(2)–2.15(1) Å (in average, 2.07 Å). The second site near another axis is occupied only by Na atoms, around which a five-vertex polyhedron М2.2O4(OH) is formed (the average distance to four oxygen atoms and the OH group is 2.24 Å). Finally, the third site is also located in a five-vertex polyhedron (М2.1–О = 2.11 Å) near the third axis and occupied by Na atoms with zirconium impurity. Thus, the occupation of M2 sites violates R centering, both because of the composition and the shapes and sizes of polyhedra. Taking into account the occupancy of these sites, Na is the dominant cation in the M2 microregion (Table 4). In combination with the M1 polyhedra with different compositions of the six-membered ring, a layer-by-layer (in the planes oriented perpendicular to the threefold axis) block isomorphism is implemented in this mineral (Fig. 3). The layered blocks also contain axial polyhedra: a SiО4 tetrahedron, a NbО6 octahedron, and a TiО6 octahedron are located in the layers at z = 0, 0.33, and 0.66, respectively; they also contribute to the violation of R centering.
Each of two other species-forming sites (M3 and M4), being located on the threefold axis near the centers of the Si9O27 nine-membered Si–O rings, is split within R symmetry into three and four subsites, spaced by short distances and occupied statistically by Si, Ti, and Nb atoms. Splitting in the P cell is retained, however, it results in the appearance of two subsites located at both sides of the nine-membered rings (Table 5). An exception is the M3.3b site, in which the Si and Nb subsites with low occupancies are overlapped. On two threefold axes, conventional orientation of SiО4 tetrahedra (into the interring cavity N5) is observed both at the M3 and M4 sites, and TiО6 and NbО6 octahedra are rotated outside from the cavity. However, on the third axis, the Si site is occupied by Ti atoms and the OH groups, which form their octahedra, occupy the N5 cavity, displacing Na atoms. On the whole, the distribution along three independent axes is nonuniform: the M4 sites contain more silicon and titanium in comparison with the M3 sites, which contain more vacancies.
The general dominance of Si atoms at the M3 and M4 sites makes it possible to relate the mineral under study to silicon-rich representatives of the minerals with the oneillite structural type (e.g., raslakite and sergevanite) (Table 6). One might suggest that, at close compositions, high-temperature crystallization yields high-symmetry structures (sp. gr. R3m and R\(\bar {3}\)m), while structures with the axial sp. gr. R3 and P3 are formed under slow-crystallization conditions. Low-temperature EGMs are often enriched with non-coherent elements (Zr, Hf, Nb), which do not enter the composition of the main rock-forming minerals but are accumulated in the late mineral-forming fluid. In particular, in many EGMs with sp. gr. R3 (and P3), zirconium occupies not only the Z octahedron but also the M2 site, which is almost completely occupied by Fe2+ in eudialyte (Table 6). From the geochemical point of view, high hafnium content in the studied EGM with the symmetry P3 is of interest (the Zr : Hf atomic ratio is about 20, although this value for EGMs is usually in the range of 60–100).
One of the specific features of minerals with microporous heteropolyhedral structures, to which EGMs belong, is splitting of sites of large cations in the zeolite-like framework cavities with partially occupied subsites. Na cations in the R cell of this sample are distributed between five independent N sites, each of which is split into two to three subsites, occupied by Na atoms with a small amount of K impurity. As the transition to the P cell shows, these sites are split not because of the ordering of cation composition (in this case, only Na) but due to the displacement of cations and formation of polyhedra with different volumes (Table 4). Among 16 independent sites, only four N5 sites are located at shortened distances from each other in the cavity, limited from above and below by Si9O27 rings and occupied (along with Na atoms) by the OH groups of axial SiО4 and TiО6 octahedra (Table 5).
The anion sites X1 and X2, located on the threefold axis, are occupied in the R cell by Cl atoms (in approximately equal parts) and water molecules. In the P3 cell, they are distributed otherwise. The largest amount of Cl (1.56 atoms) is distributed in the X1 region between three independent axes, alternating statistically with water molecules, and the smaller part (1.2 atoms) occupies only two axes in the X2 region (along with water molecules), while only water molecules are located on the third axis. All sites of water molecules are split and occupied partially.
CONCLUSIONS
On the whole, the distribution of cations between the P-structure sites of the sample corresponds to that determined within the R structure; however, the study with symmetry lowering made it possible to establish some new regularities of cation ordering not only in the structure of this unique mineral but also in the structures of other eudialyte-group representatives. The R-centering violation, which was observed in the mineral under investigation, indicates that some eudialytes may have trigonal (rather than rhombohedral) real structure with a primitive lattice and the observed splitting is caused by cation ordering not only over different cells but, primarily, within one cell.
Splitting of Na sites within the R lattice and its absence in the P structure of this Na-rich mineral indicates that it may be due to not only the distribution of impurities but also displacements of cations with the formation of polyhedra of different volumes in crystallographically independent P-structure sites.
The observed sites with mixed occupancy in the P cell indicate that impurity cations populate a specific site in different cells (which sometimes may lead to doubling of parameter c [1]) rather than are distributed uniformly in the space of one cell. Based on the results obtained, one can predict the possibility of further ordering when the symmetry of sp. gr. P3 is combined with cell doubling. The number of split sites and sites with mixed occupancy are minimum due to the cation ordering in this hypothetical structure.
Calcium-poor minerals belong generally to the oneillite or raslakite structural types or are intermediate members of their solid solutions. Table 5 contains the dominant components in the key sites of calcium-poor zirconium-rich EGMs, which belong to the typomorphic components of peculiar rocks of the Lovozero alkaline complex: the most agpaite-rich species of nepheline syenites and related pegmatites. They are characterized by low symmetry and axial sp. gr. R3. However, the tendency to horizontal ordering, which is inherent to the trigonal eudialyte structure, was implemented in this sample as a result of the combination of chemical composition (low calcium content at high zirconium content) with unusual crystallization conditions. The phenomenon of the transition from high-symmetry crystals to lower-symmetry ones with a decrease in the crystallization temperature has been demonstrated in numerous studies devoted to the synthesis of inorganic compounds. This transition is induced by kinetic rather than thermodynamic factors and is related to the decrease in the process rate with a decrease in temperature. The sample under study is anomalous from the point of view of geochemistry and genesis. On the one hand, some signs (including xenomorphism with respect to all associating minerals and high water content) indicate its late-pegmatite origin. At the same time, high hafnium content is out of the general trend, according to which the Zr : Hf ratio generally increases during the alkaline-magma evolution [17].
REFERENCES
R. K. Rastsvetaeva, N. V. Chukanov, and S. M. Aksenov, Eudialyte-Group Minerals: Crystal Chemistry, Properties, Genesis (Izd-vo NGU, Nizhny Novgorod, 2012) [in Russian].
O. Johnsen, J. D. Grice, and R. A. Gault, Can. Mineral. 37, 865 (1999).
R. K. Rastsvetaeva, N. V. Chukanov, I. V. Pekov, et al., Minerals 10, 587 (2020). https://doi.org/10.3390/min10070587
R. K. Rastsvetaeva and N. V. Chukanov, Minerals 10, 720 (2020). https://doi.org/10.3390/min10080720
R. K. Rastsvetaeva, A. P. Khomyakov, and Yu. V. Nekrasov,) Crystallogr. Rep. 44 (5), 765 (1999).
R. K. Rastsvetaeva, N. V. Chukanov, and K. V. Van, Kristallografiya 65 (4), 562 (2020). https://doi.org/10.31857/S0023476120040190
V. I. Andrianov, Kristallografiya 32 (1), 228 (1987).
N. V. Chukanov, S. M. Aksenov, I. V. Pekov, et al., Can. Mineral. 58, 421 (2020). https://doi.org/10.3749/canmin.2000006
R. K. Rastsvetaeva, N. V. Chukanov, and D. V. Lisitsyn, Crystallogr. Rep. 66 (1), 112 (2021).
R. K. Rastsvetaeva and N. V. Chukanov, Crystallogr. Rep. 66 (1), 120 (2021).
R. K. Rastsvetaeva, N. V. Chukanov, and I. A. Verin, Dokl. Akad. Nauk 409 (6), 807 (2006).
S. M. Aksenov and R. K. Rastsvetaeva, Crystallogr. Rep. 58 (5), 671 (2013).
R. K. Rastsvetaeva, K. A. Rozenberg, I. V. Pekov, et al., Crystallogr. Rep. 51 (2), 205 (2006).
I. A. Ekimenkova, R. K. Rastsvetaeva, and N. V. Chukanov, Dokl. Akad. Nauk 374 (3), 352 (2000).
O. Johnsen, J. D. Grice, and R. A. Gault, Can. Mineral. 37, 1111 (1999).
R. K. Rastsvetaeva and A. P. Khomyakov, Crystallogr. Rep. 45 (4), 591 (2000).
L. N. Kogarko, Geokhimiya 54 (1), 4 (2016).
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation within the State assignment for the Federal Scientific Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences in the part concerning the X-ray diffraction analysis and by the Russian Foundation for Basic Research in the parts concerning the crystallochemical analysis of zirconium-rich eudialyte-group minerals (project no. 18-29-12005) and the collection of mineral, analysis of its chemical composition, and diagnostics of associating minerals (project no. 18-29-12007_mk). The IR spectroscopy study was carried out within a State contract (State account no. АААА19-119092390076-7). This study was performed using equipment of the Shared Research Center of the Federal Scientific Research Centre “Crystallography and Photonics” with support by the Ministry of Science and Higher Education (project no. RFMEFI62119X0035).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Sin’kov
Rights and permissions
About this article
Cite this article
Rastsvetaeva, R.K., Chukanov, N.V. Crystal Structure of the First Representative of the Eudialyte Group with Primitive Unit Cell. Crystallogr. Rep. 66, 940–948 (2021). https://doi.org/10.1134/S1063774521060304
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774521060304