Abstract
Sm1 – ySryF3 – y (0 < y ≤ 0.31) crystals have been grown from melt by directional solidification in a fluorinating atmosphere. The crystals have been studied by X-ray diffraction and optical spectroscopy, and their fluorine-ion conductivity σdc, density ρ, and refractive indices nD have been measured. It is established that Sm3+ ions are not reduced to Sm2+ during crystal growth. The reversible polymorphic α ↔ β-SmF3 transition does not make it possible to obtain bulk (>1–3 mm3) samples of the tysonite phase (of LaF3 type) at y < 0.02. The dependences ρ(y) and nD(y) for the crystals are descending. The dependence σdc(y) exhibits nonmonotonic behavior; the maximum σdc value (1.6 × 10–4 S/cm) at 293 K is observed for the Sm0.98Sr0.02F2.98 crystal. At y = 0.31, an eutectic composite 69SmF3 × 31SrF2 is formed, whose conductivity is σdc = 6 × 10–8 S/cm, a value smaller than σdc for the crystal with y = 0.02 by a factor of ~3 × 103. The carrier concentration nmob and its mobility μmob have been calculated for Sm1 – ySryF3 – y (0.02 ≤ y ≤ 0.25) within the hopping conductivity model. For the crystal with the highest conductivity (Sm0.98Sr0.02F2.98), nmob = 4.0 × 1020 cm–3 and µmob = 2.5 × 10–6 cm2/(V s) at T = 293 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Crystals based on fluorides of rare-earth elements (REEs) with a variable oxidation state (which include samarium, europium, and ytterbium) grown from melt in graphite containers are subjected to partial reduction (Sm3+ → Sm2+, Eu3+ → Eu2+, Yb3+ → Yb2+), the degree of which is difficult to control. As a result, data on high-temperature chemistry of trifluorides of REEs that are prone to form an atypical oxidation state (R2+) are not as comprehensive as the data for trifluorides of other REEs, because the chemical composition of these crystals was always unclear.
Nonstoichiometric tysonite (LaF3 type) SmF3-based phases of Sm1 – yMyF3 – y are of interest as fluoride-conducting solid electrolytes (FSE), whose deviation from stoichiometry provided by heterovalent isomorphic substitutions of Sm3+ with M2+ (M = Ca, Sr, or Ba). At a low reduction degree of Sm3+ ions these crystals can be considered as pseudobinary ones.
Proceeding from the data on the partial reduction of a SmF3 melt by graphite [1], researchers have tried to limit experiments on fluoride crystal growth with participation of this component. As a result, there is a lacuna in the data on SmF3 (and, to a less extent, EuF3 and YbF3) concerning both its high-temperature chemistry and possibilities of forming and using materials with its participation. Researchers had to accept this limitation when studying Sm-containing fluoride crystals in some applications (especially photonics).
For the optimization of compositions of strongly nonstoichiometric FSEs with respect to ionic conductivity (the purpose of our study), the low impurity concentration in crystals is not very crucial; this circumstance stimulated us to carry out experiments on growing nonstoichiometric SmF3-based crystals. The ion-conducting properties of a Sm0.875Sr0.125F2.875 crystal were previously investigated in [2, 3]; its high electrolytic characteristics were demonstrated. The Sm1 – ySryF3 – y tysonite phase is interesting as a FSE, and its properties are comparable with those of well-known high-conductivity solid electrolytes R1 – ySryF3 – y (R = La–Nd) [4–6].
Along with the applied problem of FSE optimization for new-generation current sources, this study is of great importance for the development of high-temperature chemistry of aliovalent REE fluorides and the fluoride materials science.
The chemical family of REEs is most numerous (17 elements) in the entire periodic system of elements. It is characterized by the significant effect of the REE type on the size of R3+ ions. These effects change nonmonotonically with an increase in the atomic number: the size increases from Sc3+ to Y3+ and then to La3+; afterwards, it decreases to Lu3+ (lanthanide compression effect). The change in the ionic radius is accompanied by three transformations of the structure type of RF3 compounds (morphotropic transformations), which divide the REE trifluoride series into five structural subgroups. The correctness of phase diagrams with participation of RF3, which underlie the fluoride materials science involving REE ions (which are of great practical importance in many fields of physics), depends on the structure type, presence (absence) of polymorphic transformations in compounds of these subgroups, and phase-transition temperatures.
The first morphotropic transition in the RF3 series is in the portion of the series covering Pm, Sm, and Eu. It turned out that fluorides of all these REEs have limitations for experimental studies. Promethium fluoride is inaccessible because of natural radioactivity, while samarium and europium fluorides have hardly been fabricated for practical applications because of their partial reduction. As a result, three REEs in succession were excluded from the series, which significantly hindered the interpolation of properties of Sm, Pm, and Eu compounds, a procedure that nicely works specifically for REEs in small portions of the series.
Under the conditions conventional for thermal analysis of the heating rates in nominally pure SmF3, there is a reversible phase transition from the nonconducting orthorhombic β form (sp. gr. Pnma, Z = 4) to the low-temperature fluoride-conducting tysonite form α-l-SmF3 (sp. gr. \(P\bar {3}c1\), Z = 6).
Note that the tysonite-type structure has two forms: trigonal (sp. gr. \(P\bar {3}c1\), Z = 6) and hexagonal (sp. gr. P63/mmc, Z = 2), which were distinguished in [7]; according to these data, these forms correspond to high- and low-temperature forms, respectively. A transition between them can be assigned to the diffuse type, which affects only one (anion) sublattice. A similar transition, observed for compounds with the fluorite-type structure, is not a reconstructive polymorphic transformation. To distinguish the diffuse transition (between forms) and the polymorphic transition (between modifications), we decided to retain the generally accepted designations of modifications with Greek letters (α, β). Different types of the tysonite (in our case) structure are denoted in accordance with the their temperature stability regions: h (high-temperature) and l (low-temperature) forms. They slightly differ in X-ray powder diffraction patterns, are not always determined in studies, and may coexist in one crystal. When these forms are determined (as in this paper), one should use double designations (including modification and form).
In view of the aforesaid, the tysonite modification α-SmF3 undergoes, in turn, a diffuse transition from the low-temperature form α-l-SmF3 (sp. gr. \(P\bar {3}c1\), Z = 6) to the high-temperature form α-h-SmF3 (sp. gr. P63/mmc, Z = 2) at T ∼ 1100°C [8]. Among two REEs (Sm and Eu), the polymorphic transition in EuF3 occurs at 852 ± 8°C [7] (i.e., in the temperature range where the bulk diffusion is unfrozen), and its presence is well proven by thermal analysis. In SmF3 (the melting temperature is Tm = 1304 ± 10°C [7] and the freezing threshold of bulk diffusion is ~540°C), the phase polymorphic transformation is near 495°C [8], where the bulk diffusion is frozen. This leads to a spread of several tens of kelvins between the transformation temperatures obtained by different researchers [9]. As a result, it is difficult to locate reliably the morphotropic transition between PmF3 and SmF3.
A practical goal of our investigation was to obtain a concentration series of Sm1 – ySryF3 – y tysonite crystals, the growth of which had been hindered for a long time by considerations about the presence of destructive polymorphic α ↔ β transformation in SmF3; this problem was methodically solved for the first time.
Much interest in R1 – yMyF3 – y tysonite crystals (M = Sr or Ba, R is a REE) has been shown only recently, after the great progress in the design of new fluorine-ion current sources, which can compete now with lithium-ion current sources in many parameters [10–14].
The purpose of this study was to grow Sm1 – ySryF3 – y tysonite-phase crystals and 69SmF3 × 31SrF2 eutectic composition from melt; characterize them by X-ray diffraction (XRD) analysis and optical spectroscopy; and investigate the concentration dependences of fluorine-ion conductivity σdc(y), density ρ(y), and refractive index nD(y).
EXPERIMENTAL
Crystal Growth and Preparation of a Eutectic Composite
The phase diagram of the SrF2–SmF3 system [15] is shown in Fig. 1. This system belongs to the eutectic type with the following eutectics coordinates: temperature 1312 ± 10°C and composition 69 ± 2 mol % SmF3. The growth of crystals of the Sr1 – xSmxF2 + x fluorite phase (phase F in Fig. 1) and their complex characterization were presented in [16, 17]. The addition of SrF2 to SmF3 leads to the formation of the Sm1 – ySryF3 – y nonstoichiometric phase with a tysonite-type defect structure (LaF3), in which the SrF2 content varies in the range of 0–23 mol % at the eutectic temperature (phase T in Fig. 1). A temperature maximum (1340 ± 10°C) is observed in the liquidus curves of the Sm1 – ySryF3 – y phase for the composition with 13 mol % SrF2 (mole fraction y ≈ 0.13), which exceeds the melting temperature of the component SmF3 by ~35°C.
The growth experiments were carried out using compositions Sm1 – ySryF3 – y with the SrF2 content in the range of y = 0–0.31. The Sm1 – ySryF3 – y crystals and 69SmF3 × 31SrF2 composite were grown by directional solidification in a two-zone system with resistive heating and a graphite heating unit in a mixed atmosphere of high-purity helium and CF4 (up to 50 vol %). The initial agents were SmF3 (purity 99.99 wt %, LANHIT) and SrF2 (purity 99.995 wt %, Sigma-Aldrich) powders. Multicellular graphite crucibles with seed capillary channels were used. The temperature gradient in the growth zone was ~80°C/cm. The crucible pulling rate was ~3 mm/h. The mean crystal cooling rate after the growth was 100°C/h. The evaporation loss did not exceed 0.5–0.8 wt %.
X-ray diffraction analysis was performed with a Rigaku MiniFlex 600 X-ray powder diffractometer (radiation CuKα). X-ray diffraction patterns were recorded in the range of 2θ angles from 10° to 100°. The phases were identified using the ICDD PDF-2 (2014) database. The unit-cell parameters were refined by the Rietveld method using the X'Pert HighScore Plus software (PANanalytical, the Netherlands).
Samples with a thickness of h = 2 mm for the investigations were cut from the central portions of the Sm1 – ySryF3 – y crystals (0.03 ≤ y ≤ 0.31) and polished. The sample with y = 0.02 was 1 × 3 × 3 mm3 in size.
Crystal density ρ was measured by hydrostatic weighing in distilled water at room temperature with an error of Δρ = ±0.005 g/cm3.
Refractive indices nD (wavelength λ = 0.589 µm) were investigated by the refractometric method (immersion liquid α-bromonaphthalene) at room temperature using an IRF-454 refractometer.
Optical transmission spectra of the crystals were recorded at room temperature with a Cary 5000 spectrophotometer (Agilent Technologies) and a Nicolet Nexus 5700 IR Fourier spectrometer (Thermo Scientific) in the wavelength range of λ = 0.2–15 µm.
Electrical measurements were performed on unoriented samples because the anisotropy of conductivity of crystals of the tysonite nonstoichiometric phases can be neglected [18, 19]. Inert electrodes were made of graphite paste Dag-580 (Acheson Colloids). Dc electrical conductivity σdc was determined by impedance spectroscopy. The technique of electrical measurements was reported in [20]. The relative error in measuring σdc was 5%.
Complex impedance Z*(ω) of the concentration series of Sm1 – ySryF3 – y crystals at room temperature (T = 293 K) was measured in the frequency range of 5–5 × 105 Hz and at resistances from 1 to 107 Ω (impedancemeter Tesla BM–507) in a vacuum (~1 Pa). Temperature investigations of impedance Z*(ω) for the Sm0.97Sr0.03F2.97 crystal and 69SmF3 × 31SrF2 eutectic composite were carried out in the temperature range of 290–541 K.
The presence of the blocking effect from inert (graphite) electrodes in the low-frequency impedance spectra indicates the ionic character of electrotransfer in the crystals. For the 69SmF3 × 31SrF2 eutectic composite, the total conductivity of the sample was found; it was not separated into bulk and intercrystallite contributions.
RESULTS AND DISCUSSION
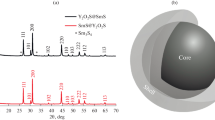
We prepared 14 Sm1 − ySryF3 − y crystal boules 12 mm in diameter and 25–35 mm long with a content of 0.005 ≤ y ≤ 0.31 (Fig. 1b). Crystals with 0.03 ≤ y ≤ 0.21 had no cracks and bulk inclusions. For the samples with 0.005 ≤ y ≤ 0.02, the transparent phase was observed only at the bottom of the boules and was less than 2% of the total length. A semitransparent rejected phase was observed at the top of the crystal for compositions in the range of 0.18 ≤ y ≤ 0.25; the sample with y = 0.31 had two phases and was visually semitransparent. Note that the Sm1 − ySryF3 − y samples with 0.005 ≤ y ≤ 0.02 were stuck in the graphite crucible cells. This mechanical sticking of Sm1 − ySryF3 − y crystals is caused by the β-SmF3 (sp. gr. Pnma) ↔ α-l-SmF3 (sp. gr. \(P\bar {3}c1\)) polymorphic transition accompanied by a decrease in the density.
An XRD analysis of the Sm1 − ySryF3 − y crystals showed that they are single-phase throughout the entire volume for the compositions of 0.03 ≤ y ≤ 0.18 (Fig. 2). Sm1 − ySryF3 − y crystal boules with 0.005 ≤ y ≤ 0.02 are two-phase. Opaque upper regions of these boules (Fig. 1a) correspond to the orthorhombic β-SmF3 phase (sp. gr. Pnma), while the transparent regions correspond to the tysonite phase α-l-Sm1 − ySryF3 − y (sp. gr. \(P\bar {3}c1\)). The low-temperature phase α-l-Sm1 − ySryF3 − y is formed during the crystallization of compositions with 0.005 ≤ y ≤ 0.08. The compositions with y > 0.08 exhibit a transition from the tysonite form α-l-Sm1 − ySryF3 − y (sp. gr. \(P\bar {3}c1\)) to the high-temperature form α-h-Sm1 − ySryF3 − y (sp. gr. P63/mmc) and its stabilization.
The semitransparent rejected substance at the top of the Sm1 − ySryF3 − y samples for 0.18 ≤ y ≤ 0.25 consists of a mixture of the “impurity” fluorite phase Sr1 − xSmxF2 + x (sp. gr. \(Fm\bar {3}m\)) with the unit-cell parameter a = 5.775(1) Å and the primary tysonite phase α-h-Sm1 − ySryF3 − y, which is in agreement with the limiting solubility of SrF2 in the SmF3 matrix (y ≈ 0.23) obtained in [15].
The sample with y = 0.31 was a two-phase (throughout the entire volume) eutectic composite consisting of a mixture of nonstoichiometric phases with boundary compositions for the fluorite (sp. gr. \(Fm\bar {3}m\), a = 5.774(1) Å) and tysonite (sp. gr. P63/mmc, a = 4.0419(1) Å, c = 7.1951(1) Å) structures.
Figure 3 shows the concentration dependences of the lattice parameters a(y) and c(y) and unit-cell volume V of the Sm1 – ySryF3 – y nonstoichiometric-phase crystals. These data are in satisfactory agreement with the results of [21]. The parameter c(y) increases according to a weak quadratic law from 7.1323(1) to 7.1951(1) Å in the composition range of 0.005 ≤ y ≤ 0.31. In the range of y = 0.08–0.10 A, one can clearly see a change in the form of the tysonite-type structure and a transition from a “large cell” (α-l-Sm1 – ySryF3 – y, sp. gr. \(P\bar {3}c1\), Z = 6) to a “small cell” (α-h-Sm1 – ySryF3 – y, sp. gr. P63/mmc, Z = 2). The observed concentration-driven structural transition with a change in the unit-cell volume and space symmetry group is characteristic of R1 – yMyF3 – y tysonite nonstoichiometric phases (R = La–Nd, M = Ca, Sr, or Ba) [22, 23].
Concentration dependences of the lattice parameters (\(\bigcirc \),  ) and unit-cell volumes (\(\blacksquare \)) of the Sm1 – ySryF3 – y tysonite phase. The dotted lines are analytical dependences a(y) and c(y) according to the data of [21].
) and unit-cell volumes (\(\blacksquare \)) of the Sm1 – ySryF3 – y tysonite phase. The dotted lines are analytical dependences a(y) and c(y) according to the data of [21].
The results of measuring density ρ(y) of the Sm1 – ySryF3 – y tysonite-phase crystals are shown in Fig. 4a. The dependence ρ(y) has a practically linear (with an error of Δρ = ±0.02 g/cm3) descending character in the range of 0.03 ≤ y ≤ 0.23. The data on the densities of the tysonite α-l-SmF3 and orthorhombic β-SmF3 modifications [24] and the Sm0.87Sr0.13F2.87 crystal [25] are shown for comparison.
Concentration dependences of the (a) density and (b) refractive indices for the Sm1 – ySryF3 – y crystals at T = 293 K. Designations are as follows: (a) (\(\bigcirc \)) the experimental values; (\(\blacksquare \), ) the X-ray density for, respectively, α-SmF3 and β-SmF3 [24]; and (Δ) the data of [25] for Sm0.87Sr0.13F2.87)); (b) (\(\bigcirc \),
) the X-ray density for, respectively, α-SmF3 and β-SmF3 [24]; and (Δ) the data of [25] for Sm0.87Sr0.13F2.87)); (b) (\(\bigcirc \),  ) the experimental values for no and ne, respectively; (\(\diamond\),
) the experimental values for no and ne, respectively; (\(\diamond\),  ) no and ne, respectively, for Sm0.87Sr0.13F2.87 at λ = 0.635 µm [25]; and (\(\square \), \(\blacksquare \), \(\square \times \)) the principal refractive indices for β-SmF3 [26].
) no and ne, respectively, for Sm0.87Sr0.13F2.87 at λ = 0.635 µm [25]; and (\(\square \), \(\blacksquare \), \(\square \times \)) the principal refractive indices for β-SmF3 [26].
Dependences of the refractive indices nD of Sm1 – ySryF3 – y crystals on the composition (0.03 ≤ y ≤ 0.25) are shown in Fig. 4b. The crystals are uniaxial and optically negative (no > ne). The no values at wavelength λ = 0.589 µm decrease monotonically from 1.601(1) to 1.569(1) in the composition range y under consideration. The dependences nD(y) can be described by second-order polynomials, whose parameters are given in Fig. 4b. The birefringence is ΔnD ~ 0.008; it barely depends on the crystal composition y. As can be seen in Fig. 4b, the optical data for the Czochralski-grown Sm0.87Sr0.13F2.87 crystal [25] differ significantly from our data, which likely indicates an error in determining its chemical composition. The principal refractive indices of the β-SmF3 crystal [26] are given for comparison.
Optical transmission spectra of the Sm1 – ySryF3 – y crystals are shown in Fig. 5. The crystals are transparent in the IR range up to 13 µm. The spectra exhibit transitions from the ground 6H5/2 state to the above multiplets of the 4f5 Sm3+ configuration. Absorption bands caused by the 4f5–4f5d1 transitions (which are characteristic of Sm2+ ions) are located in the visible and UV spectral regions [27]. For comparison, Fig. 5 (curve 3) shows a transmission spectrum of the La0.99Sm0.01F3 crystal grown in a reducing atmosphere. The spectrum of this crystal exhibits absorption at λ < 0.7 µm, which indicates the presence of a fraction of reduced Sm2+ ions, which was not observed for the Sm1 – ySryF3 – y crystals under study. Thus, under the redox conditions of growing Sm1 – ySryF3 – y crystals from melt in graphite crucibles that were implemented in our experiments, there is no significant reduction of Sm3+ ions to state 2+.
The concentration dependence of the fluorine-ion conductivity of the Sm1 – ySryF3 – y tysonite-phase crystals (0.02 ≤ y ≤ 0.25) has decays monotonically (Fig. 6). The maximum conductivity (σdc = 1.6 × 10–4 S/cm) was found for the Sm0.98Sr0.02F2.98 crystal. Its conductivity is about 3 times lower than that of the Ce0.97Sr0.03F2.97 crystal, which has the highest conductivity in the R1 – ySryF3 – y system [3, 5]. The conductivity of the 69SmF3 × 31SrF2 eutectic composite is σdc = 6 × 10–8 S/cm, which is much smaller (by a factor of 2.7 × 103) than the σdc value for the Sm0.98Sr0.02F2.98 crystal. For comparison, Fig. 6 shows data on the conductivity of Sm1 – ySryF3 – y crystals (y = 0.125 [6] and 0.13 [28]).
Concentration dependences of the ionic conductivity σdc(y) for the (1–3) Sm1 – ySryF3 – y crystals and (4) 69SmF2 × 31SrF2 composite at T = 293 K: (1, 4) this study, (2) [6], and (3) [28]. The temperature dependences of the ionic conductivity σdc(T) for the (1) Sm0.97Sr0.03F2.97 and (2) Sm0.875Sr0.125F2.875 [6] crystals and (3) 69SmF2 × 31SrF2 composite are shown in the inset.
The temperature dependences of the ionic conductivity σdc(T) for the Sm0.97Sr0.03F2.97 crystal and 69SmF2 × 31SrF2 eutectic composite in the temperature range of 290–541 K are shown in the inset in Fig. 6 (curves 1, 3). The dependence σdc(T) were processed using the Frenkel–Arrhenius equation:
where A is the pre-exponential factor of conductivity, ΔHσ is the ion-transport activation enthalpy, k is the Boltzmann constant, and T is temperature. The Frenkel–Arrhenius equation parameters are as follows: A = 3.5 × 103 S K/cm and ΔHσ = 0.30 ± 0.03 eV for the Sm0.97Sr0.03F2.97 crystal and A = 2.7 × 106 S K/cm and ΔHσ = 0.65 ± 0.02 eV for the 69 SmF2 × 31SrF2 composite.
For comparison, the dependence σdc(T) for the congruently melting Sm0.875Sr0.125F2.875 crystal studied previously in [6] in a wide temperature range (173–1073 K) is shown in the inset in Fig. 6 (curve 2). In this range, σdc increases from 2 × 10–12 to 7 × 10–1 S/cm (i.e., by 11 orders of magnitude). At 573 K, the dependence σdc(T) is divided into two portions. The Frenkel–Arrhenius equation parameters for them are as follows: A = 9.1 × 105 S K/cm and ΔHσ = 0.53 ± 0.01 eV at 173–573 K and A = 5.6 × 104 S K/cm and ΔHσ = 0.39 ± 0.02 eV at 573–1073 K.
The results of studying the R1 – yMyF3 – y tysonite phases in the MF2–RF3 systems (M = Ca, Sr, or Ba) by the 19F NMR method [29–31] indicate that ion transport therein occurs in the anion sublattice. The mechanism of ionic conductivity in R1 – yMyF3 – y crystals is related to the migration of fluorine vacancies \(V_{{\text{F}}}^{ + }\); no defect clusters were found [2–6, 28, 32–34].
Heterovalent substitutions in the Sm1 – ySryF3 – y cation sublattice induce mobile fluorine vacancies in the anion sublattice:
where \(V_{{\text{F}}}^{ + }\) is the ion charge carrier. The ion transport in the Sm1 – ySryF3 – y crystals is determined by the characteristics of mobile vacancies \(V_{{\text{F}}}^{ + }\):
where q, nmob, and µmob are, respectively, the charge, concentration, and mobility of vacancies \(V_{{\text{F}}}^{ + }\). The charge-carrier concentration in ion conductors R1 – yMyF3 – y is temperature-independent and determined by the mechanism of formation of “impurity” vacancies (2). Considering the structural data, we calculated the concentration of carriers,
and then, using (3) and (4), determined their mobility µmob.
The calculated nmob and µmob values for the Sm1 – ySryF3 – y crystals are presented in Fig. 7. The charge-carrier concentration at T = 293 K for the crystal with the highest conductivity (Sm0.98Sr0.02F2.98) is nmob = 4.0 × 1020 cm–3 at the mobility μmob = 2.5 × 10–6 cm2/(V s). When the SrF2 content increases, the dependences nmob(y) and µmob(y) behave differently. The nmob values increase by a factor of 12 with an increase in y from 0.02 to 0.25, whereas the μmob values decrease by a factor of 2 × 104. The decrease in σdc for the Sm1 – ySryF3 – y crystals in the composition range of 0.02 ≤ y ≤ 0.25 is caused by a decrease in the carrier mobility due to ion–ion interactions between carriers.
CONCLUSIONS
A series of Sm1 – ySryF3 – y crystals (0 < y ≤ 0.31) was grown from melt by the Bridgman method for the first time. It was shown that the α ↔ β polymorphic transformation in SmF3 does not make it possible to prepare bulk Sm1 – ySryF3 – y tysonite-phase samples (type LaF3) for the compositions of y < 0.02. Under the implemented conditions of growing Sm1 – ySryF3 – y crystals from melt in a graphite crucible in a fluorinating atmosphere (CF4), no significant reduction of Sm3+ ions to Sm2+ occurred.
The low-temperature α-l-Sm1 – ySryF3 – y tysonite phase (sp. gr. \(P\bar {3}c1\)) is formed for the Sm1 – ySryF3 – y crystals with 0.005 ≤ y ≤ 0.08. Beginning with the content of y > 0.08, X-ray diffraction analysis revealed stabilization of the high-temperature α-h-Sm1 – ySryF3 – y tysonite phase (sp. gr. P63/mmc). The sample with y = 0.31 is a two-phase eutectic composite consisting of a mixture of nonstoichiometric phases with saturated (at the eutectic temperature) compositions for the fluorite (Sr1−xSmxF2 + x) and tysonite (Sm1 – ySryF3 – y) structures.
It was established that the concentration transition in the tysonite modifications (α-l-Sm1 – ySryF3 – y ↔ α-h-Sm1 – ySryF3 – y) at y = 0.08–0.1 does not manifest itself in the concentration dependences of density ρ(y), refractive index nD(y), and conductivity σdc(y). For the nonstoichiometric Sm1 – ySryF3 – y tysonite phase (0.03 ≤ y ≤ 0.25), the density ρ(y) and refractive index nD(y) monotonically decrease in the composition range of 0.03 ≤ y ≤ 0.25.
The concentration dependence of the ionic conductivity was investigated for the Sm1 – ySryF3 – y nonstoichiometric-phase crystals in the composition range of 0.02 ≤ y ≤ 0.25 and the 69SmF2 × 31SrF2 composite. The room-temperature conductivity of the 69SmF2 × 31SrF2 eutectic composite is σdc = 6 × 10–8 S/cm; for the most conducting solid-solution crystal, Sm0.98Sr0.02F2.98 (y = 0.02), σdc = 1.6 × 10–4 S/cm.
Within the hopping-conductivity model, the concentration and mobility of carriers (fluorine vacancies) were calculated for the Sm1 – ySryF3 – y crystals; their values for the Sm0.98Sr0.02F2.98 crystal are, respectively, nmob = 4.0 × 1020 cm–3 and µmob = 2.5 × 10–6 cm2/(V s) (T = 293 K). The antibatic behavior of dependences nmob(y) and µmob(y) determines the decrease in the σdc value of the Sm1 – ySryF3 – y crystals with an increase in the content y.
REFERENCES
A. D. Kirhenbaum and J. A. Cahill, J. Inorg. Nucl. Chem. 14 (1–2), 148 (1960).
N. I. Sorokin, M. V. Fominykh, E. A. Krivandina, et al., Crystallogr. Rep. 41 (2), 292 (1996).
N. I. Sorokin and B. P. Sobolev, Elektrokhimiya 43 (4), 420 (2007).
N. I. Sorokin and B. P. Sobolev, Kristallografiya 39 (5), 889 (1994).
B. P. Sobolev and N. I. Sorokin, Crystallogr. Rep. 59 (6), 807 (2014).
B. P. Sobolev, N. I. Sorokin, and N. B. Bolotina, Photonic and Electronic Properties of Fluoride Materials, Ed. by A. Tressaud and K. Poeppelmeier (Elsevier, Amsterdam, 2016), p. 465.
O. Greis and M. S. R. Cader, Thermochim. Acta 87, 145 (1985).
K. Rotereau, Ph. Daniely, A. Desert, and J. Y. Gesland, J. Phys. Cond. Matter 10 (6), 1431 (1998).
B. P. Sobolev, The Rare Earth Trifluorides. The High Temperature Chemistry of Rare Earth Trifluorides (Institut d’Estudis Catalans, Barcelona, 2000).
A. A. Potanin, Zh. Ross. Khim. O-va im. D. I. Mendeleeva XLV (5–6), 58 (2001).
M. Anji Reddy and M. Fichtner, J. Mater. Chem. 21, 17059 (2011).
C. Rongeat, M. Anji Reddy, R. Witter, and M. Fichtner, J. Phys. Chem. 117, 4943 (2013).
F. Gschwind, G. Rodriguez-Garsia, D. J. S. Sandbeck, et al., J. Fluor. Chem. 182, 76 (2016).
B. Dieudonne, J. Chable, F. Mauvy, et al., J. Phys. Chem. C 119, 25170 (2015).
B. P. Sobolev and K. B. Seiranian, J. Solid State Chem. 39 (3), 337 (1981).
B. P. Sobolev, D. N. Karimov, D. N. Sul’yanov, et al., Crystallogr. Rep. 54 (1), 122 (2009).
N. I. Sorokin, D. N. Karimov, E. A. Sul’yanova, et al., Crystallogr. Rep. 55 (4), 662 (2010).
A. Roos, A. F. Aalders, J. Schoonman, et al., Solid State Ionics 9–10, 571 (1983).
N. I. Sorokin and B. P. Sobolev, Elektrokhimiya 43 (4), 420 (2007).
A. K. Ivanov-Shits, N. I. Sorokin, P. P. Fedorov, and B. P. Sobolev, Fiz. Tverd. Tela 25 (6), 1748 (1983).
B. P. Sobolev, V. B. Aleksandrov, P. P. Fedorov, et al., Kristallografiya 21 (1), 95 (1976).
N. B. Bolotina, T. S. Chernaya, A. I. Kalyukanov, et al., Crystallogr. Rep. 60 (3), 346 (2015).
O. N. Khrykina, N. I. Sorokin, I. A. Verin, et al., Crystallogr. Rep. 62 (4), 545 (2017).
L. R. Batsanova, Usp. Khim. 40 (6), 945 (1971).
G. V. Anan’eva, E. N. Baranova, M. N. Zarzhitskaya, et al., Izv. Akad. Nauk SSSR, Neorg. Mater. 16 (1), 68 (1980).
E. Staritzky and L. B. Asprey, Anal. Chem. 29 (5), 855 (1957).
E. A. Radzhabov and V. A. Kozlovskii, Bull. Russ. Acad. Sci.: Phys. 79 (2), 251 (2015).
I. V. Murin, O. V. Glumov, I. G. Podkolzina, et al., Zh. Prikl. Khim. 55 (2), 300 (1982).
A. I. Livshits, V. M. Buznik, P. P. Fedorov, and B. P. Sobolev, Neorg. Mater. 18 (1), 135 (1982).
A. F. Aalders, A. Polman, A. F. M. Arts, and H. W. de Wijn, Solid State Ionics 9–10, 539 (1983).
M. A. Denecke, W. Gunser, A. V. Privalov, and I. V. Murin, Solid State Ionics 52, 327 (1992).
T. Takahashi, H. Iwahara, and T. Ishikawa, J. Electrochem. Soc. 124 (2), 280 (1977).
I. V. Murin, O. V. Glumov, and Yu. V. Amelin, Zh. Prikl. Khim. 53 (7), 1474 (1980).
A. Roos, F. C. M. van de Pol, R. Keim, and J. Schoonman, Solid State Ionics 13, 191 (1984).
ACKNOWLEDGMENTS
We are grateful to N.A. Ivanovskaya, B.V. Nabatov, and T.B. Kosova for their help in carrying out experiments.
FUNDING
This study was supported by the Russian Foundation for Basic Research, project nos. 16-03-00707 and 17-00-00118, in the part concerning growth of crystals and by the Ministry of Higher Education and Science of the Russian Federation within the State assignment in the part concerning the analysis of crystal characteristics, using equipment of the Shared Research Center of the Federal Scientific Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Sin’kov
Rights and permissions
About this article
Cite this article
Sorokin, N.I., Karimov, D.N., Samsonova, N.V. et al. Growth of Sm1 – ySryF3 – y (0 < y ≤ 0.31) Crystals and Investigation of Their Properties. Crystallogr. Rep. 64, 488–495 (2019). https://doi.org/10.1134/S1063774519030246
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774519030246