Abstract
It is shown that the ordered phase of titanium carbohydride TiC0.50H0.31 with a face-centered cubic lattice (sp. gr. Fd\(\bar {3}\)m), obtained by self-propagating high-temperature synthesis, has an ordered antiphase domain structure with a period P = 16. This period is much larger than that in TiC0.50Hy (y ≤ 0.22), with a lower hydrogen content (P ≈ 11). It is shown that TiC0.50H0.31 decomposes at temperatures T ≤ 900°C into the cubic phase of titanium carbide TiCx (x ≥ 0.70) (sp. gr. Fm\(\bar {3}\)m) and the trigonal phase of titanium carbohydride Ti2Cv′Hz′ (sp. gr. P\(\bar {3}\)m1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The nonstoichiometric fcc phase of titanium carbide TiCx is widely applied in technology [1] and medicine [2, 3]. Hydrogen is the most difficult-to-remove impurity in the fcc phase of titanium carbide, which strongly affects its structure [4] and properties [5]. To apply titanium carbide TiCx with desired functional characteristics, one should know its structural features and the structure of hydrogen-containing product (titanium carbohydride TiCхHy). It was shown in [4] that titanium carbohydride TiCхHy (y ≤ 0.22) of variable composition with a wide homogeneity range with respect to carbon x = 0.47–1.00 at T ≥ 900°C is a high-temperature disordered fcc phase (sp. gr. Fm\(\bar {3}\)m). It was also shown that a low-temperature ordered fcc phase occurs at temperatures T ≤ 600°C, which is described within the sp. gr. Fd\(\bar {3}\)m, with antiphase domains (ADs) ordered along different [hkl] crystallographic directions (h, k, and l = 2n + 1). The mean domain period is Pmean = 11. The AD period is measured by the mean number of unit cells of the initial crystal structure in one period of the domain-structure antiphase state. In the disordered fcc phase, hydrogen atoms statistically (in a disordered way) occupy interstitial sites in octahedral and tetrahedral voids, and carbon atoms statistically occupy octahedral interstitial sites. In the ordered fcc phase, carbon and hydrogen atoms occupy orderly a part of interstitial sites in octahedral voids. It was also shown in [4] that the period of ordered ADs in titanium carbohydrides TiCхHy barely depends on the carbon concentration x in the range of x = 0.47–0.70 at hydrogen concentrations close to y ≤ 0.21. It is interesting to analyze the influence of the concentration of impurity hydrogen, dissolved in interstitial sites of the lattice, on the structure at the lower boundary of the homogeneity region of the fcc phase of nonstoichiometric titanium carbide TiC0.50. The purpose of this study was to perform neutron diffraction analysis of phase transformations in titanium carbohydride TiC0.50Hy.
EXPERIMENTAL
To find the dependence of the AD-structure period on the hydrogen concentration in titanium carbohydride, we synthesized a sample with a composition of TiC0.50H0.31, having a higher hydrogen concentration than the previous samples [4]. The initial reagents were fine-grained carbon powder of special purity grade and titanium powder of the PTM grade (purity 98.92 wt %), containing 0.38 wt % hydrogen impurity. According to the certified data, other impurities (N2, C, Fe + Ni, Si, Ca, and Cl) comprise 0.70 wt %. The mean size of powder particles is \({\bar {r}}\) = 40 µm. The sample was prepared at the Institute of Structural Macrokinetics and Materials Science Problems, Russian Academy of Sciences, by self-propagating high-temperature synthesis (SHS) [6] in a constant-pressure bomb in a hydrogen atmosphere. Note that the temperature of the solid-phase synthesis reaction may be as high as 1500–3200°C during the SHS because of the exothermic reaction of the initial reagents. At the same time, the reaction product is cooled for several seconds. Before the synthesis, the initial titanium and carbon powders were taken in the corresponding proportions by weighing on an analytical balance and carefully mixed in an agate mortar; afterwards, a cylindrical pellet was prepared under a pressure of 3 × 106 N/m2. The carbon and hydrogen atomic concentrations in the sample were determined by chemical analysis at the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences. The hydrogen concentration was determined by burning a sample weight in an oxygen flow, with subsequent weighing of water formed during the burning. The carbon atomic concentration in the samples was also determined by burning a sample weight, with absorption of released CO2. The chemical analysis error was 0.3%.
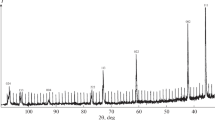
The main investigation method was neutron diffraction, which yields the most complete and reliable information about the position of light carbon and hydrogen atoms in the lattice. Neutron diffraction patterns of the powder samples were obtained on a DN-500 neutron diffractometer mounted on the thermal column of the VVR-SM nuclear reactor at the Institute of Nuclear Physics, Uzbekistan Academy of Sciences (λ = 1.085 Å). Neutron diffraction patterns were calculated by the full-profile Rietveld method using the DBW 3.2 program [7]. To estimate the single-phase character and homogeneity of the samples, we used X-ray diffraction analysis because of a higher angular resolution. Diffraction patterns of the samples were obtained on a DRON-3M X-ray diffractometer using CuKα radiation in the angular range of 2θ = 10°–110° with a detector rotation speed of 1 deg/min (Ni filter, λmean = 1.5418 Å). The lattice parameters were determined by carrying out measurements in a narrow angular range of 2θ > 101° near the diffraction maximum with a detector rotation speed of 0.25 deg/min. According to the diffraction pattern corresponding to the fcc lattice of NaCl type, the synthesized sample is single-phase and homogeneous, which is evidenced by clear separation of the doublet (CuKα1 and CuKα2 radiations) at the Bragg angles 2θ > 60°. The lattice parameter of the sample is a0 = 4.315(2) Å. However, a series of superstructural peaks (Fig. 1) with the Miller indices satisfying the condition h + k + l = 2n + 1 is observed in the neutron diffraction pattern of TiC0.50H0.31 (in contrast to the neutron diffraction pattern of the TiC0.50H0.22 sample with a low hydrogen concentration, obtained under similar conditions [8]). The presence of superstructural reflections in the neutron diffraction pattern indicates ordering of interstitials [4]. The neutron diffraction pattern of this phase with the ordered arrangement of interstitials in interstitial lattice sites is indexed within the fcc cell with a parameter a = 8.643(2) Å ≈ 2a0. According to the neutron diffraction pattern, the crystal structure of the ordered TiC0.50H0.31 phase is described within the sp. gr. Fd\(\overline 3 \)m, as well as the structure of the ordered TiC0.50H0.22 phase after low-temperature annealing at temperatures of 600–500°C [4]. According to the model of [4], carbon atoms mainly occupy octahedral sites 16c, while the remaining small amount of carbon atoms and hydrogen atoms occupy octahedral interstitial sites 16d within the sp. gr. Fd\(\overline 3 \)m. However, we could not obtain an acceptable reliability factor by neutron diffraction analysis within this model. The experimental and calculated intensities are listed in Table 1. According to these data, the total reliability factor for structural and superstructural Bragg reflections was Rtot = 10.0%, the reliability factor for only structural (str) maxima was Rstr = \({\frac{{\sum {\left| {I_{{{\text{str}}}}^{{{\text{exp}}}} - I_{{{\text{str}}}}^{{{\text{calc}}}}} \right|} }}{{\sum {\left| {I_{{{\text{str}}}}^{{{\text{exp}}}}} \right|} }}}\) = 5.2%, and the reliability factor for only superstructural (ss) maxima was Rss = \({\frac{{\sum {\left| {I_{{{\text{ss}}}}^{{{\text{exp}}}} - I_{{{\text{ss}}}}^{{{\text{calc}}}}} \right|} }}{{\sum {\left| {I_{{{\text{ss}}}}^{{{\text{exp}}}}} \right|} }}}\) = 59.0%. The poor agreement between the experimental and calculated intensities of superstructural diffraction maxima in the proposed model can be explained by the formation of ordered ADs in the sample under study. Indeed, as can be seen in Fig. 1, there is splitting (distortion) of superstructural reflections (reflections with odd Miller indices), and their profiles differ from the calculated ones. Figure 2 shows profiles of the 111 and 133 superstructural reflections on an enlarged scale. The splitting of the superstructural reflections indicates that a periodic ordered AD structure is formed in the sample [4]. The distortion of the shape of the superstructural reflections (in contrast to the structural ones) is caused by slightly different systems of atomic planes, corresponding to different ADs. Note that the AD ordering in TiC0.50Hy with a hydrogen concentration of H/Ti ≤ 0.22 requires long-term annealing at a temperature T ≤ 600°C [4]. The period of the ordered AD structure P was determined based on the distance between satellites of the 133 reflection from the formula [9]

where λ is the neutron wavelength, a0 is the lattice parameter determined by X-ray diffraction within the sp. gr. Fm\(\bar {3}\)m, and θ+ and θ– are the Bragg angles of the satellites arising due to the increase in the AD-structure period. The AD-structure period P was 16, which is larger than the period of ordered ADs in titanium carbohydrides with an H/Ti concentration of 0.22 (Pmean = 11) [4]. This is likely due to the higher temperature of the disorder–order transition in titanium carbohydride TiC0.50H0.31 in comparison with the titanium carbohydrides having lower hydrogen concentrations.
It is of interest to study the influence of low-temperature annealing on the phase composition of carbohydride TiC0.50H0.31, i.e., to analyze the formation conditions for the equilibrium phase composition. To this end, the sample was first annealed in an evacuated and sealed quartz ampoule at a temperature of 900°C for 5 h. According to the neutron diffraction pattern (Fig. 3a), this annealing leads to segregation of the ordered carbon–hydrogen solid solution in the α-Ti lattice (sp. gr. P\(\overline {\text{3}} \)m1) from the main cubic phase of titanium carbohydride:
Neutron diffraction pattern of TiC0.50H0.31: (a) after annealing at 900°C for 5 h (the Miller indices of the reflections corresponding to the disordered fcc phase (sp. gr. Fm\(\bar {3}\)m) and the trigonal ordered phase (sp. gr. P\(\bar {3}\)m1) are indicated above and below, respectively) and (b) after annealing at a temperature of 800–700°C for 72 h (the Miller indices of the reflections corresponding to the ordered fcc phase (sp. gr. Fd\(\bar {3}\)m) and the trigonal ordered phase (sp. gr. P\(\bar {3}\)m1) are indicated above and below, respectively).
A similar decomposition scheme is observed after annealings at 550°C for 24 h and 450°C for 24 h. An increase in the annealing time to 100 h at these temperatures did not lead to a further change in the phase composition. Therefore, the temperature range of decomposition for titanium carbohydride TiC0.50H0.31 is much wider (900–450°C) from the high-temperature side, as compared with titanium carbohydrides TiC0.50Hy with a lower hydrogen concentration (Tdec ≈ 800–450°C for a concentration H/Ti ≤ 0.22 [4]).
Furthermore, to obtain the equilibrium phase composition, we annealed initial titanium carbohydride TiC0.50H0.31 at a temperature of 800–600°C with a step of 100°C for 72 h. The neutron diffraction pattern of the sample after this annealing is shown in Fig. 3b. The Ti–C system has no hexagonal ordered phase [10]. It exists in the Ti–C–H system [11]. According to [11], the chemical formula Ti2CvHz should be assigned to the hexagonal ordered phase in the Ti–C–H system. Concerning the cubic disordered phase indexed within the sp. gr. Fm\(\bar {3}\)m, this disordered nonstoichiometric phase is not formed in the Ti–C–H system after annealing at temperatures of 800–500°C; an ordered phase with the structure described within the sp. gr. Fd\(\bar {3}\)m is observed instead [4]. The disordered fcc phase (sp. gr. Fm\(\bar {3}\)m) at these temperatures can be titanium carbide with a higher carbon concentration (x ≥ 0.70) [10]. Therefore, there are grounds to suggest that a further phase transformation is observed after annealing at temperatures of 800–600°C. According to the neutron diffraction pattern (Fig. 3b), the ordered fcc phase of titanium carbohydride TiC0.50H0.31 (sp. gr. Fd\(\bar {3}\)m) observed at T > 900°C is decomposed into a disordered fcc phase of titanium carbide TiCx (x ≥ 0.70) (sp. gr. Fm\(\bar {3}\)m) and a trigonal ordered Ti2Cv'Hz' phase (sp. gr. P\(\bar {3}\)m1):

As follows from the experimental data, the decomposition process begins in titanium carbohydride TiC0.50H0.31 at a temperature of 900°C or lower (at 500–450°C). However, the conditions for the first case (chosen time of 5 h) and the second case (temperature of 500–450°C) were insufficient for the complete transition of the system to the two-phase (Fm\(\bar {3}\)m + P\(\bar {3}\)m1) equilibrium state. The optimal temperature for the complete transition to the equilibrium state for an acceptable time lies in the range of 800–600°C.
Thus, titanium carbohydride TiC0.50H0.31 has higher ordering and decomposition temperatures, in comparison with titanium carbohydride at the lower boundary of the homogeneity region with a much lower hydrogen atomic concentration (H/Ti ≤ 0.22). In the high-temperature state, titanium carbohydride TiC0.50H0.31 has an ordered crystal structure (sp. gr. Fd\(\bar {3}\)m) and an ordered AD structure (AD period P = 16). The high-temperature ordered cubic phase of titanium carbohydride TiC0.50H0.31 at temperatures of 900–700°C in the equilibrium state consists of a disordered cubic phase of titanium carbide (sp. gr. Fm\(\bar {3}\)m) and a trigonal (sp. gr. P\(\bar {3}\)m1) ordered Ti2CxHz phase. The system passes to the equilibrium state through a two-phase region: an ordered fcc phase (sp. gr. Fd\(\bar {3}\)m) and a trigonal ordered phase (sp. gr. P\(\bar {3}\)m1) of titanium carbohydride. At the same time, for the composition of TiC0.50Hy (y ≤ 0.22), one can observe decomposition with segregation of pure titanium having a hexagonal structure upon annealing in the temperature range of 750–600°C for 24 h [4]. This decomposition can be described by the following scheme:

The hydrogen concentration in the TiC0.50Hy phase (y ≤ 0.22) is likely insufficient for the formation of the ordered trigonal Ti2CvHz phase. To generalize sche-mes (2)–(4), we can propose the following scheme of decomposition (phase transformation) in titanium carbohydride TiC0.50Hy, which depends on the hydrogen concentration:
The lower boundary of the homogeneity region of the fcc phase of titanium carbide TiCx is known to correspond to the carbon concentration x = 0.50 [9]. It follows from the results of the study that hydrogen, incorporated into the lattice of the fcc phase of titanium carbide at the lower boundary of the TiCx homogeneity region in the equilibrium state (at low temperatures T ≤ 900°C), leads to a decrease in its homogeneity region and decomposition into two phases. The phase composition of the decomposed alloy depends on the hydrogen concentration (5).
CONCLUSIONS
It was shown that the crystal structure of titanium carbohydride TiC0.50H0.31 synthesized by the SHS method differs from the disordered structure of TiC0.50Hy (y ≤ 0.22) (sp. gr. Fm\(\bar {3}\)m) [4]. It is described within the sp. gr. Fd\(\bar {3}\)m and has an ordered AD structure with a period P = 16. The found difference can be explained by the fact that titanium carbohydride with a higher hydrogen concentration is characterized by a higher ordering temperature. It was established that hydrogen impurity incorporated into interstitial sites of the fcc phase of titanium carbide TiC0.50 in the equilibrium state (at low temperatures T ≤ 900°C) narrows its homogeneity range. At y ≤ 0.22, α-Ti (sp. gr. P63/mmc) is segregated from the high-temperature cubic phase of titanium carbohydride TiC0.50Hy (sp. gr. Fd\(\bar {3}\)m) [4], whereas at y = 0.31 it decomposes into the cubic phase of titanium carbide TiCx (sp. gr. Fm\(\overline 3 \)m) and the ordered trigonal phase of titanium carbohydride Ti2Cv′Hz′ (sp. gr. P\(\bar {3}\)m1).
REFERENCES
S. S. Kiparisov, Yu. V. Levinskii, and A. P. Petrov, Titanium Carbide: Formation, Properties, and Applications (Metallurgiya, Moscow, 1987) [in Russian].
I. M. Bairikov, A. P. Amosov, O. V. Tyumina, et al., Vopr. Chelyustnoo Litsevoi, Plast. Khir., Implantol. Klin. Stomatol., Nos. 1–2, 23 (2011).
Cardio-Vascular Set with a Titanium Carbide Coating. http://www. rmed.ru/ tem./info/22030.html.
I. Khidirov, Crystallogr. Rep. 60 (5), 706 (2015).
I. Khidirov and D. I. Sotvoldiev, Al’tern. Energ. Ekol., No. 3, 94 (2008).
A. P. Amosov, I. P. Borovinskaya, and A. G. Merzhanov, Powder Technology of Self-Propagating High-Temperature Materials Synthesis (Mashinostroenie-1, Moscow, 2007) [in Russian].
R. A. Yang and D. B. J. Wilas, J. Appl. Crystallogr. 15, 430 (1982).
I. Khidirov, Russ. J. Inorg. Chem. 60 (10), 1263 (2015).
T. S. Boyarshinova, O. D. Shashkov, and V. D. Sukhanov, Fiz. Met. Metalloved. 78 (5), 85 (1994).
A. I. Gusev, Phys.-Usp. 57 (9), 905 (2014).
I. Khidirov, B. B. Mirzaev, N. N. Mukhtarova, et al., Al’tern. Energ. Ekol., No. 5, 47 (2007).
ACKNOWLEDGMENTS
We are grateful to the employees of the Laboratory of Chemistry of Hydrides and Boron (which now enters the Laboratory of Energy-Consuming Materials) of the Kurnakov Institute of General and Inorganic Chemistry (Russian Academy of Sciences), especially to L.N. Padurets for the chemical analysis of the carbon and hydrogen contents in the samples.
This study was performed within the Program of Fundamental Investigations of the Uzbekistan Academy of Sciences, project no. F2-FA-F119.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Sin’kov
Rights and permissions
About this article
Cite this article
Khidirov, I., Rakhmanov, S.J. Neutron Diffraction Study of the Phase Transformations in Titanium Carbohydride TiC0.50Hy. Crystallogr. Rep. 63, 1147–1152 (2018). https://doi.org/10.1134/S106377451807012X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106377451807012X