Abstract
The synthesis and X-ray diffraction study of the ruthenium(II) complex, [{trans-(dppe)2(Cl) Ru–C≡C–H} · 0.5CDCl3] (trans-Ru · 0.5CDCl3), are presented. The molecule of trans-Ru · 0.5CDCl3 crystallizes in the triclinic sp. gr. \(P\bar {1}\) with two chemically equivalent but crystallographic independent molecules. The intramolecular C–H···Cl–C hydrogen bonds build with the deuterated chloroform. In addition, the intermolecular C–H···Cl–Ru hydrogen bonds have been also found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, an interest in designing new carbon-rich organometallic compounds has been increased, especially those containing an σ-alkynyl-metal link, because these kinds of structures allow the metal to be connected to a different functional groups thus enabling direct electronic communication between them and the metal through the alkyne moiety (–C≡C–) [1–4]. The insertion of a ruthenium-containing group into acetylide moieties stabilized by bis(diphenylphosphanyl)ethane (dppe) ligands and bis(diphenylphosphanyl)methane (dppm) ligands has been investigated [5–8]. The Cl(dppe)2Ru–C≡C– end groups behave as strongly electron-releasing groups that compare favorably with purely organic electron-releasing substituents such as the methoxy or amino substituents. Additionally, these mononuclear organometallic acetylide complexes exhibit usually a very electron-rich chemistry and constitute a remarkable model for potential development of new molecular electronic devices [9, 10]. We have reported previously the X-ray structure of the Ru(II) complex, namely [trans-(dppe)2(Cl) Ru–C≡C–H] [11]. Then, as a second step toward their study, here, we report an additional structural determination for the complex [{trans-(dppe)2(Cl) Ru–C≡C–H} · 0.5CDCl3]. They differ in that a deuterated chloroform solvent molecule has been found in the new structure.
EXPERIMENTAL
Synthesis of [trans-(dppe)2 (Cl) Ru–C≡C–H], trans-Ru
Under argon atmosphere, a Schlenk tube was loaded with the [Ru(dppe)2Cl]+ (0.450 g, 0.451 mmol), ethynyltrimethylsilane (108 mL, 0.798 mmol), and 30 mL CH2Cl2. The resulting mixture was stirred over night. Then, 30 mL triethylamine were added and the mixture was stirred during 2 h. Then, the solvent was evaporated and the oil residue was resuspended in 40 mL of CH2Cl2 and filtered through a 2 × 4 cm Al2O3 column chromatography. The filtered product was evaporated and the remaining solid was washed with the n-pentane and dried in vacuo, to afford trans-Ru as a yellow-greenish powder (0.245 g, 55.2%). The reaction scheme can be seen in Fig. 1. Single crystals of trans-Ru · 0.5CDCl3 were obtained by vapor diffusion of deuterated chloroform from the NMR tube solution of trans-Ru. The 1H NMR (CDCl3, 400 MHz; δ, ppm): 1.37 (1H, s, C≡C–H), 2.62 (8H, m, –PCH2CH2P), 6.86–7.63 (40H, –C6H4); 13C NMR (100.6 MHz, CDCl3; δ, ppm): 30.73 (PCH2CH2P), 100.88 (–C≡C–H), 135.59 (Ru–C≡C–H), 126.78–136.80 (–C6H4); 31P{1H} NMR (161.98 MHz, CDCl3; δ, ppm): 50.68.
Crystal Structure Determination
The compound was studied at 296.15 K on a D8 Smart Apex II Bruker AXS diffractometer using a X-ray source MoKα radiation (λ = 1.71073 Å). The X-ray-quality crystal of trans-Ru · 0.5CDCl3 was mounted with epoxy cement on the tip of a glass fiber in a random orientation. Using OLEX2 [12], the structure was solved with the SHELXS structure solution program [13] using direct methods and refined with the SHELXL refinement package [14] using full-matrix least squares techniques based on F2. All non-hydrogen atoms were refined with anisotropic displacement parameters. The H atoms were finally included in their calculated positions. CCDC 1400416 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. A summary of the experimental and crystallography data for the compound are given in Table 1. Selected bond distances and bond angles are listed in Tables 2 and 3, respectively.
RESULTS AND DISCUSSION
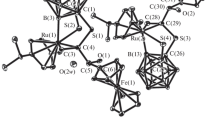
The molecular structure of trans-Ru · 0.5CDCl3 is shown in Fig. 2. The molecule has a Ci symmetric point and crystallizes in the triclinic sp. gr. \(P\bar {1}\) with two chemically equivalent but crystallographic independent molecules. Additionally, one distorted deuterated chloroform molecule is present in the asymmetric unit. The structure of trans-Ru · 0.5CDCl3 showed for both independent structures the phosphorus atoms occupying the equatorial plane of the octahedron and the chloride and acetylide ligands occupying the axial position in a trans geometry. Since both independent structures exhibit a similar crystal and molecular structures, they will be described together. The Cl1A–Ru1A, Ru1A–C2A, Cl1B–Ru1B, Ru1B–C2B, and C1A–C2A, C1B–C2B distances for this complex differ slightly from those reported previously for the structurally related octahedral trans-bis(bidentate phosphine) ruthenium alkynyl complexes [15–17]. In our case, in both independent molecules, the –C≡CH and the chlorine ligands are distorted over two equivalent positions (0.5 occupancy each, see Table 4).
In both independent structures, a non-conventional intramolecular hydrogen bonds were found involving both the dppe arene carbon atoms, C28A and C24B, and chlorine ligand atoms, Cl1A and Cl1B, respectively. These interactions have significant consequences for the structure of trans-Ru · 0.5CDCl3: the Ru–Cl, namely Ru1A–Cl1A and Ru1B–Cl1B bond distances, are 2.580(2) and 2.556(2) Å, respectively, which are larger compared to those found in other ruthenium complexes, namely Ru–Cl = 2.478(13) Å [18]. In addition, the Ru–C and C–C bond distances in the ethylene fragment for both structures, are shorter than those previously reported in the literature for Ru–C = 2.001 and C–C = 1.188 Å [19]: Ru1A–C2A = 1.937(8), C1A–C2A = 1.181(11), Ru1B–C2B = 1.955(7), and C1B–C2B = 1.184 (9) Å.
Finally, the compound trans-Ru · 0.5CDCl3 exhibits an intermolecular C16A–H16A···Cl3S hydrogen bond linking one chlorine of the deuterated chloroform solvent molecule and the aryl moiety of the dppe ligand (Fig. 2, Table 5).
CONCLUSION
A new structure of the ruthenium(II) complex, [{trans-(dppe)2(Cl) Ru–C≡C–H} · 0.5CDCl3] (trans-Ru · 0.5CDCl3) was determined. The molecule crystallizes in the triclinic sp. gr. \(P\bar {1}\) with two chemically equivalent but crystallographic independent molecules. Intra and intermolecular hydrogen bonds, C–H···Cl and Ru–Cl···H, were observed by crystal X-ray diffraction analysis, finding variations in the Ru–Cl and C–C bond distances of ethynyl fragment with those previously reported in the literature.
REFERENCES
T. X. Neenan and G. M. Whitesides, J. Org. Chem. 53, 2489 (1988).
O. Lavastre, L. Ollivier, and P. H. Dixneuf, Tetrahedron 52, 5495 (1996).
P. Chuentragool, K. Vongnam, P. Rashatasakhon, et al., Tetrahedron 67, 8177 (2011).
N. J. Long and C. K. Williams, Angew. Chem. Int. Ed. Engl. 42, 2586 (2003).
P. H. Dixneuf, Acc. Chem. Res. 32, 311 (1999).
D. Touchard, M. Christophe, V. Cadierno, et al., J. Chem. Soc., Chem. Commun. 2, 859 (1994).
D. Touchard, P. Haquette, A. Daridor, et al., Organometallics 17, 3844 (1998).
D. Touchard, P. Haquette, S. Guesmi, et al., Organometallics 6, 3640 (1997).
B. G. Ellis, M. I. Bruce, L. Toupet, et al., Organometallics 25, 649 (2006).
M. I. Bruce, Chem. Rev. 91, 197 (1991).
A. Trujillo, M. Fuentealba, J. A. K. Howard, and R. Arratia-Perez, Acta Crystallogr. E 68, m1445 (2012).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009).
G. M. Sheldrick, Acta Crystallogr. A 64, 112 (2008).
G. M. Sheldrick, Acta Crystallogr. C 71, 3 (2015).
M. A. Fox, J. E. Harris, S. Heider, et al., J. Organomet. Chem. 694, 2350 (2009).
C. J. Jeffery, M. P. Cifuentes, G. T. Dalton, et al., Macromol. Rapid Commun. 31, 846 (2010).
R. F. Winter and K.-W. Klinkhammer, Organometallics 2, 1317 (2001).
M. Younus, N. J. Long, P. R. Raithby, et al., J. Organomet. Chem. 578, 198 (1999).
I. Ouerfelli, R. Gatri, M. Lotfi Efrit, et al., J. Organomet. Chem. 696, 670 (2011).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the UNAB (grant no. DI-5-17/RG). In addition, the authors gratefully acknowledge the use of the services and facilities of the Laboratorio de Análisis de Sólidos (L.A.S-UNAB) at the Universidad Andrés Bello. The authors thank Poldie Oyarzun for technical assistance using the D8 Smart Apex II Bruker AXS diffractometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Escobar, C.A., Madrid-Moliné, F., Fuentealba, M. et al. Molecular and Crystalline Structure of the Ruthenium(II) Complex [{trans-(dppe)2(Cl) Ru–C≡C–H} · 0.5CDCl3]: An Additional Determination. Crystallogr. Rep. 63, 1133–1137 (2018). https://doi.org/10.1134/S106377451807009X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106377451807009X