Abstract
In this paper, we investigate the kinetics and mechanisms of reactive-ion etching of Si and SiO2 in the plasma of an HBr + O2 mixture with a variable initial composition under conditions of the high-frequency (13.56 MHz) inductive discharge. In the experimental and theoretical (model) analysis of the plasma parameters, the key plasma-chemical processes that form the stationary composition of the gas phase are identified and the densities of active particle fluxes onto the surface under processing are determined. It is found that the increase in the O2 concentration in the plasma-forming mixture is accompanied by a decrease in the kinetic coefficients (probability of effective interaction and etching yield) that characterize the heterogeneous stages of the etching process. It is assumed that the main mechanism of this effect is the oxidation of SiBrx etching products to low volatile compounds of the SiBrxOy type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Plasma of halogen-containing gases is widely used in integrated micro- and nanoelectronics for the dimensional structuring of semiconductor wafer surfaces and various functional layers [1, 2]. In particular, for reactive-ion etching of silicon and its compounds (SiO2, Si3N4, and SiC), fluorocarbon gas plasma is traditionally employed, while the wide nomenclature of the corresponding compounds (CF4, C2F6, C4F8, CHF3, etc.) makes it possible to effectively optimize the yield characteristics of the process: etching rate, anisotropy, and selectivity with respect to the abovelying or underlying layer. Meanwhile, a significant disadvantage of fluorine chemistry is the possibility of a spontaneous reaction between fluorine atoms and silicon, which results in an isotropic-like etch profile of this material [2, 3]. Thus, searching for alternative plasma-forming media for the reactive-ion etching of Si and SiO2 is an important problem focused on optimizing both the technological and functional parameters of the end products. An integral part of solving this problem, in our opinion, is the investigation of the relationships between the external parameters of the plasma, its composition, and the kinetics of the processes on the surface under processing. This provides a comprehensive understanding of the interaction mechanism in the plasma–surface system and, hence, makes it possible to govern its result.
There are a number of works devoted to the characteristics of Si and SiO2 etching in HBr-based plasmas [5–12]. The contribution of these works can be summarized as follows.
− A spontaneous chemical reaction in the SiO2 + Br system is thermodynamically not possible [4, 5] due to the fact that the energy of the Si–O bond (∼800 kJ/mol [6]) is much higher than that of Si–Br (∼358 kJ/mol [6]). Hence, SiO2 etching in HBr plasma follows the mechanism of an ion-stimulated chemical reaction in which ion bombardment provides the formation (by breaking the Si–O bonds) and purification (by sputtering practically nonvolatile unsaturated products of the SiBrx interaction) of adsorption centers for bromine atoms [3, 4, 8, 9].
− The probability of a spontaneous chemical reaction in the Si + Br system is much lower than that in Si + F [5, 7]. This is most likely due to the large size of the bromine atom, which hinders its implantation into the silicon lattice. That is why the stationary mode of Si etching in HBr plasma is also provided by an ion-stimulated chemical reaction [10–12] and is characterized by significantly lower absolute rates of the process compared to fluorine chemistry.
− Adding oxygen to HBr reduces the rate of silicon etching but increases the anisotropy of the process [10, 12]. It was experimentally shown that this effect is achieved by masking the side walls of the formed relief with compounds of the SiBrxOy type, which have even lower volatility (and, therefore, lower sputtering coefficients) compared to SiBrx [10].
Unfortunately, almost all of these investigations were carried out in a purely experimental approach and did not analyze heterogeneous effects on the surface under processing with respect to the gas phase parameters. Thus, most of the conclusions about the etching mechanisms, including Si and SiO2 etching in HBr + O2 mixture plasma, prove declarative and require independent verification. In our previous works [13, 14], we carried out a comprehensive (using plasma diagnostics and simulation methods) analysis of the kinetics and mechanisms of Si and SiO2 etching in the plasma of a three-component mixture HBr + Cl2 + O2, which is characterized by the simultaneous action of chemically active particles of two types: bromine and chlorine atoms. In this study, we use a similar approach to investigate the binary HBr + O2 system in order to determine the effect of initial mixture composition on the kinetics of Si and SiO2 etching, the electrophysical parameters and composition of plasma, and mechanisms of heterogeneous interaction.
2 METHODOLOGY
2.1 Experimental Equipment and Technique
The experiments on etching and plasma parameter investigation were carried out in a planar reactor [15] with a processing chamber of stainless steel upon excitation of a high-frequency (13.56 MHz) inductive discharge. As constant external plasma parameters, we used the total flow rate of the plasma-forming gas q = 40 std. cm3/min, its pressure p = 6 mTorr (∼0.8 Pa), and input power W = 700 W. A variable parameter was the initial composition of the plasma-forming mixture HBr + O2, which was specified by the ratio of individual flow rates of its components. In particular, a variation in \({{q}_{{{{{\text{O}}}_{2}}}}}\) in the range of 0–30 std. cm3/min provided the oxygen concentration of \({{y}_{{{{{\text{O}}}_{2}}}}}\) = 0–75% in the supplied gas. The data on the electrophysical parameters of the plasma were obtained using a double Langmuir probe (DLP2000, Plasmart Inc., Korea). The data on the electron temperature (Te) and ion current density (J+) were obtained by processing the measured current–voltage (I–V) characteristics based on the well-known provisions of the double probe theory [16]. The negative bias at the bottom electrode (–Udc), which was set by an independent high-frequency (12.56 MHz) generator with a constant bias power Wdc = 200 W, was measured with a high-voltage probe (AMN-CTR, Youngsin Eng, Korea). The experiments showed that varying Wdc in the range of 0 to 200 W slightly affected the shape of the probe I–V curves and, therefore, the discharge gas phase parameters.
For etching, we used fragments of unoxidized and oxidized Si(100) wafers with an average area of ∼4 cm2. The samples to be processed were located in the central part of the lower electrode the temperature of which was maintained at a constant level by using a water cooling system. The etching rate was determined as R = Δh/τ, where τ is the etching time and Δh is the etching step height at the boundary between the masked and unmasked regions of the sample’s surface. As a mask coating, we used an AZ1512 photoresist. The parameter Δh was measured with a profilometer (Alpha-step D-500, KLA-Tencor, United States). The preliminary experiments showed that even a fivefold increase in the area of the processed surface slightly (within the experimental error) affects the probe I–V characteristics and does not cause a decrease in the rates of Si and SiO2 etching. Thus, under these conditions, the etching process for both the materials corresponds to the kinetic regime of an ion-stimulated chemical reaction and is characterized by a negligible effect of the etching products on the gas phase parameters.
2.2 Plasma Simulation
To obtain data on the kinetics of plasma-chemical processes and composition of the HBr + O2 plasma, we used a zero-dimensional kinetic model described in detail in our previous works [13, 14]. The basic kinetic scheme (set of reactions and corresponding rate constants) for the HBr + O2 mixture was borrowed from [14]. The adequacy of this scheme for describing the kinetics of plasma-chemical processes in individual gas components of the mixture was previously confirmed in [17, 18] with a satisfactory agreement between the results of plasma diagnostics and simulation. The input parameters of the model were the plasma probe diagnostics data for Te and J+. The output parameters of the model were the reactor-volume-averaged rates of the particle birth and death processes, as well as the particle concentration and flux density onto the surface that is in contact with the plasma.
3 RESULTS AND DISCUSSION
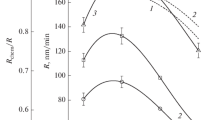
Figure 1 shows the effect of the initial composition of the HBr + O2 mixture on the kinetics of Si and SiO2 etching. It can be seen (Fig. 1a) that the etching rates of both the materials decrease monotonically with increasing \({{y}_{{{{{\text{O}}}_{2}}}}}\) (27.8–2.7 nm/min or by a factors of ∼10 for Si and 8.0–2.1 nm/min and ∼4 for SiO2 at 0–75% O2) with the decrease being sharper in the region \({{y}_{{{{{\text{O}}}_{2}}}}}\) < 30%. This behavior of the etching rates corresponds to the decrease in the selectivity of the Si/SiO2 etching in the range of 3.1 to 1.2 at 0–75% O2. Thus, the addition of oxygen to HBr promotes the leveling of the etching rates for the materials under investigation, which is qualitatively similar to the effects achieved in the plasma of fluorocarbon gases with a high level of polymerization capability [1–3, 19]. The analysis of the works [19–21] suggests that the change in the rate of the reactive-ion process when varying the ratio of the components in the plasma-forming mixture can be due to several factors, namely, the changes in the concentrations and flux densities of the active particles (bromine atoms and positive ions) because of the changes in the kinetics of the plasma-chemical processes in the gas phase and the effect of the initial composition of the mixture on the kinetic characteristics (probabilities and rate constants) of the heterogeneous interaction. That is why the correct interpretation of the data shown in Fig. 1a requires information on the electrophysical parameters and plasma composition.
Kinetics of Si and SiO2 etching in the plasma of the HBr + O2 mixture with variable initial composition: (a) etching rate R (solid lines) and etching selectivity \({{{{R}_{{{\text{Si}}}}}} \mathord{\left/ {\vphantom {{{{R}_{{{\text{Si}}}}}} {{{R}_{{{\text{Si}}{{{\text{O}}}_{{\text{2}}}}}}}}}} \right. \kern-0em} {{{R}_{{{\text{Si}}{{{\text{O}}}_{{\text{2}}}}}}}}}\) (dashed line); (b) kinetic coefficients γR = R/ΓBr and YR = R/\(\sqrt {{{M}_{i}}{{\varepsilon }_{i}}} {{\Gamma }_{ + }},\) which characterize the chemical and physical components of heterogeneous interaction.
During the plasma diagnostics, it was found that the dilution of HBr with oxygen at p = const causes a slight increase in the electron temperature (3.2–3.3 eV at 0–75% O2) with a more noticeable increase in the ion current density (1.7–2.5 mA/cm2 or ∼1.5 times at 0–75% O2) (see Fig. 2a). The first effect is caused by the fact that the decrease in the electron energy loss for the excitation and ionization of the main molecular components of the pure HBr plasma is almost completely compensated by similar processes for O2. At the same time, the latter are characterized by lower energy losses for vibrational excitation compared to HBr and H2 [22], which ensures a weak increase in Te. The behavior of J+ with increasing \({{y}_{{{{{\text{O}}}_{2}}}}}\), as expected, follows the behavior of the total concentration of positive ions (7.5 × 1010–9.0 × 1010 cm–3 at 0–75% O2; see Fig. 2b), while the formal difference in the shapes of the corresponding curves is due to the decrease in the effective masses of the ions. Our computations showed that the main reason for the increase in n+ is the decrease in the frequency of volumetric loss of positive ions in the processes of the A+ + B– → A + B type (due to n– = 3.5 × 1010–1.9 × 1010 cm–3 at 0–75% O2) against the background of the small changes in the total ionization frequency. The latter is provided by the similar values of the ionization rate constants for Br (∼1.9 × 10–10 cm3/s at Te = 3 eV) and O2 (∼1.3 × 10–10 cm3/s at Te = 3 eV) as the dominant neutral components of the gas phase (see Fig. 3a). Figure 2b shows that the concentration of electrons under the condition n–/ne < 1 follows the change in n+. In fact, this is caused by a decrease in the effective diffusion coefficient and frequency of the diffusion loss of electrons with a decrease in the plasma’s electronegativity (n–/ne = 0.85–0.26 at 0–75% O2) due to the corresponding differences in the rate constants of the processes R1: HBr + e → H + Br– (k1 ∼ 3.0 × 10–10 cm3/s for Te = 3 eV) and R2: O2 + e → O + O– (k2 ∼ 2.1 × 10–11 cm3/s for Te = 3 eV). It should also be noted that the total effect of the decrease in the negative bias at the lower electrode (–Udc = 296–280 V at 0–75% O2; see Fig. 2c) and effective mass of the ions is more than compensated for by the increase in the ion flux density (Fig. 3b). As a result, with increasing \({{y}_{{{{{\text{O}}}_{2}}}}}\), the parameter \(\sqrt {{{M}_{i}}{{\varepsilon }_{i}}} {{\Gamma }_{ + }}\) (Fig. 2c), which characterizes the intensity of ion bombardment of the processed surface [13, 14], increases.
Electrophysical parameters for the plasma of the HBr + O2 mixture with variable initial composition: (1) electron temperature; (2) ion current density; (3) total concentration of positive ions; (4) electron concentration; (5) negative bias at the substrate holder; and (6) parameter \(\sqrt {{{M}_{i}}{{\varepsilon }_{i}}} {{\Gamma }_{ + }},\) which characterizes the ion energy flux density.
When analyzing the kinetics of neutral particles, it was found that, in the investigated range of conditions, the HBr plasma preserves almost all its specific features noted previously in [17, 23, 24]. In particular, our computations show that the reactions R3: HBr + e → H + Br + e (k3 ∼ 1.6 × 10–9 cm3/s at Te = 3 eV) and R4: Br2 + e → 2Br + e (k4 ∼ 1.2 × 10–8 cm3/s at Te = 3 eV) are bromine atom sources similar in their efficiencies even under the condition \({{n}_{{{\text{B}}{{{\text{r}}}_{2}}}}} < {{n}_{{{\text{HBr}}}}}.\) At the same time, the low rate constant of R5: H2 + e → 2H + e (k5 ∼ 8.1 × 10–10 cm3/s at Te = 3 eV) together with the rapid loss of hydrogen atoms in the atomic-molecular processes R6: HBr + H → H2 + Br (k6 ∼ 1.2 × 10–11 cm3/s) and R7: Br2 + H → HBr + Br (k7 ∼ 1.2 × 10–10 cm3/s) lead to \({{n}_{{\text{H}}}} \ll {{n}_{{{\text{Br}}}}}.\) In addition, the fulfillment of the condition \({{k}_{4}} \gg {{k}_{6}}\) and effective formation of H2 molecules in R6 ensure \({{n}_{{{{{\text{H}}}_{2}}}}} > {{n}_{{{\text{B}}{{{\text{r}}}_{2}}}}}\) (Fig. 3a). The dilution of HBr with oxygen is accompanied by both the increase in the dissociation frequencies of molecular particles in electron impact processes (e.g., k3ne = 72–137 s–1 and k4ne = 512–938 s–1 at 0–75% O2) and the emergence of stepwise dissociation mechanisms with the participation of O and OH. Among the latter, the most effective are R8: HBr + OH → H2O + Br (k8 ∼ 8.0 × 10–12 cm3/s), R9: Br2 + O → BrO + Br (k9 ∼ 1.3 × 10–11 cm3/s), and R10: Br2 + OH → HOBr + Br (k10 ∼ 3.1 × 10–11 cm3/s). This causes a rapid decrease in the HBr and Br2 concentrations (by factors of ∼4 and ∼7, respectively, at 0–50% O2) with a much slower change in nBr (by a factor of ∼1.5 at 0–50% O2). A similar behavior is also typical for the density of the bromine atom flux onto the processed surface ΓBr (see Fig. 3b).
The comparison of the shown data in Figs. 1–3 allows us to conclude that the monotonic decrease in the Si and SiO2 etching rates with increasing \({{y}_{{{{{\text{O}}}_{2}}}}}\) contradicts the behavior of \(\sqrt {{{M}_{i}}{{\varepsilon }_{i}}} {{\Gamma }_{ + }},\) but it is qualitatively consistent with the behavior of ΓBr. This is typical for the ion-stimulated chemical reaction in mode of limitation by a flux of neutral particles [19, 20]; the rate of this reaction can be represented as γRΓBr, where γR is the effective interaction probability. Figure 1b shows that the increase in the oxygen concentration in the HBr + O2 mixture causes a monotonic decrease in γR for Si and SiO2 with the decrease slowing down significantly in the region \({{y}_{{{{{\text{O}}}_{2}}}}}\) > 30–40%. Taking into account that the latter effect correlates with saturation on the corresponding dependence of the ion energy flux density (see Fig. 2c), the mechanisms of Si and SiO2 etching in the investigated range of conditions can be interpreted as follows. In oxygen-free plasma, etching is provided by the chemical reaction of silicon with bromine atoms, followed by the ion-stimulated desorption of the interaction products:
where (s.) corresponds to the state of the particle on the surface. The introduction of oxygen into the plasma-forming gas initiates the oxidation of silicon bromides and, hence, adds two heterogeneous stations to the etching process:
Obviously, the increase in \({{y}_{{{{{\text{O}}}_{2}}}}}\) causes a similar change in the rate of R13 and, hence, contributes to the increase in the concentration of SiBrxOy among the interaction products. The low volatility of SiBrxOy compared to SiBrx causes a decrease in the effective yield of the ion-stimulated desorption of the products, which, in the region \({{y}_{{{{{\text{O}}}_{2}}}}}\) < 30–40%, more than compensates for the increase in the intensity of ion bombardment of the surface. This causes a decrease in γR due to the reduction in the free surface available for the adsorption of Br atoms. It can be assumed that, in the region \({{y}_{{{{{\text{O}}}_{2}}}}}\) > 30–40%, SiBrxOy becomes a dominant product of interaction with the effect of R13 on the composition of the interaction products and effective yield of their ion-stimulated desorption being significantly weakened. Under these conditions, small changes in γR are consistent with the corresponding dependence of the parameter \(\sqrt {{{M}_{i}}{{\varepsilon }_{i}}} {{\Gamma }_{ + }}.\) It should also be noted that, in the region \({{y}_{{{{{\text{O}}}_{2}}}}}\) < 30–40%, \({{y}_{{R,{\text{Si}}{{{\text{O}}}_{2}}}}}\) is characterized by a weaker dependence on the oxygen concentration in the mixture as compared to γR, Si (see Fig. 1b). In our opinion, this is due to the fact that SiO2 etching is initiated by the ion process
the effectiveness of which is not due to the change in the composition of the interaction products. Figure 1b also illustrates the effect of the initial composition of the HBr + O2 mixture on the effective etching yield YR defined as a ratio of the etching rate to the parameter \(\sqrt {{{M}_{i}}{{\varepsilon }_{i}}} {{\Gamma }_{ + }}.\) With the latter taking into account the variability of the energy and effective mass of the ions, the dependence YR = f(\({{y}_{{{{{\text{O}}}_{2}}}}}\)) reflects only the change in the properties of the sputtered surface. Thus, the monotonic decrease in YR with increasing concentration and flux density of oxygen atoms confirms our assumption that the etching products are oxidized into low-volatile compounds of the SiBrxOy type.
4 CONCLUSIONS
In this paper, we have investigated the effect of the initial composition of the HBr + O2 mixture on the kinetics and mechanisms of reactive-ion etching of Si and SiO2 under the conditions of a high-frequency (13.56 MHz) inductive discharge. It has been found that the increase in the oxygen concentration in the mixture is accompanied by a monotonic decrease in both the etching rates and kinetic coefficients that characterize the chemical (effective interaction probability) and physical (effective etching yield) components of the heterogeneous interaction. The latter is provided by the monotonic—but slower than the etching rate—decrease in the flux density of bromine atoms and increase in the ion energy flux density. The satisfactory correlation between the changes in the heterogeneous kinetic coefficients and the flux density of the oxygen atoms suggests that the etching products are oxidized into low-volatile compounds of the SiBrxOy type. This is accompanied by the decrease in the coefficient of the ion-stimulated desorption (sputtering) of the etching products and the reduction in the free surface available for the adsorption of bromine atoms.
REFERENCES
Advanced Plasma Processing Technology, New York: Wiley, 2008.
Wolf, S. and Tauber, R.N., Silicon Processing for the VLSI Era, Vol. 1: Process Technology, New York: Lattice Press, 2000.
Nojiri, K., Dry Etching Technology for Semiconductors, Tokyo: Springer Int., 2015.
Coburn, J.W., Plasma Etching and Reactive Ion Etching, AVS Monograph Series, New York: IOP Publ., 1982.
Vitale, S.A., Chae, H., and Sawin, H.H., Silicon etching yields in F2, Cl2, Br2, and HBr high density plasmas, J. Vac. Sci. Technol., A, 2001, vol. 19, no. 5, pp. 2197–2206.
Handbook of Chemistry and Physics, New York: CRC, 2003–2004.
Belen, R.J., Gomez, S., Kiehlbauch, M., and Aydil, E.S., Feature scale model of Si etching in SF6/O2/HBr plasma and comparison with experiments, J. Vacuum. Sci. Technol. A, 2006, vol. 24, no. 2, pp. 350–361.
Tokashiki, K., Ikawa, E., Hashimoto, T., Kikkawa, T., Teraoka, Y., and Nishiyama, I., Influence of halogen plasma atmosphere on SiO2 etching characteristics, Jpn. J. Appl. Phys., 1991, vol. 30, no. 11b, pp. 3174–3177.
Donnelly, V.M., Klemens, F.P., Sorsch, T.W., Timp, G.L., and Baumann, F.H., Oxidation of Si beneath thin SiO2 layers during exposure to HBr/O2 plasmas, investigated by vacuum transfer X-ray photoelectron spectroscopy, Appl. Phys. Lett., 1999, vol. 74, no. 9, pp. 1260–1262.
Cunge, G., Kogelschatz, M., Joubert, O., and Sadeghi, N., Plasma-wall interactions during silicon etching processes in high-density HBr/Cl2/O2 plasmas, Plasma Sources Sci. Technol., 2005, vol. 14, no. 2, pp. S42–S52.
Yeom, G.Y., Ono, Y., and Yamaguchi, T., Polysilicon etchback plasma process using HBr, Cl2, and SF6 gas-mixtures for deep-trench isolation, J. Electrochem. Soc., 1992, vol. 139, no. 2, pp. 575–579.
Kim, D.K., Kim, Y.K., and Lee, H., A study of the role of HBr and oxygen on the etch selectivity and the post-etch profile in a polysilicon/oxide etch using HBr/O2 based high density plasma for advanced DRAMs, Mater. Sci. Semicond. Proc., 2007, vol. 10, no. 1, pp. 41–48.
Lee, B.J., Efremov, A., Kim, J., Kim, C., and Kwon, K.-H., Peculiarities of Si and SiO2 etching kinetics in HBr + Cl2 + O2 inductively coupled plasma, Plasma Chem. Plasma Process., 2019, vol. 39, no. 1, pp. 339–358.
Lee, B.J., Efremov, A., and Kwon, K.-H., Plasma parameters, gas-phase chemistry and Si/SiO2 etching mechanisms in HBr + Cl2 + O2 gas mixture: effect of HBr/O2 mixing ratio, Vacuum, 2019, vol. 163, pp. 110–118.
Son, J., Efremov, A., Yun, S.J., Yeom, G.Y., and Kwon, K.-H., Etching characteristics and mechanism of SiNx films for nano-devices in CH2F2/O2/Ar inductively coupled plasma: effect of O2 mixing ratio, J. Nanosci. Nanotechnol., 2014, vol. 14, pp. 9534–9540.
Shun’ko, E.V., Langmuir Probe in Theory and Practice, Boca Raton, FL: Universal, 2008.
Kwon, K.-H., Efremov, A., Kim, M., Min, N.K., Jeong, J., and Kim, K., A model-based analysis of plasma parameters and composition in HBr/X (X = Ar, He, B2) inductively coupled plasmas, J. Electrochem. Soc., 2010, vol. 157, no. 5, pp. H574–H579.
Hsu, C.C., Nierode, M.A., Coburn, J.W., and Graves, D.B., Comparison of model and experiment for Ar, Ar/O2 and Ar/O2/Cl2 inductively coupled plasmas, J. Phys. D: Appl. Phys., 2006, vol. 39, no. 15, pp. 3272–3284.
Lieberman, M.A. and Lichtenberg, A.J., Principles of Plasma Discharges and Materials Processing, New York: Wiley, 2005.
Jin, W.D., Vitale, S.A., and Sawin, H.H., Plasma-surface kinetics and simulation of feature profile evolution in Cl2 + HBr etching of polysilicon, J. Vac. Sci. Technol., A, 2002, vol. 20, no. 6, pp. 2106–2114.
Gray, D.C., Tepermeister, I., and Sawin, H.H., Phenomenological modeling of ion-enhanced surface kinetics in fluorine-based plasma-etching, J. Vac. Technol. B, 1993, vol. 11, no. 4, pp. 1243–1257.
Chistophorou, L.G. and Olthoff, J.K., Fundamental Electron Interactions with Plasma Processing Gases, New York: Springer Science, 2004.
Efremov, A., Kim, Y., Lee, H.W., and Kwon, K.-H., A comparative study of HBr-Ar and HBr-Cl2 plasma chemistries for dry etch applications, Plasma Chem. Plasma Process., 2011, vol. 31, no. 2, pp. 259–271.
Efremov, A., Lee, J., and Kwon, K.-H., A comparative study of CF4, Cl2 and HBr + Ar inductively coupled plasmas for dry etching applications, Thin Solid Films, 2017, vol. 629, pp. 39–48.
Funding
This work was carried out as part of a state task for the Scientific Research Institute of System Analysis, Russian Academy of Sciences (fundamental scientific research) on topic no. 0065-2019-0006 “Fundamental and Applied Research in the Field of Subwavelength Holographic Lithography, Physicochemical Processes of Etching 3D Nanometer Dielectric Structures for the Development of Crucial Electronic Component Manufacturing Technologies.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Yu. Kornienko
Rights and permissions
About this article
Cite this article
Efremov, A.M., Betelin, V.B. & Kwon, KH. Kinetics and Mechanisms of Reactive-Ion Etching of Si and SiO2 in a Plasma of a Mixture of HBr + O2 . Russ Microelectron 49, 379–384 (2020). https://doi.org/10.1134/S1063739720060037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063739720060037