Abstract
Hemolymph is one of the main tissues providing invertebrates’ immunity and homeostasis. Earlier, we showed that some of the hemolymph immune parameters of the common Far Eastern mollusk Modiolus kurilensis are sensitive to bacterial and anthropogenic challenges. In this work, a number of cellular parameters such as total hemocyte count (THC), hemocyte phagocytic activity (PA), and reactive oxygen species (ROS) and humoral parameters such as hemagglutination (HA) and hemolytic (HL) activity of plasma, as well as its protein concentration (CP) and profiling in M. kurilensis were studied depending on the season and environmental indicators (temperature, salinity, and oxygen concentration). The total hemocyte count was weakly correlated with water oxygen concentration (R = –0.25) and temperature (R = 0.27), and the 36 kDa plasma protein was weakly correlated with temperature (R = –0.33). Also, there was a correlation (R = 0.38) between hemagglutination activity and the 19 kDa protein fraction. The minimum values for a number of parameters were noted in January (THC, PA, ROS, HA, HL), while the maximum values were observed in late spring (THC, PA, ROS, HA, HL). At the same time, in other months, these and most other parameters had metastable values. Thus, the indicators that were identified in previous studies (THC, PA, ROS, HA, HL, CP, protein fractions 55 and 124 kDa) are suitable for assessing the physiological state of M. kurilensis during most of the year.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In coastal ecosystems, bivalved mollusks are one of the key species, which are considered the main indicators for assessing the condition of marine areas. Many of them are valuable commercial species. Most of physiological, and especially immune, parameters of Bivalvia depend on seasonal abiotic (temperature and salinity) and biotic (pathogens or food availability) factors and endogenous processes (reproduction). This has been shown in different studies of Mytilus spp. [1–5]. Environmental factors may exhibit short- and long-term fluctuations depending on the particular geographic area and the characteristics of streams. Such fluctuations affect the health of marine bivalves, which can be estimated through investigation of the dynamics of the cellular and humoral defense-related parameters [6].
Bivalves have both cellular and humoral immune mechanisms that in many cases co-operate to eliminate invading pathogens [7, 8]. The immune system is also involved in many biological functions, such as wound and shell repair, digestion and nutrient transport, and excretion [9]. The correlations of abiotic factors with the main cell-mediated parameters, namely the total hemocyte count, phagocytosis, and the production of oxygen metabolites, have been described for Mytilus spp. [2, 10, 11]. The humoral immune system includes bioactive factors such as agglutinins, opsonins, lytic and cytotoxic substances [12], and they can also be used in bioassays for evaluating the deleterious effects of contaminants on the well-being of organisms and the quality of their habitat [13–15].
The influence of environmental factors on the dynamics of immune parameters has been studied in many invertebrates [16–19]. However, the available data on mollusk immune response to seasonal changes still do not cover all questions due to deep specific immunological responses from species to species [20].
The bivalve Modiolus kurilensis is widespread in the northwestern Pacific and is one of the dominant species in benthic communities of the Sea of Japan. In previous studies, we have investigated the immune state, histopathological level, and integral health index (IHI) of M. kurilensis from different areas with different levels of ecotoxicological stress [21, 22], as well as its reactions to bacterial stimulation [23]. These studies revealed several highly sensitive immune and histological parameters to changes under chronic and impulse stress. The aim of the present study was to examine the dynamics of the hemolymph parameters of M. kurilensis, reveal correlations between immune activity and environmental factors, and summarize the key aspects of development of the Integral Health Index concept.
MATERIALS AND METHODS
Collection of Animals and Environmental Conditions
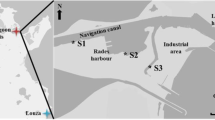
Sexually mature specimens of M. kurilensis with a shell length of 75–95 mm were collected from wild populations in Vostok Bay of the Sea of Japan (42.892078° N, 132.737502° E) in different temperature seasons. Temperature, salinity, and the concentration of oxygen were measured on the day of collection of mollusks at the depth of their habitat (2–4 m). Data on the dates of measurement of environmental parameters and the number of mollusks are given in Table 1. A total of 276 bivalves were collected.
Assessment of Humoral and Cellular Parameters of M. kurilensis
For assessment of the immune status of M. kurilensis, the hemolymph was sampled from the posterior adductor muscle hemal sinus. Total hemocyte count (THC), hemocyte phagocytic activity (PA), hemagglutination (HA) and hemolytic (HL) activity of plasma, and plasma protein concentration (Cp) were determined according to the methods described previously [23]. Hemocyte production of reactive oxygen species (ROS) was measured using the nitroblue tetrazolium (NBT) reduction spontaneous test, and plasma protein profiling was determined as the percentage of the optical density (POD) of each detected fraction [21].
Data Analysis
The values of both the humoral and cellular immune parameters were checked by the Kolmogorov–Smirnov test and had a non-normal distribution (p < 0.05) for all samples. Further analysis was performed using non-parametric statistics. The basic dynamics of distribution of immunity parameters in different seasons were analyzed using basic statistics such as the mean, median, standard deviation, quartile range, and minimum and maximum values for each sample. Significant changes in parameter dynamics were assessed by the Kruskal–Wallis H test. Differences between pairs of samples were assessed by the Mann–Whitney U test. Correlations between immune parameters themselves and environmental conditions were revealed using the Spearman’s rank-order correlation test (p < 0.05).
RESULTS
Total Hemocyte Count (THC)
The H test confirmed the significance of changes in the total number of hemocytes circulating in the hemolymph (p < 0.001). The maximum number of hemocytes was observed in April (5.6 × 106 cells/mL) in one individual. There was a gradual decrease in THC values during the low temperatures period from November to March (Fig. 1). The minimum value of THC (0.5 × 106 cells/mL) was found in March. The most characteristic values were from 1.1 × 106 to 1.9 × 106 cells. The U test confirmed the differences in THC values between March and most other months (p < 0.05).
The dynamics of M. kurilensis parameters in different seasonal periods (the mean value ±95% confidence interval). (a) Total hemocyte count (THC); (b) phagocytic activity (PA); (c) the percentage of ROS-positive cells (NBT-test); (d) hemolysis (HL); (e) hemagglutination (HA); (f) protein concentration (Cp).
Phagocytic Activity (PA)
In general, during the year, the PA values ranged from 8.3 × 103 RFU in January to 42.4 × 103 RFU in May. Changes in the seasonal dynamics of PA were supported by the H test (p < 0.001). The maximum values of PA were observed in late spring (April, May); phagocytic activity was decreased in other months, with minimum values in January (Fig. 1). PA was also increased in November, when the temperature is close to April and May values. The U test confirmed the differences in the values of April, May, and November compared to other months (p < 0.001).
Nitroblue Tetrazolium Reduction (NBT) Test
The significant changes in cell-mediated immunity parameters, namely hemocyte production of reactive oxygen species (ROS), were supported by the H test (p < 0.05). Primarily, the NBT-test was not stable, varying with a wide scatter of the minimum and maximum values in general and for each month (Fig. 1). The maximum decrease in ROS was in January, which was consistent with paired comparisons (p < 0.05). The maximum values of the NBT-test were 54% ROS positive cells out of all hemocytes. However, most of the values of the total sample lay in the range of quartiles: from 3% for the lower to 15% for the upper quartile.
Hemolysis (HL)
The seasonal fluctuations in the activity of lytic hemolymph factors were expressed in values of hemolysis; the significance of changes was supported by the H test (p < 0.05). The dynamics of hemolytic activity in M. kurilensis reflected the general tendency of decreasing immune activity in the winter period with minimum average values of HL (24.6%) in January (Fig. 1). The paired test confirmed the significance of differences between the values of January and other months (p < 0.05). Moreover, the HL values were highest in May and April, which was also characteristic for most other parameters. In general, the range of minimum and maximum values of HL was wide, from 0 to 100%.
Hemagglutination (HA)
The least variability of HA values was noted in June, which was associated with a larger sample size than in other months. A value of –log2 (titer) = 1 was found only for one individual; and HA was equal to 2 for 4 individuals out of the whole sample. The maximum activity of plasma agglutinins in M. kurilensis was –log2 (titer) = 11. This value was observed in 5 individuals from samples collected in April, June, and May. For the total sample, a value of 4 was the lower quartile (25%) and a value of 8 the upper quartile (75%). There was a tendency for HA values to decrease in January (Fig. 1). However, the H test showed no differences in HA values during the year (p > 0.05). The exception was the differences between January and March that were supported by the paired U test (p < 0.05).
Protein Concentration (Cp)
The plasma protein content varied in a wide range from 0.35 to 3.00 mg/mL and differed almost by an order of magnitude within a single sample. The limits of the quartile range, which characterizes the most typical values, were 0.92 mg/mL for the lower quartile (25%) and 1.58 mg/mL for the upper quartile75%. Regarding the variability between samples, values for the summer-autumn period were more stable, while the scatter for the winter-spring samples was much higher (Fig. 1). The dynamics of PC in plasma differed from those of other immune factors and had a clear tendency to decrease from June to November, which was confirmed by both the paired U and the H tests (p < 0.001).
Protein Profiling
Fifteen protein fractions in plasma of M. kurilensis were detected: 196, 145, 124, 107, 78, 55, 48, 41, 36, 33, 29, 26, 22, 19, and 16 kDa. Proteins with a molecular weight of 145, 124, 107, and 29 kDa were observed in less than 50% of the specimens. Proteins of 55, 41, 36, and 16 kDa were found most frequently, in more than 86% of the animals. The major fraction was 55 kDa, which was found in all analyzed specimens and constituted two-thirds of all proteins in plasma. The U test (p < 0.05) supported an increase in POD55 kDa in May (68.0 ± 4.5), April (69.3 ± 4.3), and June (67.5 ± 2.7) in comparisons with minimum values in March (58.8 ± 6.4) (Fig. 2).
Among all PODs, there were highly significant (H test, p < 0.001) within-year variations in POD124 kDa, POD107 kDa, POD48 kDa, POD41 kDa, and POD36 kDa (Fig. 2).
The mean value of POD124 kDa was highest in September (1.4 ± 0.4), the paired test confirmed differences from other months. The percentage of this fraction in plasma of M. kurilensis slightly decreased in November (0.9 ± 0.6) and January (0.7 ± 0.6) and had consistently low values in April (0.4 ± 0.3), June (0.4 ± 0.2), March (0.3 ± 0.1), and May (0.3 ± 0.3).
POD107 kDa showed consistently higher values from September (1.2 ± 0.5) to March (1.3 ± 0.6); its values decreased in January (0.9 ± 0.5) and were low in April (0.3 ± 0.3), June (0.3 ± 0.2), and May (0.3 ± 0.3). The dynamics of POD48 kDa showed a peak in March (4.6 ± 2.4) and lowest values in April (1.6 ± 0.5) and May (1.6 ± 0.7).
The dynamics of POD41 kDa and POD36 kDa were opposite. For POD41 kDa, a dramatic decline in values was found in January (1.7 ± 0.7), compared to November (3.9 ± 1.1) and March (4.0 ± 1.3), while POD36 kDa had maximum values in January (10.6 ± 3.1), which were more than twice as high, compared to other months.
Correlation Analysis
The analysis of environmental parameters showed a higher direct correlation between water temperature and salinity (R = 0.66), an inverse correlation between temperature and oxygen concentration (R = –0.91), as well as between salinity and oxygen concentration (R = –0.54).
Further correlation analysis demonstrated a positive relationship between THC and water temperature (R = 0.27, n = 276) and an inverse dependence between THC and oxygen concentration in water (R = –0.25, n = 276). An inverse correlation between water temperature and POD36 kDa was obtained (R = –0.33, n = 276).
Data from correlation analysis of hemolymph parameters for each seasonal period and in total are given in Table 2. Among cellular immune parameters, there was a positive correlation between PA and the NBT-test, with a higher value in May, April, and June. In addition, a high positive correlation was found between PA and THC in March. Also, a moderate positive correlation was observed between THC and NBT-test parameters in September.
Among humoral immune parameters, Cp and HL were positively correlated for the total sample, with higher values in September, November, and March. An average degree of positive correlation between Cp and HA was found only in September. Positive relationships between Cp and POD36 kDa as well as between Cp and POD16 kDa were also obtained, with maximum values in January for the POD36 kDa fraction and in March, April, and June for the POD16 kDa fraction. An inverse correlation was observed between Cp and POD55 kDa in all months except November, as well as between Cp and POD196 kDa in April, June, and March.
Among all correlations of humoral immune parameters, most interesting were the relationships of HL and HA with particular protein fractions. Correlation analysis showed a positive dependence between HL and POD16 kDa for the June–November samples. For March and April, positive relationships were found between HL and POD33 kDa and between HL and POD78 kDa. A positive correlation was observed between HA and POD19 kDa for the total sample (R = 0.38, n = 276), with higher values in June and January.
No significant correlations were found between cellular and humoral immune parameters for the total sample; the correlation coefficients in certain months were non-significant too and had a disparate character (data not shown).
DISCUSSION
Environmental seasonal changes directly affect many organisms, especially in areas where temperature fluctuations are significant. In this research, we examined the immune activity of the bivalve M. kurilensis from Vostok Bay (Peter the Great Bay, Sea of Japan) during the year and revealed possible correlations between immune parameters and environmental factors such as temperature, salinity, and oxygen concentration. In addition to the above environmental factors, the reproductive cycle directly associated with the season strongly influences the physiology and immune system of mollusks [6, 15, 24, 25].
A general scheme of characteristics of immune parameters of M. kurilensis measured during the year along with temperature data and approximate stage of the reproductive cycle (according to [26, 27]) is presented in Fig. 3.
The maximum immune activity was observed in late spring during gametogenesis and at the beginning of rapid increase in temperature. The summer-autumn period was most stable, and only in late autumn, the values of THS and ROS decreased and there was a significant increase in PA.
During the period of low temperatures (under zero degree), the lowest immune activity and the minimum values of most parameters were observed. The exception was Cp, which had low values from June to November. The dynamics of each immune parameter are discussed more precisely further.
According to current results, there was a positive correlation between THC and temperature, with the highest values in January and March, when both parameters were decreased. Such a relationship between THC and temperature was also obtained for Mytilus galloprovincialis and Paphia malabarica [28, 29]. The minimum value of THC in the winter period was also typical for Crassostrea gigas from the Mediterranean Sea and the Sea of Japan [24, 30] and M. galloprovincialis from the northern part of the Atlantic Ocean [29].
For several species, the presence or absence of any correlations between THC and temperature was shown to depend on the environmental characteristics. For example, for Ruditapes philippinarum [25] inhabiting the Gulf of Morbihan (southwest of France, Atlantic Ocean) such correlation was positive, while for R. philippinarum [15] in the northern and southern areas of Venice (Adriatic Sea) the authors found an inverse dependence.
Such fluctuations in correlations might be associated with particular characteristics of temperature range during the year. Thus, the temperature range of waters in the Gulf of Morbihan (from 6 to 23°C) is fairly wide, as is the temperature range of the studied area of the Sea of Japan (from –1.5 to 19.3°C). At the same time, the temperature range for waters in the Adriatic Sea is narrower (from 13 to 23°C).
In this regard, the wider variation in temperature supported the stronger correlation between THC and temperature. In contrast, waters with a more stable temperature have a lesser influence on it, and for such areas, the characteristics of the sexual cycle have a stronger effect on THC, as it was shown for R. philippinarum [15].
The decrease in THC in winter might also be caused by a limited availability of food. This was confirmed for M. galloprovincialis, and a positive correlation between THC and the availability of nutrition was obtained [29].
A positive correlation between THC and salinity was also observed for M. kurilensis, with the minimum of both parameters in March, when the melting of coastal ice caused decreased salinity. The same tendency of relationship between THC and water salinity was also shown for P. malabarica [28], Mytilus edulis [31], and Ostrea edulis [32].
Although the current research revealed an inverse correlation between THC and oxygen concentration, another tendency was obtained for Mactra veneriformis. THC dropped when the mollusks had been exposed to anoxic conditions [33]. The opposite dependence between cellular immune parameters and the level of oxygen was apparently due to the extremely high stress of the mollusks.
Dynamics of THC with a significant increase in April and May and a decrease in autumn could also be linked with the reproductive cycle of M. kurilensis. The spawning period of M. kurilensis begins at a temperature of 16°C and lasts during July–August in the studied area, while the processes of gametogenesis start in April and May [27]. Strong influence of the reproductive cycle on the dynamics of hemocyte circulation and THC was shown for C. gigas [24] and R. philippinarum [6, 15, 25]. The THC was decreased during the spawning period of C. gigas and R. philippinarum, which lasts from the middle of August to the end of September. Nonetheless, the number of granular hemocytes and THC were high during the gametogenesis period of these species (spring and the first half of summer). According to the authors, the decrease in THC in hemolymph was related to that most of the cells were mobilized to repair the reproductive tissue in the gonads of mollusks. This could also explain the results on THC for M. kurilensis.
The PA of M. kurilensis had maximum values in April and May and minimum in January. Moreover, an increase in PA was noted in November, when the temperature was close to that in spring months. A similar dynamics of maximum and minimum values of PA were reported for P. malabarica [28].
The PA dynamics of M. kurilensis, especially high values in spring, could be related to the high bacterial activity and the onset of higher temperatures in late spring. In addition, PA probably depends on the reproductive cycle. During the summer and September, there was a decrease in PA possibly related to the spawning period in M. kurilensis, as was the case for THC values at that period. This was suggested in related research by other authors [24].
A study of the immune parameters of C. gigas from the Sea of Japan revealed maximum PA in June and minimum in February. The shift in the results, compared to our findings, might be explained by a much later beginning of the spawning period in C. gigas [24].
The minimum values of PA of M. kurilensis were observed in winter at the lowest temperature and possibly reflected the overall decrease in metabolic rate and low bacterial activity in that period. In the current research, the correlation between PA and temperature was non-significant, which was also noted in a study of C. gigas [24]. However, a high positive correction was observed for R. philippinarum [15].
The unstimulated NBT-test allows detecting ROS in hemocytes and estimates the most stressful periods for species. Moreover, ROS plays a key role in oxygen-dependent phagocytosis. This was reflected in the positive correlation between NBT-test and PA during the year, with maximum values in April, May, and June.
In general, the correlation analysis showed a non-significant positive dependence between temperature and ROS for M. kurilensis; however, more significant values were found for P. malabarica [28]. Studies of Chlamys farreri and M. galloprovincialis emphasized that the activity of the antioxidant systems might decrease at high temperatures. As a consequence of inactivation of these factors, the quantity of ROS could increase in mollusks tissues [34, 35]. In a recent study of the immune response of C. gigas, M. galloprovincialis, and Katelysia rhytiphora, both the concentration of ROS and antioxidant system parameters increased with increasing temperature in the experiments [36]. However, no correlation between ROS and environmental parameters was found for some species, e.g., M. edulis [31, 37].
The minimum NBT-test values of M. kurilensis were in winter, which was also shown for R. philippinarum. According to the authors, this dynamics could be related to reduced nutrition availability during low temperatures [6].
Like PA, the maximum ROS was observed in spring and in June, which might be due to the specific features of gametogenesis and reproductive period of M. kurilensis. A study of R. philippinarum suggested that the reproductive period involves a huge consumption of oxygen, which leads to a high level of production of ROS and, consequently, increased antioxidant activity [15].
The general tendency reflected in the dynamics of HL with the minimum values in winter was most probably connected with the lower bacterial activity, lower quantity of nutrition in water, and a decrease in the general metabolic rate of M. kurilensis. Similar suggestions concerning the reasons and factors influencing on dynamics of HL were made in a study of R. philippinarum [6].
Similarly to the above-discussed immune parameters, HL reached the maximum in April and May. In several studies of R. philippinarum [6] and M. galloprovincialis [3, 38], the year-round trend of lysozyme activity was quite similar to our results on HL. The maximum values of lysozyme activity were observed in May, June, and September for M. galloprovincialis, and the minimum values were recorded in winter for both R. philippinarum and M. galloprovincialis [3, 6, 38].
In the present work, HL of M. kur Ailensis and the total protein concentration positively correlated, as was also shown in a study of the year-round lysozyme dynamics of R. philippinarum [6].
The correlation analysis in the current work showed a significant dependence between HL and POD16 kDa. These results suggest the presence of lytic factors in the 16 kDa fraction. Moreover, the range of molecular mass of lytic factors (especially lysozymes) varies from 12 to 22 kDa for many mollusks: M. galloprovincialis (C-type lysozyme 14.4 kDa), R. philippinarum (invertebrate type lysozyme 20.87 kDa), Crassostrea virginica (i-type lysozymes 12.98 and 17.86 kDa), Cristaria plicata (i-type lysozyme 18.2 kDa), Meretrix meretrix (i-type lysozyme 14.6 kDa) [39–44].
The HA is most stable among all immune parameters studied. Nevertheless, there was a non-significant increase in HA mean values (H test) in March, which is possibly associated with the low values of THC and PA (factors of cell-mediated immunity) and, consequently, with the necessity to enhance the humoral immune system of M. kurilensis as a compensatory mechanism. A slight decrease in HA mean values was observed in January, when the temperature was lowest. On the other hand, the dynamics of HA in M. galloprovincialis [38] were not stable and had a significant minimum in January and a maximum in July. Moreover, a positive correlation was found between the total protein concentration and HA and lysozyme activity of M. galloprovincialis, as was also observed for M. kurilensis in the current study.
In the present research, we found a positive correlation between HA and POD19 kDa for the total sample of specimens (R = 0.38, n = 276), with higher values in June and January suggesting the presence of agglutinins in this fraction. Besides, the main role of this fraction in agglutination was described in a previous study of M. kurilensis (unpublished data).
The Cp of M. kurilensis tended to decrease in summer and autumn and increase in January, March, April, and May. However, an opposite tendency of Cp was shown for M. galloprovincialis. The maximum of Cp was in June, during the recovery period after spawning and low nutrition content in the water, which was the main reason for increasing Cp in hemolymph of M. galloprovincialis [38]. In Argopecten irradians and C. farreri, Cp increased with increasing temperature up to 28°С, after that a reverse correlation was observed. Thus, the dynamics of Cp in this case decreased during the period of high temperatures [45]. In R. philippinarum, the protein concentration was high in spring-summer and low in winter. Although the protein concentration was decreasing during the winter, the specimens from northern areas had, in general, higher values. It was suggested that this depended on a wider temperature range in northern areas [6]. Such differences in Cp dynamics are probably associated with the different temperature characteristics of the habitats of mollusks.
The increase in Cp of M. kurilensis in January and March at the lowest temperatures is most likely associated with the activation of cryoprotective proteins, which are responsible for the sustainability of mollusks at temperatures below zero. In this context, the 36 kDa protein is most interesting due to a sudden jump of values in winter. We found a positive correlation between POD36 kDa and Cp (R = 0.31, n = 276) with the maximum in January (R = 0.62, n = 31) and an inverse correlation between temperature and POD36 kDa (R = –0.33, n = 276). The antifreeze protein is actively investigated in different species. Thus, proteins with cryoprotective function (molecular mass from 2.6 to 33.7 kDa) were described for fishes as multi-repeated motives of several amino acids [46]. Antifreeze proteins of Megaphorura arctica also have a wide range of molecular mass from 6.5 to 16.9 kDa [47]. Thus, the molecular mass of such proteins varies even within one species. The role of the 36 kDa protein of M. kurilensis in the antifreeze function requires further analysis and is currently hypothetical.
For hemolymph plasma of M. kurilensis, 15 protein fractions were characterized. The major fraction was 55 kDa. The H test supported significant variations for POD124 kDa, POD107 kDa, POD48 kDa, POD41 kDa, and POD36 kDa during the year. Previously, a protein profiling analysis of mollusks from two areas was performed: non-polluted Vostok Bay and polluted Sportivnaya Gavan Bay (Amursky Bay, the Sea of Japan) [21]. That study showed that POD78 kDa and POD124 kDa were significantly increased and POD55 kDa decreased in polluted areas. Moreover, there was a correlation between these protein fractions and immune and histopathological parameters, suggesting the diagnostic importance of these fractions [21]. In addition, the immune parameters PA, NBT-test, HL, and Cp were also sensitive to the level of pollution.
In previous studies, we found and described the parameters of hemolymph of M. kurilensis that were sensitive to impulse bacterial stimulation (THC, PA, HA, HL, and Cp) [22] and to chronic pollution (THC, PA, NBT, HL, Cp, POD55 kDa, POD78 kDa, and POD124 kDa) [21, 23]. In the present article, we have shown that the immune parameters studied have significant fluctuations during the year. Thus, the scheme of comprehensive assessment of the physiological state of animals using the previously accepted Integral Health Index includes the valid identification of parameters that are sensitive to the effects of interest, as well as the determination of reference boundaries for them, depending on environmental factors, certainly including the seasonal aspect (Fig. 4).
Scheme of Integral Health Index development. Asterisk* marks parameters discussed by Kumeiko et al., 2018 [21].
Thus, not only the obvious stress factors such as bacterial activity and presence of pollutants, but also the seasonal characteristics of immune parameters dynamics should be considered for the evaluation and diagnostics of Bivalvia. The variation in immune factors shows a specific tendency during the year. Besides, there is a tendency that the wider the variation in a particular environmental factor, the more pronounced its effect on the dynamics of sensitive immunological parameters. This was observed for individuals of one species from various water areas with a different temperature range [2, 15, 25, 48, 49].
On the other hand, the presence of sensitivity of various immune parameters to environmental factors can be specific, regarding the biology of species, the characteristics of metabolic activity, lifestyle, nutrition, sexual cycle, and others.
In this work, we have shown the within-year dynamics of immunity parameters in M. kurilensis from Vostok Bay (the Sea of Japan) taking into account distribution of temperature, salinity, and oxygen concentration in Peter the Great Bay of the Sea of Japan. Thus, summarizing previous research on the sensitivity of immunological parameters for assessing the physiological state of M. kurilensis, the present study is a continuation and a necessary step in the development of effective methods for assessing the physiological state of marine bivalves.
REFERENCES
Carballal, M.J., López, C., Azevedo, C., et al., Enzymes involved in defense functions of hemocytes of Mussel Mytilus galloprovincialis, J. Invertebr. Pathol., 1997, vol. 70, pp. 96–105. https://doi.org/10.1006/jipa.1997.4670
Carballal, M.J., Villalba, A., and López, C., Seasonal variation and effects of age, food availability, size, gonadal development, and parasitism on the hemogram of Mytilus galloprovincialis, J. Invertebr. Pathol., 1998, vol. 72, pp. 304–312. https://doi.org/10.1006/jipa.1998.4779
Malagoli, D., Casarini, L., and Ottaviani, E., Monitoring of the immune efficiency of Mytilus galloprovincialis in Adriatic sea mussel farms in 2006: Regular changes of cytotoxicity during the year, Invertebr. Survival J., 2007, vol. 4, pp. 10–12.
Novas, A., Barcia, R., and Ramos-Martínez, J.I., Nitric oxide production by haemocytes from Mytilus galloprovincialis shows seasonal variations, Fish Shellfish Immunol., 2007, vol. 23, pp. 886–891. https://doi.org/10.1016/j.fsi.2007.04.007
Schmidt, W., Power, E., and Quinn, B., Seasonal variations of biomarker responses in the marine blue mussel (Mytilus spp.), Mar. Pollut. Bull., 2013, vol. 74, pp. 50–55. https://doi.org/10.1016/j.marpolbul.2013.07.033
Soudant, P., Paillard, C., Choquet, G., et al., Impact of season and rearing site on the physiological and immunological parameters of the Manila clam Venerupis (=Tapes, =Ruditapes) philippinarum, Aquaculture, 2004, vol. 229, pp. 401–418. https://doi.org/10.1016/S0044-8486(03)00352-1
Mitta, G., Vandenbulcke, F., and Roch, P., Original involvement of antimicrobial peptides in mussel innate immunity, FEBS Lett., 2000, vol. 486, pp. 185–190. https://doi.org/10.1016/S0014-5793(00)02192-X
Montes, J.F., Durfort, M., and García-Valero, J., Cellular defence mechanism of the clam Tapes semidecussatus against infection by the protozoan Perkinsus sp., Cell Tissue Res., 1995, vol. 279, pp. 529–538. https://doi.org/10.1007/BF00318165
Cheng, T.C., Bivalves, in Invertebrate Blood Cells, Ratcliffe, N.A. and Rowley, A.F., Eds., London: Academic Press, 1981, pp. 233–300.
Ottaviani, E., Franchini, A., Barbieri, D., et al., Comparative and morphofunctional studies on Mytilus galloprovincialis hemocytes: Presence of two aging-related hemocyte stages, Ital. J. Zool., 1998, vol. 65, pp. 349–354. https://doi.org/10.1080/11250009809386772
Wootton, E.C., Dyrynda, E.A., and Ratcliffe, N.A., Bivalve immunity: Comparisons between the marine mussel (Mytilus edulis), the edible cockle (Cerastoderma edule) and the razor-shell (Ensis siliqua), Fish Shellfish Immunol., 2003, vol. 15, pp. 195–210. https://doi.org/10.1016/S1050-4648(02)00161-4
Ottaviani, E., Molluscan immunorecognition, Invertebr. Survival J., 2006, vol. 15, pp. 50–63.
Luna-Acosta, A., Bustamante, P., Budzinski, H., et al., Persistent organic pollutants in a marine bivalve on the Marennes–Oléron Bay and the Gironde Estuary (French Atlantic Coast)-Part 2: Potential biological effects, Sci. Total Environ., 2015, vol. 514, pp. 511–522. https://doi.org/10.1016/j.scitotenv.2014.10.050
Espinosa Ruiz, C., Morghese, M., Renda, G., et al., Effects of BDE-47 exposure on immune-related parameters of Mytilus galloprovincialis, Aquat. Toxicol., 2019, vol. 215. https://doi.org/10.1016/j.aquatox.2019.105266
Matozzo, V., Da Ros, L., Ballarin, L., et al., Functional responses of haemocytes in the clam Tapes philippinarum from the Lagoon of Venice: Fishing impact and seasonal variations, Can. J. Fish. Aquat. Sci., 2003, vol. 60, pp. 949–958. https://doi.org/10.1139/f03-084
Donaghy, L. and Volety, A.K., Functional and metabolic characterization of hemocytes of the green mussel, Perna viridis: In vitro impacts of temperature, Fish Shellfish Immunol., 2011, vol. 31, pp. 808–814. https://doi.org/10.1016/j.fsi.2011.07.018
Franco-Martínez, L., Martínez-Subiela, S., Escribano, D., et al., Alterations in haemolymph proteome of Mytilus galloprovincialis mussel after an induced injury, Fish Shellfish Immunol., 2018, vol. 75, pp. 41–47. https://doi.org/10.1016/j.fsi.2018.01.038
Wang, Y., Hu, M., Cheung, S.G., et al., Chronic hypoxia and low salinity impair anti-predatory responses of the green-lipped mussel Perna viridis, Mar. Environ. Res., 2012, vol. 77, pp. 84–89. https://doi.org/10.1016/j.marenvres.2012.02.006
Wang, Y., Hu, M., Cheung, S.G., et al., Immune parameter changes of hemocytes in green-lipped mussel Perna viridis exposure to hypoxia and hyposalinity, Aquaculture, 2012, vols. 356–357, pp. 22–29. https://doi.org/10.1016/j.aquaculture.2012.06.001
Duchemin, M.B., Fournier, M., and Auffret, M., Seasonal variations of immune parameters in diploid and triploid Pacific oysters, Crassostrea gigas (Thunberg), Aquaculture, 2007, vol. 264, pp. 73–81. https://doi.org/10.1016/j.aquaculture.2006.12.030
Kumeiko, V.V., Sokolnikova, Y.N., Grinchenko, A.V., et al., Immune state correlates with histopathological level and reveals molluscan health in populations of Modiolus kurilensis by integral health index (IHI), J. Invertebr. Pathol., 2018, vol. 154, pp. 42–57. https://doi.org/10.1016/j.jip.2018.03.014
Sokolnikova, Y.N., Trubetskaya, E.V., Beleneva, I.A., Grinchenko, A.V., and Kumeiko, V.V., Fluorescent in vitro phagocytosis assay differentiates hemocyte activity of the bivalve molluscs Modiolus kurilensis (Bernard, 1983) inhabiting impacted and non-impacted water areas, Russ. J. Mar. Biol., 2015, vol. 154, pp. 118–126. https://doi.org/10.1134/S106307401502011X
Grinchenko, A.V., Sokolnikova, Y.N., Korneiko, D.D., et al., Dynamics of the immune response of the horse mussel Modiolus kurilensis (Bernard, 1983) following challenge with heat-inactivated bacteria, J. Shellfish Res., 2015, vol. 34, pp. 909–917. https://doi.org/10.2983/035.034.0321
Ishikawa, H., Takahashi, G., and Katsuyoshi, M., Annual changes in maturation of the gonad and phagocytic activity in hemocytes of the pacific oyster, Crassostrea gigas, in Onagawa Bay, Miyagi prefecture, Aquacult. Sci., 1999, vol. 47, pp. 519–525.
Flye-Sainte-Marie, J., Soudant, P., Lambert, C., et al., Variability of the hemocyte parameters of Ruditapes philippinarum in the field during an annual cycle, J. Exp. Mar. Bio. Ecol., 2009, vol. 377, pp. 1–11. https://doi.org/10.1016/j.jembe.2009.06.003
Vaschenko, M.A., Kovaleva, A.L., Syasina, I.G., et al., Reproduction-related effects of green alga Coccomyxa sp. infestation in the horse mussel Modiolus modiolus, J. Invertebr. Pathol., 2013, vol. 113, pp. 86–95. https://doi.org/10.1016/j.jip.2013.02.003
Rodovets, A.V. and Khristoforova, N.K., Density dynamics of commercial bivalves larvae in plankton of the Minonosok Bight and Vostok Bay (Japan Sea), Izv. Tikhookean. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2008, vol. 153, pp. 201–214.
Gajbhiye, D.S. and Khandeparker, L., Immunoecology of the short neck clam Paphia malabarica (Chemnitz, 1782) in a tropical monsoon-influenced estuary, Mar. Environ. Res., 2019, vol. 143, pp. 60–70. https://doi.org/10.1016/j.marenvres.2018.11.004
Carballal, M.J., Villalba, A., and López, C., Seasonal variation and effects of age, food availability, size, gonadal development, and parasitism on the hemogram of Mytilus galloprovincialis, J. Invertebr. Pathol., 1998, vol. 72, pp. 304–312. https://doi.org/10.1006/jipa.1998.4779
Duchemin, M.B., Fournier, M., and Auffret, M., Seasonal variations of immune parameters in diploid and triploid Pacific oysters, Crassostrea gigas (Thunberg), Aquaculture, 2007, vol. 264, pp. 73–81. https://doi.org/10.1016/j.aquaculture.2006.12.030
Bussell, J.A., Gidman, E.A., Causton, D.R., et al., Changes in the immune response and metabolic fingerprint of the mussel, Mytilus edulis (Linnaeus) in response to lowered salinity and physical stress, J. Exp. Mar. Biol. Ecol., 2008, vol. 358, pp. 78–85. https://doi.org/10.1016/j.jembe.2008.01.018
Hauton, C., Hawkins, L.E., and Hutchinson, S., The effects of salinity on the interaction between a pathogen (Listonella anguillarum) and components of a host (Ostrea edulis) immune system, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2000, vol. 127, pp. 203–212. https://doi.org/10.1016/S0305-0491(00)00251-0
Yu, J., Choi, M., Park, K., et al., Effects of anoxia on immune functions in the surf clam Mactra veneriformis, Zool. Stud., 2010, vol. 49, pp. 94–101.
Chen, M., Yang, H., Delaporte, M., et al., Immune condition of Chlamys farreri in response to acute temperature challenge, Aquaculture, 2007, vol. 271, pp. 479–487. https://doi.org/10.1016/j.aquaculture.2007.04.051
Mosca, F., Narcisi, V., Calzetta, A., et al., Effects of high temperature and exposure to air on mussel (Mytilus galloprovincialis, Lmk 1819) hemocyte phagocytosis: Modulation of spreading and oxidative response, Tissue Cell, 2013, vol. 45, pp. 198–203. https://doi.org/10.1016/j.tice.2012.12.002
Rahman, M.A., Henderson, S., Miller-Ezzy, P., et al., Immune response to temperature stress in three bivalve species: Pacific oyster Crassostrea gigas, Mediterranean mussel Mytilus galloprovincialis and mud cockle Katelysia rhytiphora, Fish Shellfish Immunol., 2019, vol. 86, pp. 868–874. https://doi.org/10.1016/j.fsi.2018.12.017
Bibby, R., Widdicombe, S., Parry, H., et al., Effects of ocean acidification on the immune response of the blue mussel Mytilus edulis, Aquat. Biol., 2008, vol. 2, pp. 67–74. https://doi.org/10.3354/ab00037
Santarem, M.M., Robledo, J.A.F., and Figueras, A., Seasonal changes in hemocytes and serum defense factors in the blue mussel Mytilus galloprovincialis, Dis. Aquat. Organ., 1994, vol. 18, pp. 217–222. https://doi.org/10.3354/dao018217
Wang, Q., Wang, C., Mu, C., et al., A novel C-type lysozyme from Mytilus galloprovincialis: Insight into innate immunity and molecular evolution of invertebrate C-type lysozymes, PLoS One, 2013, vol. 8. https://doi.org/10.1371/journal.pone.0067469
Zhao, J., Qiu, L., Ning, X., et al., Cloning and characterization of an invertebrate type lysozyme from Venerupis philippinarum, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2010, vol. 156. pp. 56–60. https://doi.org/10.1016/j.cbpb.2010.02.001
Xue, Q.G., Itoh, N., Schey, K.L., et al., A new lysozyme from the eastern oyster (Crassostrea virginica) indicates adaptive evolution of i-type lysozymes, Cell Mol. Life Sci., 2007, vol. 64, pp. 82–95. https://doi.org/10.1007/s00018-006-6386-y
Itoh, N., Xue, Q.G., Li, Y., et al., cDNA cloning and tissue expression of plasma lysozyme in the eastern oyster, Crassostrea virginica, Fish Shellfish Immunol., 2007, vol. 23, pp. 957–968. https://doi.org/10.1016/j.fsi.2007.03.006
Dai, W., Wu, D., Zhang, M., et al., Molecular cloning and functional characterization of a novel i-type lysozyme in the freshwater mussel Cristaria plicata, Microbiol. Immunol., 2015, vol. 59, pp. 744–755. https://doi.org/10.1111/1348-0421.12341
Yue, X., Liu, B., and Xue, Q., An i-type lysozyme from the Asiatic hard clam Meretrix meretrix potentially functioning in host immunity, Fish Shellfish Immunol., 2011, vol. 30, pp. 550–558. https://doi.org/10.1016/j.fsi.2010.11.022
Liu, S., Jiang, X., Hu, X., et al., Effects of temperature on non-specific immune parameters in two scallop species: Argopecten irradians (Lamarck 1819) and Chlamys farreri (Jones & Preston 1904), Aquacult. Res., 2004, vol. 35, pp. 678–682. https://doi.org/10.1111/j.1365-2109.2004.01065.x
Kim, H.J., Lee, J.H., Hur, Y.B., et al., Marine antifreeze proteins: Structure, function, and application to cryopreservation as a potential cryoprotectant, Mar. Drugs, 2017, vol. 15. https://doi.org/10.3390/md15020027
Graham, L.A., Boddington, M.E., Holmstrup, M., et al., Antifreeze protein complements cryoprotective dehydration in the freeze-avoiding springtail Megaphorura arctica, Sci Rep., 2020, vol. 10. https://doi.org/10.1038/s41598-020-60060-z
Ciacci, C., Fabbri, R., Betti, M., et al., Seasonal changes in functional parameters of the hemolymph of Mytilus galloprovincialis, Invertebr. Survival J., 2009, vol. 6, pp. 44–48.
Lacroix, C., Duvieilbourg, E., Guillou, N., et al., Seasonal monitoring of blue mussel (Mytilus spp.) populations in a harbor area: A focus on responses to environmental factors and chronic contamination, Mar. Environ. Res., 2017, vol. 129, pp. 24–35. https://doi.org/10.1016/j.marenvres.2017.04.008
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (project no. 0657-2020-0004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Grinchenko, A.V., Sokolnikova, Y.N., Ilyaskina, D.V. et al. Seasonal Changes in Hemolymph Parameters of the Bivalve Modiolus kurilensis Bernard, 1983 from Vostok Bay, Sea of Japan. Russ J Mar Biol 47, 300–311 (2021). https://doi.org/10.1134/S1063074021040052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063074021040052