Abstract
New data on the effect of additional lighting on the lipid profile of Atlantic salmon 0+ fingerlings during growth and development under artificial cultivation in fish farming conditions were obtained. The influence of different lighting modes, such as standard farming lighting conditions (control), experimental modes of 16 h of light and 8 h of dark (16 : 8), and constant lighting (24 : 0), were studied. Specific modifications of the lipid profile—mainly in the content of structural lipids (phospholipids) as well as indexes of the ratios of structural and reserve lipids: the cholesterol/phospholipids (Chol/PL) and triacylglycerols/phospholipids (TAG/PL)—were found in the fingerlings whose development took place under the conditions of experimental photoperiod regimes. These changes were more pronounced in young salmons cultivated under round-the-clock lighting (24 : 0).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Light, as an abiotic factor, affects various aspects of fish life. Each species of fish is characterized by an optimal regime of lighting, which improves the physiological state of the body and accumulation of body mass. Because of this, regulated light modes that affect the growth and development of young fish are often used in artificial cultivation of young salmon and other fish species (Villarreal et al., 1988; Mäkinen and Ruohonen, 1992; Boeuf and Le Bail, 1999; Taylor et al., 2006; Björnsson et al., 2011). In studies on juvenile fish (trout, silver carp, carp, pike) grown under variable lighting conditions (artificial and natural light), the difference in higher growth rate and maximum survival is shown (Vlasov et al., 2013). For example, in cyprinids, the efficiency of food conversion increases with 24-h lighting (Ruchin, 2012); in sturgeon muscles under variable conditions (12-h light change), the content of lipids and proteins increases, while their water content decreases (Ruchin, 2007). It is shown that increasing the lighting regime from 8 to 16 h stimulates the growth and development of young salmon fish and increases the efficiency of food consumption and its conversion (Clarke, 1981; Brett, 1983). The previous life conditions of young salmon (0+ fingerlings), associated with changes in temperature and the duration of the lighting period, may further affect the timing of smoltification of 2-year-old salmon (1+) (Metcalfe and Thorpe, 1990), which is important for their farming since the release of young fish into the natural environment to maintain the natural population of Atlantic salmon occurs at this age.
It is known that lipids largely provide adaptive abilities of the organism in response to specific conditions of the artificial environment, including photoperiod, temperature, aeration of the environment, etc. (Murzina et al., 2009; Nemova et al., 2020) and, thus, can be considered as indicators of growth and development of viable juvenile salmons. In this paper, we evaluated the effect of different lighting modes (standard farming lighting as control and experimental modes: 16 h of light and 8 h of dark (prolonged lighting), 24 h of constant lighting on the lipid profile and growth of Atlantic salmon fingerlings (0+) under artificial cultivation conditions.
MATERIALS AND METHODS
In July, when the corresponding weight (0.96–1.00 g) was reached, fingerlings (0+) of Atlantic salmon were transferred from the incubation center to the growth pools of the Vygsky Fish Factory (Republic of Karelia). After a week of adaptation, an experiment on the effect of different photoperiod modes (standard farming conditions as control, modes 16 : 8 and 24 : 0) on the lipid profile of salmon fingerlings was conducted in early August. Later, samples of young salmon for biochemical analysis of lipids were taken twice: in September and October. Young salmon were kept in 2 × 2 m pools. Two pools were determined for each of the experimental lighting study modes as well as for the standard farming mode (control). An equal number of fish were taken from each pool with the appropriate lighting mode to form a mixed sample (n = 20). Thus, three groups of fish were studied: control group (the standard farming lighting mode), experimental groups with 16 : 8 (16 h of light and 8 h of dark), and 24 : 0 (24 h of constant light). The description of lighting modes was previously presented in the work of Nemova et al. (2020), in which data on fatty acids and body mass in juvenile salmons in the photoperiod experiment were discussed. The scheme of the experiment is represented in Fig. 1.
The experimental pools were equipped with two LED lights (Aquael leddy smart led sunny, 6 W, 6500 K), while to create dark, the pools were covered with a black, nontransparent film. Mode switching was performed automatically with the help of timer sockets (Feron TM-50). All other conditions of maintenance for all pools were identical: planting density, feeding regime and nutrition (according to the needs of the age group), preventive actions, and care. We used commercial BioMar Inicio plus feed (Biomar, Danmark) (starter feed for 0+ fingerlings) for young salmon grown in the factory.
The number of fingerlings contained in the pools was: 7541 in control, 7361 in the 16 : 8 mode, and 7400 the 24 : 0 mode. The water supply to the pools was from Matkozhnensky reservoir (Nizhny Vyg River). The temperature regime was natural. The temperature values for the study period were 18.2–13.8°C in August, 13.8–9.8°C in September, and 9.8–2.4°C in October. The experiment was conducted until the end of October. Fish samples (fish carcasses) for lipid analysis were taken at the end of each month.
The fish weighing was carried out every month (3–5 times a month) at the fish farm. Chipping of fingerlings individually was not carried out due to their small size, so data on the mass of fish were obtained from the results of repeated weighing of 70–100 individuals together. The average weight of young salmons (0+) at the beginning of the study in different pools was: 0.96 ± 0.07 in the control, 1.00 ± 0.04 in the 16 : 8 mode, and 0.96 ± 0.03 in the 24 : 0 mode. During the entire period of the experiment, 465 samples of fingerlings were collected. In addition, it should be noted that the “waste” in the pools with experimental lighting was lower compared to the control (139 animals or 1.84%); it was 45 animals (0.61%) in the 16 : 8 mode and 72 (0.97%) in the 24 : 0 mode.
Lipid extraction from samples (flesh) of juvenile salmons was performed using the Folch method (Folch et al., 1957) and Kates method (Kates, 1975). The lipids were then concentrated using a Heidolph Hei-Vap rotary vacuum evaporator (Germany). The extracted total lipids and fat-free residue, including proteins, carbohydrates, nucleic acids, amino acids and microelements were dried to a constant mass.
Qualitative and quantitative determination of individual lipid classes was carried out using the method of high-performance thin-layer chromatography (HPLC). The fractionation of total lipids was performed on glass-based plates, HPTLC Silicagel 60 F254 Premium Purity (Merck, Germany). The application of the lipid extract was carried out using a semiautomatic applicator Linomat 5 (CAMAG, Switzerland) with a 100 µL microinjector by the streak technique. The solvent system hexane-diethyl ether-acetic acid (32 : 8 : 0.8 by volume) was used as eluent as well as a solution for saturation of the ADC2 chromatographic chamber (CAMAG, Switzerland) (Olsen and Henderson, 1989). Saturation of the chromatographic chamber was carried out for 20 min with simultaneous humidity control (10 min), after which the plate was saturated (20 min). The distance of the mobile phase was 80 mm (Rf final = 80 mm), and the plate was dried for 5 min. The appearance of lipid spots was carried out in a solution of copper sulfate (CuSO4) with orthophosphoric acid (H3PO4) and by heating the plate to 160°C for 15 min (Hellwig, 2008). Qualitative and quantitative determination of lipid components was performed in the chamber of the TLC Scanner 4 densitometer (CAMAG, Switzerland) on a deuterium lamp at a wavelength of 350 nm in the adsorption mode (Hellwig, 2008). Identification of lipid classes was carried out according to the reference standards of the corresponding components (Sigma-Aldrich, United States), taking into account the compliance of Rf values. Total lipids were analyzed as total phospholipids (PL), triacylglycerols (TAG), diacylglycerol (DAG), cholesterol (Chol), cholesterol esters (Chol esters), and non-esterified fatty acids (NEFA).
The composition of individual phospholipids: phosphatidylcholine (PC), phosphatidylethanolamine (PEA), phosphatidylserine (PS), phosphatidylinositol (PI), sphingomyelin (SM) and lysophosphatidylcholine (LPC) was analyzed by high-performance liquid chromatography on a Nucleosil 100-7 steel column (Elsico, Moscow) using acetonitrile–hexane-methanol-orthophosphoric acid (918 : 30 : 30 : 17.5) eluent. Detection was performed by the degree of light absorption at 206 nm (Arduini et al., 1996). The ratio between the components was estimated by the values of the peak areas on the chromatograms.
Data in Tables 1 and 2 are represented as M ± SE (arithmetic mean error). The results were processed using the nonparametric Wilcoxon–Mann–Whitney Rank Sum Test method in the open R software environment (Korosov and Gorbach, 2007). The differences are considered significant at p ≤ 0.05.
The research was carried out on the basis of the laboratory of ecological biochemistry and using the equipment of the Equipment Sharing Facility of the Karelian Research Center (Russian Academy of Sciences).
RESULTS
The results of studies on the effect of different photoperiod modes on the lipid profile of salmon fingerlings during its artificial cultivation are presented in Tables 1 and 2.
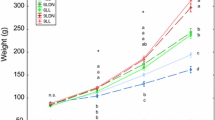
The lipid profile did not significantly differ in the content of total lipids (within 26.59–27.13% of dry weight), including total PL (3.69–4.02%) and DAG (1.22–1.24%) in fingerlings from three pools with different lighting conditions, including the control one, picked in September. Statistically significant decrease of Chol, minor PI and PS in the 16 : 8 and 24 : 0 modes was observed as were and the reduction of LPC and Chol esters in the 24 : 0 mode and also decrease in Chol/PL index in the 16 : 8 mode. Moreover, the content of TAG and the TAG/PL index increased in fingerlings in the case of the 16 : 8 lighting mode, and the content of SM and NEFA increased in the case of the 24 : 0 mode compared to the control. The greatest changes in the lipid profile of fingerlings in September were found at the 24 : 0 mode. The decrease at the different degrees of certain lipid classes (PI, PS, LPC, Chol esters) in juvenal fish positively correlated with an increase of NEFA. At the same time, salmon fingerlings in September showed an increase in size and weight characteristics at the 24 : 0 mode: 2.62 g and 6.18 cm vs. 2.51 g and 6.09 cm in the 16 : 8 mode and 2.47 g and 6.12 cm in the control.
In the fingerlings selected in October, in general, under all modes, including control, an increase in the content of TL (within 32.72–34.23% of dry weight), including the dominant reserve TAG (18.26–19.77%), as well as PL and Chol, compared to September was noted. The main changes in the lipid spectrum under the influence of two photoperiod modes (relative to the control) were observed in fingerlings adapted to the 24 : 0 mode and were associated with a decrease in the content of structural PL (including PC, LPC), DAG, and Chol esters and an increase in NEFA, Chol/PL, and TAG/Phl indexes. At the same time, a significant increase in the content of Chol and NEFA and a decrease in Chol esters was found in fish at the 16 : 8 mode relative to the control. In fingerlings whose growth and development were at the condition of 24 : 0 photoperiod mode, there was a tendency to increase in weight: 3.74 g vs. 3.50 g at the 24 : 0 mode and vs. 3.68 g at control mode.
DISCUSSION
Lipids are not only structural components of any organisms and a source of energy but also perform signaling functions, modulating the activity of cellular enzymes. Therefore, modifications of the lipid environment of organisms caused by the influence of various factors affect several physiological processes, including growth and development (Hochachka and Somero, 2002; Arts and Kohler, 2009). The results of studies on the influence of lightning modes on the lipid status of Atlantic salmon fingerlings indicate that, in fingerlings in September, with the background of no changes in the content of TL, total PL, and DAG, a decrease in Chol and its reserve form Chol esters, as well as minor PL (PI, PS, LPC) was observed, which positively correlates with an increase (1.2 times) in NEFA. These changes are more pronounced in fingerlings kept in pools with a 24 : 0 photoperiod. The established modifications indicate more active metabolic processes in fish organisms in conditions of 24-h lighting. When physiological processes are activated in fish, fatty acids are rapidly mobilized from lipids, forming a pool of NEFA, providing them with an influx of energy (Sautin, 1989). Increased oxidative reactions in individual lipids could well lead to an increase in SM (two fold), which contributes to the rigidity of biomembranes and can be considered as a compensatory reaction to prolonged lightening.
The combination of changes in individual classes of Phl, especially minor ones, which are known to mediate many signaling mechanisms of regulation of the most important metabolic processes, is apparently aimed at ensuring the adaptation of salmon fingerlings in response to the impact of such a powerful physical factor as continuous lighting. With an increase in the duration of daily light, young fish most effectively metabolize food and are characterized by higher viability and growth rate (Vlasov et al., 2013). In our experiment, salmon fingerlings in September at the 24 : 0 mode had a relatively better weight and length: 2.62 g and 6.18 cm vs. 2.51 g and 6.09 cm in the 16 : 8 mode and 2.47 g and 6.12 cm in the control.
It should be mentioned that in all studied photoperiod regimes, including the control, an increase in TL in October compared to September, was noted due to reserve TAG and structural lipids: Chol and PL, including PS, PEA, PC, and SM, which indicates the effect of not only different photoperiod modes but, possibly, also of the temperature factor. In October, the temperature decreased by 4°C (to 9.8°–2.4°C) compared to September (13.8°–9.8°C). The adaptation of fingerlings to the experimental lighting modes at the lipid level can be realized by different biochemical mechanisms. On the one hand, under experimental lighting conditions, the intensity of lipolysis associated with the mobilization of energy components of lipids increases in fingerlings, which is indicated by a decrease in the content of Chol esters, DAG, and PL, including PC and LPC, and an increase in NEFA as well as of Chol/PL, TAG/PL indexes (in the 24 : 0 mode). It should be noted that the decrease in DAG in October in 0+ fingerlings at the 24 : 0 mode may be due to the complex effect of the duration of lighting and the temperature decrease. Diacylglycerols are multifunctional substances, precursors not only of TAG but also of many PL, and the direction of their metabolic pathways is regulated by both internal and external environmental factors (Sautin, 1989). In this case, it is necessary to take into account not only the content of Chol and PL but also their ratio Chol/PL. Thus, in October, under the studied lighting modes, the index of the ratio of membrane lipids Chol/PL was increased in fingerlings (up to 0.97 in the 24 : 0 mode and up to 0.85 in the 16 : 8 mode vs. 0.79 in the control) due to a decrease in the content of Phl (mainly PC and also LPC). The change in the Chol/PL ratio is one of the key mechanisms for regulating the physicochemical state of biomembranes and their ion permeability, which affects various metabolic processes, such as the rate of ion transport, metabolites, and water (Netyukhailo and Tarasenko, 2001; Perevozchikov, 2008). The result can be an inhibition of oxidation of both Chol and individual minor classes of PL (PI, PS, PEA, and SM). All the observed changes in the lipid profile of salmon fingerlings during development under experimental conditions were more pronounced in October with the 24 : 0 mode. Also, by this period, both in the control and in both lighting modes, the content of spare TAG increases in fingerlings, and the TAG/PL index increases at 24 : 0, which indicates an increase in the energy potential of fish, which is maintained by more efficient feed conversion in the condition of additional lighting, and can also be associated with the temperature factor. It is possible that the decrease in water temperature in October affected the increase in lipase activity in the gastrointestinal tract of fish, which contributed to the best food conversion. Thus, the results of the work of Chinese scientists showed that young Amur sturgeon digests lipids well at low temperature (14°C), and proteins at 21 and 28°C (Hong-jie et al., 2007).
The observed changes in the content of the main lipid classes positively correlate with the tendency to increase the growth rate of fingerlings in the 24 : 0 lighting mode. It should be noted that the data are consistent with the previously published (Nemova et al., 2020) results of the study of the fatty acid composition of young salmon with varying light modes in a similar experiment.
CONCLUSIONS
Additional lighting during the farming of Atlantic salmon juveniles at the stage of fingerlings leads to quantitative and qualitative modifications of structural and reserve lipids and their ratios (indexes), aimed at regulating the vital functions of salmon fingerlings by such compensatory changes in lipids that provide optimal adaptive opportunities for their survival. Variation of light modes when growing salmon juveniles in the conditions of Northern fish breeding farms can affect the production of more resilient, ready-to-release juveniles into the natural environment due to the acceleration of their growth processes. Additional lighting can be used to accelerate the growth of young salmons, the onset of the period of smoltification, and to delay maturation. In addition, the results obtained on the modification of the lipid composition of fingerlings in additional lighting in the development process can be used in the practice of growing young salmons in farming conditions when calculating feed coefficients and the amount of food and to characterize their physiological state.
REFERENCES
Arduini, A., Peschechera, A., Dottori, S., et al., High-performance liquid chromatography of long-chain acylcarnitine and phospholipids in fatty acid turnover studies, J. Lipid Res., 1996, vol. 37, no. 2, pp. 684–689.
Arts, M.T. and Kohler, C.C., Health and conditions in fish: the influence of lipids on membrane competency and immune response, in Lipids in Aquatic Ecosystems, Dordrecht: Springer, 2009, pp. 237–257.
Björnsson, B.T., Stefansson, S.O., and McCormick, S.D., Environmental endocrinology of salmon smoltification, Gen. Comp. Endocrinol., 2011, vol. 170, pp. 290–298.
Boeuf, G. and Le Bail, P.-Y., Does light have an influence on fish growth?, Aquaculture, 1999, vol. 177, no. 3, pp. 129–152.
Brett, D., Environmental factors and fish growth, in Bioenergetika i rost ryb (Bioenergetics and Fish Growth), Khoar, U., Rendoll, D., and Brett, J., Eds., Moscow: Legkaya Pishchevaya Prom-st’, 1983, pp. 275–345.
Clarke, W.C., Sheinbourn, J.E., and Brett, J.N., Effect of artificial photoperiod cycles, temperature and salinity on growth and smolting in underyearlings coho. Chinook, Aquaculture, 1981, pp. 105–116.
Folch, J., Lees, M., and Sloan-Stanley, G.H., A simple method for the isolation and purification of total lipids animal tissue (for brain, liver and muscle), J. Biol. Chem., 1957, vol. 226, pp. 497–509.
Hellwig, J., Definig parameters for a reproducible TLC-separation of phospholipids using ADC 2, Diploma Thesis, University of Applied Sciences Northwestern Switzerland (FHNW), 2005.
Hochachka, P.W. and Somero, G.N., Biochemical Adaptation: Mechanism and Process Physiological Evolution, New York: Oxford Univ. Press, 2002.
Hong-jie, T., Ping, Z., Long-zhen, Z., et al., The influence of water temperature on the activity of digestive enzymes of juvenile Amur sturgeon, Acipenser schrenckii, J. Fish. Sei. China, 2007, vol. 14, no. 1, рр. 126–131.
Keits, M., Techniques of Lipidology: Isolation, Analysis, and Identification of Lipids, Amsterdam: Elsevier, 1972.
Korosov, A.V. and Gorbach, V.V., Komp’yuternaya obrabotka biologicheskikh dannykh (Computer Processing of Biological Data), Petrozavodsk: Petrozav. Gos. Univ., 2007.
Mäkinen, T. and Ruohonen, K., Effect of delayed photoperiod on the growth of a Finnish rainbow trout (Oncorhynchus mykiss Walbaum) stock, J. Appl. Ichthyol., 1992, vol. 8, pp. 40–50.
Metcalfe, N.B. and Thorpe, J.E., Determinants of geographical variation in the age of seaward-migrating salmon, Salmo salar, J. Anim. Ecol., 1990, vol. 59, pp. 135–145.
Murzina, S.A., Nefedova, Z.A., Ruokolainen, T.R., et al., Dynamics of lipid content during early development of freshwater salmon Salmo salar L., Russ. J. Dev. Biol., 2009, vol. 40, pp. 165–170. https://doi.org/10.1134/S1062360409030059
Nemova, N.N., Nefedova, Z.A., Pekkoeva, S.N., et al., The effect of the photoperiod on the fatty acid profile and weight in hatchery-reared underyearlings and yearlings of Atlantic salmon Salmo salar L., Biomolecules, 2020, vol. 10, no. 6, p. 845. https://doi.org/10.3390/biom10060845
Netyukhailo, L.G. and Tarasenko, L.M., Features of the lipid composition of the plasma membranes of lung tissues in acute emotional and pain stress in rats, Ukr. Biokhim. Zh., 2001, vol. 73, no. 1, pp. 115–117.
Olsen, R.E. and Henderson, R.J., The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry, J. Exp. Mar. Biol. Ecol., 1989, vol. 129, pp. 189–197.
Perevozchikov, A.P., Sterols and their transport in animal development, Russ. J. Dev. Biol., 2008, vol. 39, pp. 131–150. https://doi.org/10.1134/S1062360408030016
Ruchin, B.A., Effect of photoperiod on growth, physiological, and hematological indices of juvenile Siberian sturgeon Acipenser baerii, Biol. Bull. (Moscow), 2007, vol. 34, no. 6, pp. 583–589.
Ruchin, B.A., Influence of the photoperiod on the energy indices of cyprinids, Astrakhan. Vestn. Ekol. Obraz., 2012, no. 4(22), рр. 144–150.
Sautin, Yu.Yu., The problem of regulation of adaptive changes in lipogenesis, lipolysis, and lipid transport in fishes, Usp. Sovrem. Biol., 1989, vol. 107, no. 1, pp. 131–147.
Taylor, J.F., North, B.P., Porter, M.J.R., et al., Photoperiod can be used to enhance growth and improve feeding efficiency in farmed rainbow trout, Oncorhynchus mykiss, Aquaculture, 2006, vol. 256, pp. 216–234.
Villarreal, C.A., Thorpe, J.E., and Miles, M.S., Influence of photoperiod on growth changes in juvenile Atlantic salmon, Salmo salar L., J. Fish. Biol., 1988, vol. 33, pp. 15–30.
Vlasov, V.A., Maslova, N.I., Ponomarev, S.V., and Bokaneva, Yu.M., Influence of light on the growth and development of fishes, Vestn. Astrakhan. Gos. Techn. Univ., Ser. Rybn. Khoz., 2013, no. 2, pp. 24–34.
Funding
The study was financially supported by Russian Science Foundation, project no. 19-14-00081.
Author information
Authors and Affiliations
Contributions
N.N. Nemova, Z.A. Nefedova, S.A. Murzina: discussion of the research results, preparation of the publication. S.N. Pekkoeva: analysis of lipids and fatty acids in the tissues of the studied juvenile fish, participation in the preparation of the publication. V.P. Voronin: collection of the samples during the expedition, analysis of lipids and fatty acids in the tissues of the studied juvenile fish, statistical analysis. T.R. Ruokolainen: analysis of individual classes of phospholipids by high-performance liquid chromatography.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the use of animals in experiments and their care conditions have been met.
Additional information
Translated by A. Ermakov
Rights and permissions
About this article
Cite this article
Nemova, N.N., Nefedova, Z.A., Murzina, S.A. et al. The Effect of the Photoperiod on the Lipid Profile in Hatchery-Reared Atlantic Salmon Salmo salar L. Fingerlings (0+). Russ J Dev Biol 52, 105–111 (2021). https://doi.org/10.1134/S1062360421020053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360421020053