Abstract
The content of endogenous hormones (auxin IAA, cytokinines, ABA) in explants of various types (segments of the leaf, bud, and stem), primary calluses induced from them, and morphogenic and nonmorphogenic calluses at the initial stages of in vitro culture by the immunoassay method was studied for the first time for Lavandula angustifolia Mill. The maximum value of the hormone levels in explants such as segments of a bud was shown. An increase in the content of hormones in primary calluses was revealed in comparison with similar characteristics in all types of explants. The higher level of the active form of cytokinin (trans-zeatin) and ABA, as well as the lower level of the inactive form of cytokinin (zeatin-nucleotide) and auxin IAA, were identified in the morphogenic callus compared with the nonmorphogenic one. It is suggested that the content of endogenous hormones in explants and calluses of L. angustifolia is due to their histological status. A conclusion is made about the unified histophysiological mechanisms of callusogenesis and morphogenesis in vitro in the studied plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The callus in vitro is defined as integrated tissue system resulting from disorganized proliferation of dedifferentiated explant cells (Ikeuchi et al., 2022). As a rule, morphogenic calli are distinguished, the competent cells of which under optimal culture conditions in vitro are capable of further morphogenesis along various pathways, and nonmorphogenic calluses that are not capable of such processes.
Of particular interest are morphogenic calluses such as experimental model systems for studying the patterns and features of morphogenesis in intact plants (Feher, 2023; Kruglova et al., 2023). In addition, based on the use of morphogenic calluses, biotechnologies have been developed for obtaining full-fledged regenerants of valuable crops (generalization, Efferth, 2019).
Numerous experimental data indicate that callus formation and morphogenesis in vitro in them are determined by a complex of interrelated factors with the dominant role of exogenous hormones added to the culture medium. The development of this problem using the example of auxins and cytokinins began as early as in the 1950s (Skoog and Miller, 1957). To date, the leading role of exogenous polyfunctional auxins and cytokinins in the induction of callus formation and the implementation of various morphogenetic programs for the development of callus cells in vitro has been confirmed in many works carried out on the example of plants from various families (Raspor et al., 2021). A number of works show the fundamental importance of an optimal balance between the concentration of exogenous auxins and cytokinins and their endogenous content in explants (when inducing the formation of calluses) and calluses (in the processes of morphogenesis in vitro) (Kruglova, 2022). In recent years, researchers have paid significant attention to abscisic acid (ABA), a hormone also characterized by its effects on various aspects of morphogenesis in calluses in vitro (Kruglova et al., 2018; Bidabadi and Jain, 2020). In general, auxins, cytokinins, and ABA should be considered the leading hormones of callusogenesis and morphogenesis in vitro.
All the issues discussed above are of great theoretical and practical interest. At the same time, studies devoted to identifying the content of endogenous auxins, cytokinins, and ABA in explants and calluses are relatively few (publications in recent years: Hisano et al., 2016; Seldimirova et al., 2019; Mostafa et al., 2020), and a comparative analysis of the levels of these hormones in the initial explants and calluses of various types formed from them is absent in the available literature.
The focus of this study was lavender Lavandula angustifolia Mill., an essential oil plant, widely used in pharmacology, perfumery, and cosmetics (Salehi et al., 2018). For this valuable plant, a number of biotechnologies have been developed for obtaining regenerants through the stage of callus formation with subsequent induction of morphogenesis in vitro in them (Leelavathi et al., 2020; Yegorova, 2021). We have begun research on the content of a number of endogenous hormones in calluses of L. angustifolia variety Vdala (Yegorova et al., 2020). Thus, it was revealed that morphogenic calluses of passage 4 obtained from leaf explants, in comparison with nonmorphogenic calluses of the same passage, were characterized by a higher content of cytokinins and a lower content of the auxin indole-3-acetic acid (IAA) and ABA. At the same time, it is necessary to conduct further research in this direction, involving other biotechnologically promising varieties of L. angustifolia. Data on the endogenous content of these hormones in calluses at the very initial stages of culture in vitro may be especially important, because it can largely determine the methods for regulating the morphogenetic potential of callus cells in a specific cell technology.
In this regard, the purpose of this study was a comparative analysis of the content of endogenous hormones (auxin IAA, cytokinins, ABA) in various explants of L. angustifolia, in the primary calluses obtained from them, and in morphogenic and nonmorphogenic calluses at the initial stages of culture in vitro.
MATERIALS AND METHODS
The material for this study was plants of lavender. Lavandula angustifolia Mill. variety Stepnaya grown in closed ground conditions at a temperature of 22–24°C, an illumination of 3–5 klx, and a 16-hour photoperiod.
Culture methods were used for the in vitro plant organs, both those generally accepted (Kalinin et al., 1980) and those developed by the authors for various cellular technologies of lavender angustifolia (Yegorova, 2021). Segments of leaves, stems, and buds of donor plants were used as explants. Explants were sterilized in 70% ethanol (40 s) and 50% solution of the drug Bradofen 10N (FLORIN JSC, Hungary) (12 min) and washed three times with autoclaved distilled water. Aseptic work was carried out in a BAVnp-01-Laminar-S-1,2 laminar flow hood (Lamsystems, Russia).
To obtain the primary calluses, explants were placed on an agar nutrient medium containing macrosalts, microsalts, and vitamins according to the recipe of Murashige and Skoog (Murashige and Skoog, 1962) with the addition of α-naphthylacetic acid (NAA, Sigma-Aldrich, United States) at a concentration of 1.0 mg/L and 6-benzylaminopurine (BAP, Sigma-Aldrich, United States) at a concentration of 0.5 mg/L (MC160 medium, according to Yegorova, 2021). Cultivation of explants was carried out in test tubes at a temperature of 26 ± 2°C, relative air humidity of 70%, illumination of 2–3 klx, and a 16-hour photoperiod.
After 4–6 weeks of culture, the primary calluses were separated from explants and transferred into test tubes onto agar media of various compositions. Nonmorphogenic calluses were obtained on MC160 medium (the composition of the medium is given above), and morphogenic calluses, on MC594 medium (the medium was prepared according to the recipe of Murashige and Skoog with the addition of BAP at a concentration of 0.5 mg/L and 0.5 mg/L thidiazuron (TDZ, Sigma, United States), by Yegorova, 2021). Callus transplants under the above physical conditions were cultivated for 4–6 weeks to obtain and increase the mass of calluses of the first passage.
The quantitative content of endogenous hormones (IAA, cytokinins, ABA) was determined by enzyme immunoassay (ELISA) in explants before their introduction into an aseptic culture in vitro, in the primary calluses after their separation from explants, and in the morphogenic and nonmorphogenic calluses of the first passage at the end of the growing cycle (30–35 days). The weights of plant material were combined so that the total mass of explants of each type was 2–3 g, and that of calluses of each type was 5–6 g.
The samples were placed in penicillin vials and frozen in a Thermo Scientific Forma 900 low-temperature freezer (Thermo Scientific, United States) at ‒80°C. Next, the vials were transferred to the chamber of a FreeZone 2.5 L lyophilizer (Labconco, United States) and freeze-dried at –50°C and a vacuum level of 0.1 mBar for 3 days. After freeze-drying, the vials with the material were stored at 4°C in a pharmaceutical refrigerator HYC-580 (Haier, United States). Next, the lyophilized material was homogenized, extracted with 70% ethanol, and incubated in a pharmaceutical refrigerator HYC-580 (Haier, United States) at 4°C for 12 h. The homogenizate was filtered using paper filters to separate the liquid phase and further evaporated the filtrate to an aqueous residue.
Extraction of IAA and ABA from an aliquot of the aqueous residue was carried out according to a modified scheme with a decrease in volume (Vysotskaya et al., 2008). Extraction was performed with diethyl ether and methylated with diazomethane. The resulting ether phase was evaporated to a dry residue, which was then reduced with 80% ethanol; an aliquot of it in a series of dilutions was added to the wells of the plates and ELISA was performed in test systems using antibodies specific to IAA and ABA.
The content of cytokinins was determined according to (Kudoyarova et al., 2014). To do this, cytokinins from the aqueous residue were concentrated on a C18 cartridge (Sep-Pak Classic C18) and eluted with ethanol, then evaporated in a rotary evaporator. The dry residue was reconstituted in 80% ethanol and applied to thin-layer chromatography silica gel plates to separate the cytokinin metabolites. Various forms of cytokinins (trans-zeatin, zeatin riboside, and zeatin nucleotide) were eluted from the corresponding zones with the phosphate buffer, then an aliquot in a series of dilutions was added to the wells of the plates and ELISA was performed in test systems using antibodies specific to zeatin.
Each experimental variant was analyzed in 3–5 repetitions. Statistical processing of the results was carried out using Microsoft Office Excel 2010. The figures show the arithmetic means and the errors of the means. The significance of differences was determined using Student’s t-test.
RESULTS
The explants (Fig. 1a) were segments of leaves, stems, and buds of L. angustifolia plants. Figure 2 presents the results of identifying the content of IAA, ABA, and cytokinins in various explants after their separation from donor plants before inoculation onto a nutrient medium. The highest detected levels of IAA (414.92 ng/g) and ABA (21.38 ng/g) were observed in the bud segments, while in the leaf/stem segments, these indicators are lower: IAA 354.81/185.04 ng/g and ABA 15.28/14.55 ng/g, respectively. As for cytokinins, the level of trans-zeatin in the bud segments (72.60 ng/g) significantly exceeds the same indicators in the leaf/stem segments (4.77/20.10 ng/g, respectively), while the levels of other forms of cytokinins are approximately the same, with a slight increase in indicators in stem segments. Thus, the values of the zeatin riboside/zeatin nucleotide were 27.30/49.41 ng/g in stem segments, 16.39/20.34 ng/g in leaf segments, and 37.89/41.65 ng/g in the buds, respectively.
The primary calluses of L. angustifolia formed on the surface of various explants 2–3 weeks after the introduction of the in vitro culture to the nutrient medium. According to the morphological parameters, primary calluses were loose, undifferentiated formations with many invaginations, as shown in the example of such calluses obtained from leaf segments (Fig. 1b). The results of identifying the content of endogenous IAA, ABA, and cytokinins in the primary calluses are presented in Fig. 3. Their analysis indicates approximately equal levels of IAA (2806.50, 2563.60, and 3189.54 ng/g) and ABA (82.33, 98.52, and 88.99 ng/g) in calluses obtained from various explants (leaf, bud, and stem, respectively). At the same time, a significant increase in the zeatin nucleotide cytokinin index was detected in calluses obtained from stem segments (377.15 ng/g), in comparison with similar indicators in calluses obtained from bud segments (142.86 ng/g) and especially leaf segments (62.28 ng/g).
After 4–6 weeks, the primary calluses were separated from explants and transferred to fresh MC160 medium to obtain nonmorphogenic calluses. Calluses proliferating under such conditions were cultured for 5–6 weeks until the stationary growth phase (first passage). According to the morphological data, nonmorphogenic calluses of the first passage, like primary calluses, were characterized by a loose structure and the presence of many invaginations on their surface (Fig. 1c). To obtain morphogenic calluses, the primary calluses were transferred to MC594 medium and cultivated for 5–6 weeks to obtain and increase the mass of calluses (first passage). According to the morphological data, morphogenic calluses of the first passage were characterized by the presence of buds (Fig. 1d) and leaves on their surface. It should be noted that, under the conditions of the experiments, morphogenic calluses of the first passage were obtained from primary calluses induced from the leaf and bud, but not the stem.
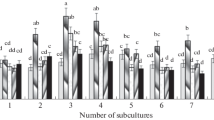
The content of endogenous IAA, ABA, and cytokinins in nonmorphogenic and morphogenic calluses of the first passage is shown in Fig. 4. As the analysis shows, in morphogenic calluses, a lower content of IAA was revealed compared to that in nonmorphogenic ones, respectively, 3321.32 and 4294.57 ng/g when using leaf segments and 2833.06 and 4084.17 ng/g when using bud segments. At the same time, the ABA levels are higher in morphogenic calluses compared to nonmorphogenic calluses for all explants used: 149.6 ng/g versus 132.47 ng/g when using leaf segments and 211.45 ng/g versus 175.89 ng/g when using bud segments.
Content of endogenous hormones in morphogenic and nonmorphogenic calli of the first passage of L. angustifolia, obtained from various explants: (a) IAA; (b) ABA; and, (c) cytokinins. Note: there are no data on the content of endogenous hormones in morphogenic calli of the first passage obtained from the stem; see the text for explanation.
In morphogenic calluses obtained from leaf segments, in comparison with nonmorphogenic ones of similar origin, higher levels of all forms of the cytokinin studied were identified. Thus, the indicator of zeatin-nucleotide in morphogenic calluses is almost two times higher than that of nonmorphogenic calluses (235.16 and 116.84 ng/g, respectively). The indicator of trans-zeatin is almost four times higher (178.30 and 45.03 ng/g), and the indicator of zeatin-riboside is almost fivefold higher (170.44 and 35.92 ng/g, respectively). This trend continues in morphogenic calluses obtained from bud segments: the levels of trans-zeatin (87.86 ng/g) and zeatin-riboside (110.40 ng/g) are higher than those of nonmorphogenic calluses (46.52 and 57.56 ng/g, respectively). However, in such morphogenic calluses, a lower level of the zeatin-nucleotide (119.15 ng/g) was observed in comparison with the nonmorphogenic ones (144.05 ng/g).
DISCUSSION
Various parts of donor plants are used as explants to obtain calluses. Numerous experimental data indicate that the highest “yield” of primary calluses is achieved, as a rule, when introduced into the organs in the in vitro culture in the early stages of development (Zinatullina, 2021; Kruglova et al., 2021; Wang et al., 2021; Kharel et al., 2022). This empirical observation can be explained by the histological status of such explants—the presence in them of meristematic or still weakly specialized cells capable of transitioning to a state of dedifferentiation, successive divisions with proliferation of dedifferentiated cells, i.e., callus formation (Feher, 2019; Kruglova, 2022). Callus formation is associated with structural restructuring of the initial cells of explants with the participation of a number of genes (YUC1, YUC4, WOX5, WOX11, etc.) (Ikeuchi et al., 2019; Feher, 2023). A major role in this process is played by the hormonal status of explants in the early stages of development and especially by the presence of the most important hormones of morphogenesis: auxins, cytokinins, and ABA (Bidabadi and Jain, 2020).
Analysis of the content of endogenous hormones in explants of L. angustifolia confirms these observations. Thus, the maximum identified level of IAA, ABA, and especially cytokinin trans-zeatin was noted in the bud segments, which are actively developing structures, while in the segments of specialized structures like leaves and stems, a reduced content of these hormones is shown (Fig. 2). These results are consistent with our findings that in L. angustifolia it was the buds that were characterized by the highest frequency of formation of primary calluses compared to leaves and stems, as well as on a wider range of nutrient media (unpublished data).
Of course, it would be interesting to study the content of endogenous hormones in those organs of intact plants of L. angustifolia, segments of which served as explants during the experiments, and compare the indicators obtained with similar indicators of explants. However, this kind of research turned out to be impossible due to the lack of available techniques. At the same time, based on general approaches, it can be assumed that the content of, for example, ABA in the studied explants is increased in comparison with the organs of intact plants. Indeed, ABA is defined as the “stress hormone” (Fidler et al., 2022), and separation of an organ from a donor plant is regarded as a kind of wound stress (Feher, 2023; Kruglova et al., 2023). In general, the influence of hormonal signals in explants on stress induction of callus formation in vitro is of great interest to researchers, largely due to wound regeneration of organs of intact plants, which at the initial stages also consists of callus formation (Ikeuchi et al., 2022).
Increased ABA levels in explants of L. angustifolia may also be due to the presence of significant amounts of secondary metabolites in intact plants of this object (Salehi et al., 2018; Yegorova, 2021). Thus, the work of Dong et al. (Dong et al., 2022) shows the influence of the transcription factor LaMYC4, which is responsible for the biosynthesis of terpenoids in L. angustifolia to increase the ABA content in transgenic terpenoid-overexpressing tobacco plants. In general, the influence of various secondary metabolites in explants on the processes of callusogenesis in vitro has been demonstrated for many plants (Ozyigit et al., 2023).
Comparison of the levels of endogenous hormones in all types of explants (Fig. 2) and the primary calluses obtained from them (Fig. 3) indicates an increase in the absolute value of all indicators in the calluses. Such results, in our opinion, are due to an increase in the mass of the primary calluses during a fairly long (4–6 weeks) culture in vitro, which is accompanied by their significant proliferation, and therefore, histological changes. A particularly significant increase was noted in the content of cytokinin zeatin-nucleotide: the level of this hormone in the primary calluses obtained from stems (Fig. 3c) exceeded the same indicator in the original explants (Fig. 2c) by more than 70 times and far exceeded similar indicators in calluses obtained from leaves and buds (Fig. 3c). This work, carried out using transcriptome analysis and quantitative hormonal analysis, showed that, in intact plants of Arabidopsis thaliana, wounding of the stem, inducing callus formation, changes the expression of genes involved in the biosynthesis of cytokinins, which leads to increased accumulation of these hormones (Ikeuchi et al., 2017). It is possible that in the case of the primary calluses of L. angustifolia the same mechanism works.
As a result of the experiments, two contrasting types of calluses of the first passage were obtained from primary calluses—nonmorphogenic and morphogenic, differing in their morphological parameters (Figs. 1c, 1d). It is important to emphasize that contrasting types of calluses from passage 1 of L. angustifolia also differ in the histological parameters, as we established earlier (Kruglova et al., 2024). Thus, nonmorphogenic calluses are represented by parenchymal tissue, while in morphogenic ones such morphogenesis pathways have been identified in vitro, like organogenesis de novo (leaf buds at different stages of gemmo/caulogenesis) and somatic embryogenesis in vitro (somatic embryos in the early stages of embryogenesis). In addition, in the thickness of morphogenic calluses, numerous morphogenetic foci were noted—groups of undifferentiated meristematic cells that are gradually transformed into meristems of future shoots and organs of somatic embryos. Such morphogenetic foci are regarded as the histological basis of morphogenesis pathways in vitro in calluses (Zinatullina, 2023; Kruglova et al., 2023).
Analysis of the content of endogenous hormones in morphogenic and nonmorphogenic calluses of the first passage of L. angustifolia showed the following.
In morphogenic calluses compared to nonmorphogenic ones, there is a higher level of the active form of cytokinin, trans-zeatin, when using leaf explants. The content of this form of cytokinin in the morphogenic callus was almost four times higher than its content in the nonmorphogenic callus (Fig. 4c). The increased content of this active form of cytokinin can be explained by the morphogenetic activity of morphogenic calluses and by the formation and development in them of multiple morphogenetic foci, somatic embryos, and especially organs, buds. It is endogenous cytokinins that are considered the main candidates for receiving specialized gene signals of postembryonic organogenesis de novo, the primarily bud-specific homeodomain regulator WUSCHEL (Smeringai et al., 2023). At the same time, in nonmorphogenic calluses obtained from bud segments, a higher level of the zeatin-nucleotide was noted in comparison with morphogenic calluses. Since the zeatin-nucleotide is classified as an inactive form of cytokinins, these data once again confirm the nonmorphogenic nature of such calluses.
In morphogenic calluses, a lower content of endogenous auxin IAA was revealed compared to nonmorphogenic ones, when both leaf and bud segments were used as explants (Fig. 4a). This result may be due to the hormonal characteristics of the morphogenesis-pathways identified in morphogenic calluses namely organogenesis de novo and somatic embryogenesis in vitro. It is suggested that, in the process of organogenesis in calluses, endogenous auxins generally play a less important role than endogenous cytokinins (Raspor et al., 2021). Moreover, inhibition biosynthesis and polar transport of endogenous auxin IAA in calluses of Arabidopsis thaliana led to increased organ regeneration de novo (Ohbayashi et al., 2022).
At the same time, it is endogenous auxins are important in the such pathway of morphogenesis in calluses as somatic embryogenesis in vitro (Wojcik et al., 2020). However, the need for endogenous auxins arises mainly in the late stages of development of somatic embryos in vitro, as shown in the example of Arabidopsiss thaliana (Karami et al., 2023). Our results confirm this observation, since the development of somatic embryos of L. angustifolia in morphogenic calluses of the first passage stopped at a fairly early heart-shaped stage (Kruglova et al., 2024), which apparently determined the relatively low level of auxin IAA in them.
The levels of endogenous ABA in morphogenic calluses of the first passage should also be associated with the formation and development of somatic embryos of L. angustifolia. As evidenced by the results obtained, the content of endogenous ABA is higher in morphogenic calluses compared to similar indicators in nonmorphogenic ones, for all explants used (Fig. 4b). Perhaps, in this case, the increase in the ABA content is due to the role of this “stress hormone” in the formation and development of somatic embryos, since in recent years somatic embryogenesis in vitro is considered as a stress response of the explant to wound damage (Spinoso-Castillo and Bello-Bello, 2022).
It should be emphasized that an important direction of modern research is to study the synergistic/antagonistic influence (in the English literature, crosstalk) of endogenous hormones in the regulation of morphogenesis pathways in callus cultures in vitro. It has been established, for example, that in calluses of wheat and barley the ability for somatic embryogenesis in vitro was determined by the balance of the content of endogenous IAA and ABA in them (Seldimirova et al., 2019). In calluses of Fouquieria splendens (Salinas-Patino et al., 2018) and Brassica juncea (Lu et al., 2020), cytokinin influenced the expression of a number of genes in the auxin signaling pathways. In calluses of Arabidopsis thaliana, the early stages of shoot primordia formation depended on auxin signals, while the formation of the shoot apical meristem at later stages of primordia development was regulated by cytokinin, but under the influence of auxin signaling (Cosic and Raspor, 2022).
This kind of research in relation to L. angustifolia remains to be done, since the identified absolute values of the content of the studied endogenous hormones in calluses most likely, do not play as important a role as their mutual influence.
CONCLUSIONS
The results received for the first time for L. angustifolia confirm the data presented in the literature on the active participation of endogenous hormones (auxin IAA, cytokinins, and ABA) both in the induction of callus formation from various types of explants and in callusogenesis at the initial stages of culture in vitro.
A comparative analysis of the levels of the studied endogenous hormones indicates (1) their maximum value in explants such as bud segments; (2) an increase in their content in the primary calluses in comparison with the explants; (3) higher levels of the active form of cytokinin (trans-zeatin) and ABA, as well as lower levels of the inactive form of cytokinin (zeatin-nucleotide) and auxin IAA morphogenic calluses of the first passage in comparison with nonmorphogenic ones of the same passage. In our opinion, the content of the studied hormones in explants, the primary alluses, and the morphogenic and nonmorphogenic calluses of L. angustifolia is directly determined by their histological status. In general, we can talk about common histophysiological mechanisms of the callus and morphogenesis in vitro in the studied plant.
REFERENCES
Bidabadi, S.Sh. and Jain, S.M., Cellular, molecular, and physiological aspects of in vitro plant regeneration, Plants, 2020, vol. 9, no. 6, p. 702. https://doi.org/10.3390/plants9060702
Cosis, T. and Raspor, M., The role of auxin and cytokinin signaling components in de novo shoot organogenesis, in Auxins, Cytokinins and Gibberellins Signaling in Plants. Signaling and Communication in Plants, Aftab, T., Ed., Cham: Springer, 2022. https://doi.org/10.1007/978-3-031-05427-3_3
Dong, Ya., Zhang, W., Li, J., Wang, D., Bai, H., Li, H., and Shi, L., The transcription factor LaMYC4 from lavender regulates volatile terpenoid biosynthesis, BMC Plant Biol., 2022, vol. 22, no. 1, p. 289. https://doi.org/10.1186/s12870-022-03660-3
Efferth, T., Biotechnology applications of plant callus cultures, Engineering, 2019, vol. 5, no. 1, pp. 50–59. https://doi.org/10.1016/j.eng.2018.11.006
Feher, A., Callus, dedifferentiation, totipotency, somatic embryogenesis: what these terms mean in the era of molecular plant biology?, Front. Plant Sci., 2019, vol. 10, p. 536. https://doi.org/10.3389/fpls.2019.00536
Feher, A., A common molecular signature indicates the pre-meristematic state of plant calli, Int. J. Mol. Sci., 2023, vol. 24, no. 17, p. 13122. https://doi.org/10.3390/ijms241713122
Fidler, J., Graska, J., Gietler, M., Nykiel, M., Prabucka, B., Rybarczyk-Płońska, A., Muszyńska, E., Morkunas, I., and Labudda, M., PYR/PYL/RCAR receptors play a vital role in the abscisic-acid-dependent responses of plants to external or internal stimuli, Cells, 2022, vol. 11, no. 8, p. 1352. https://doi.org/10.3390/cells11081352
Hisano, H., Matsuura, T., Mori, I.C., Yamane, M., and Sato, K., Endogenous hormone levels affect the regeneration ability of callus derived from different organs in barley, Plant Physiol. Biochem., 2016, vol. 99, pp. 66–72. https://doi.org/10.1016/j.plaphy.2015.12.005
Ikeuchi, M., Iwase, A., Rymen, B., Lambolez, A., Kojima, M., Takebayashi, Yu., Heyman, J., Watanabe, Sh., Seo, M., De Veylder, L., Sakakibara, H., and Sugimoto, K., Wounding triggers callus formation via dynamic hormonal and transcriptional changes, Plant Physiol., 2017, vol. 175, no. 3, pp. 1158–1174. https://doi.org/10.1104/pp.17.01035
Ikeuchi, M., Favero, D.S., Sakamoto, Yu., Iwase, A., Coleman, D., Rymen, B., and Sugimoto, K., Molecular mechanisms of plant regeneration, Annu. Rev. Plant Biol., 2019, vol. 70, no. 1, pp. 377–406. https://doi.org/10.1146/annurev-arplant-050718-100434
Ikeuchi, M., Iwase, A., Ito, T., Tanaka, H., Favero, D.S., Kawamura, A., Sakamoto, Sh., Wakazaki, M., Tameshige, T., Fujii, H., Hashimoto, N., Suzuki, T., Hotta, K., Toyooka, K., Mitsuda, N., and Sugimoto, K., Wound-inducible WUSCHEL-RELATED HOMEOBOX 13 is required for callus growth and organ reconnection, Plant Physiol., 2022, vol. 188, no. 1, pp. 425–441. https://doi.org/10.1093/plphys/kiab510
Kalinin, F.L., Sarnatskaya, V.V., and Polishchuk, V.E., Metody kul’tury tkanei v fiziologii i biokhimii (Tissue Culture Methods in Physiology and Biochemistry), Kiev: Naukova dumka, 1980.
Karami, O., Philipsen, Ch., Rahimi, A., Nurillah, A.R., Boutilier, K., and Offringa, R., Endogenous auxin maintains embryonic cell identity and promotes somatic embryo development in Arabidopsis, Plant J., 2023, vol. 113, no. 1, pp. 7–22. https://doi.org/10.1111/tpj.16024
Kharel, P., Creech, M.R., Nguyen, Ch.D., Vendrame, W.A., Munoz, P.R., and Huo, H., Effect of explant type, culture medium, and BAP concentration on in vitro shoot development in highbush blueberry (Vaccinium corymbosum L.) cultivars, In Vitro Cell. Dev. Biol., Plant, 2022, vol. 58, no. 6, pp. 1057–1065. https://doi.org/10.1007/s11627-022-10299-0
Kruglova, N.N., Callus formation and callusogenesis in vitro in cereals: the role of hormonal balance, Izv. Ufimsk. Nauch. Tsentra Ross. Akad. Nauk, 2022, no. 1, pp. 52–59. https://doi.org/10.31040/2222-8349-2022-0-1-52-59
Kruglova, N.N., Seldimirova, O.A., Zinatullina, A.E., and Veselov, D.S., Abscisic acid in in vitro explant culture systems, Izv. Ufimsk. Nauch. Tsentra Ross. Akad. Nauk, 2018, no. 2, pp. 55–60. https://doi.org/10.31040/2222-8349-2018-0-2-55-60
Kruglova, N.N., Titova, G.E., Seldimirova, O.A., and Zinatullina, A.E., Cytophysiological features of the cereal-based experimental system “embryo in vivo–callus in vitro,” Russ. J. Dev. Biol., 2021, vol. 52, no. 4, pp. 199–214. https://doi.org/10.1134/s1062360421040044
Kruglova, N., Zinatullina, A., and Yegorova, N., Histological approach to the study of morphogenesis in callus cultures in vitro: a review, Int. J. Plant Biol., 2023, vol. 14, no. 2, pp. 533–545. https://doi.org/10.3390/ijpb14020042
Kruglova, N.N., Zinatullina, A.E., and Yegorova, N.A., Morphogenesis in vitro in calluses of lavender Lavandula angustifolia Mill.: histological aspects, Biol. Bull. (Moscow), 2024, vol. 51, no. 3, pp. 481–489. https://doi.org/10.1134/S1062359023605876
Kudoyarova, G.R., Korobova, A.V., Akhiyarova, G.R., Arkhipova, T.N., Zaytsev, D.Yu., Prinsen, E., Egutkin, N.L., Medvedev, S.S., and Veselov, S.Yu., Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP), J. Exp. Bot., 2014, vol. 65, no. 9, pp. 2287–2294. https://doi.org/10.1093/jxb/eru113
Leelavathi, D., Raajasubramanian, D., Ramu, L., Haseena, R., Midhila, P., Govindaraju, M.V., Lavanya, G., Chetan, H.C., and Narendra, K., Lavandula angustifolia L. plants regeneration from in vitro leaf explants-derived callus as conservation strategy, Biotecn. Veg., 2020, vol. 20, no. 2, pp. 75–82.
Lu, H., Xu, P., Hu, K., Xiao, Q., Wen, J., Yi, B., Ma, Ch., Tu, J., Fu, T., and Shen, J., Transcriptome profiling reveals cytokinin promoted callus regeneration in Brassica juncea, Plant Cell, Tissue Organ Culture, 2020, vol. 141, no. 1, pp. 191–206. https://doi.org/10.1007/s11240-020-01779-5
Mostafa, H.H.A., Wang, H., Song, J., and Li, X., Effects of genotypes and explants on garlic callus production and endogenous hormones, Sci. Rep., 2020, vol. 10, no. 1, p. 4867. https://doi.org/10.1038/s41598-020-61564-4
Murashige, T. and Skoog, F., A revised medium for rapid growth and bio assays with tobacco tissue cultures, Physiol. Plant., 1962, vol. 15, no. 3, pp. 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Ohbayashi, I., Sakamoto, Yu., Kuwae, H., Kasahara, H., and Sugiyama, M., Enhancement of shoot regeneration by treatment with inhibitors of auxin biosynthesis and transport during callus induction in tissue culture of Arabidopsis thaliana, Plant Biotechnol., 2022, vol. 39, no. 1, pp. 43–50. https://doi.org/10.5511/plantbiotechnology.21.1225a
Ozyigit, I.I., Dogan, I., Hocaoglu-Ozyigit, A., Yalcin, B., Erdogan, A., Yalcin, I.E., Cabi, E., and Kaya, Yi., Production of secondary metabolites using tissue culture-based biotechnological applications, Front. Plant Sci., 2023, vol. 14, p. 1132555. https://doi.org/10.3389/fpls.2023.1132555
Raspor, M., Motyka, V., Kaleri, A.R., Ninković, S., Tubić, L., Cingel, A., and Ćosić, T., Integrating the roles for cytokinin and auxin in de novo shoot organogenesis: from hormone uptake to signaling outputs, Int. J. Mol. Sci., 2021, vol. 22, no. 16, p. 8554. https://doi.org/10.3390/ijms22168554
Salehi, B., Mnayer, D., Özçelik, B., Altin, G., Kasapoğlu, K.N., Daskaya-Dikmen, C., Sharifi-Rad, M., Selamoglu, Z., Acharya, K., Sen, S., Matthews, K.R., Fokou, P.V.T., Sharopov, F., Setzer, W.N., Martorell, M., and Sharifi-Rad, J., Plants of the genus Lavandula: from farm to pharmacy, Nat. Prod. Commun., 2018, vol. 13, no. 10, pp. 1385–1402. https://doi.org/10.1177/1934578x1801301037
Salinas-Patino, V.A., Espinoza-Mellado, M.R., Hernandez-Pimentel, M.V., García-Pineda, M., Montes-Villafan, S., and Rodríguez-Dorantes, A., Phytohormones action on fouquieria splendens callogenesis and organogenesis processes, Int. J. Agricult. Innov. Res., 2018, vol. 7, no. 2, pp. 2319–1473.
Seldimirova, O.A., Kudoyarova, G.R., Kruglova, N.N., Galin, I.R., and Veselov, D.S., Somatic embryogenesis in wheat and barley calli in vitro is determined by the level of indoleacetic and abscisic acids, Russ. J. Dev. Biol., 2019, vol. 50, no. 3, pp. 124–135. https://doi.org/10.1134/s1062360419030056
Skoog, F. and Miller, C.O., Chemical regulation of growth and organ formation in plant tissues cultured in vitro, Symp. Soc. Exp. Biol., 1957, vol. 11, pp. 118–130.
Šmeringai, J., Schrumpfová, P.P., and Pernisová, M., Cytokinins—regulators of de novo shoot organogenesis, Front. Plant Sci., 2023, vol. 14, p. 1239133. https://doi.org/10.3389/fpls.2023.1239133
Spinoso-Castillo, J.L. and Bello-Bello, J.J., In vitro stress-mediated somatic embryogenesis in plants, Methods in Molecular Biology, Springer US, 2022, vol. 2527, pp. 223–235. https://doi.org/10.1007/978-1-0716-2485-2_16
Vysotskaya, L.B., Korobova, A.V., and Kudoyarova, G.R., Abscisic acid accumulation in the roots of nutrient-limited plants: its impact on the differential growth of roots and shoots, J. Plant Physiol., 2008, vol. 165, no. 12, pp. 1274–1279. https://doi.org/10.1016/j.jplph.2007.08.014
Wang, Ch., Ma, H., Zhu, W., Zhang, J., Zhao, X., and Li, X., Seedling-derived leaf and root tip as alternative explants for callus induction and plant regeneration in maize, Physiol. Plant., 2021, vol. 172, no. 3, pp. 1570–1581. https://doi.org/10.1111/ppl.13347
Wójcik, A.M., Wójcikowska, B., and Gaj, M.D., Current perspectives on the auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants, Int. J. Mol. Sci., 2020, vol. 21, no. 4, p. 1333. https://doi.org/10.3390/ijms21041333
Yegorova, N.A., Biotekhnologiya efiromaslichnykh rastenii: sozdanie novykh form i mikrorazmnozhenie in vitro (Biotechnology of Oilseed Plants: Creation of New Forms and in Vitro Micropropagation), Simferopol: Avtograf, 2021.
Yegorova, N., Kruglova, N., Galin, I., and Stavtzeva, I., Induction of morphogenesis in the callus culture of Lavandula angustifolia Mill., BIO Web Conf., 2020, vol. 24, p. 00098. https://doi.org/10.1051/bioconf/20202400098
Zinatullina, A.E., Structural features of in vivo eexplant cells and the formation of morphogenic callus in vitro (review), Biomika, 2021, vol. 13, no. 1, pp. 8–19. https://doi.org/10.31301/2221-6197.bmcs.2021-2
Zinatullina, A.E., Formation of morphogenetic foci as a basis for hemorrhizogenesis in vitro in wheat germ calluses, Ekobiotekh, 2023, vol. 6, no. 2, pp. 81–90. https://doi.org/10.31163/2618-964X-2023-6-2-81-90
ACKNOWLEDGMENTS
This research was carried out at the Agidel Center for Collective Use, Federal Research Center, Russian Academy of Sciences, and the Biotechnology Laboratory, Research Institute of Agriculture of Crimea.
Funding
This work was supported by the Russian Science Foundation, grant no. 23-24-00023.
Author information
Authors and Affiliations
Contributions
N.N. Kruglova, I.R. Calin, and A.E. Zinatullina prepared the initial version of the article. N.A. Yegorova analyzed the main statements of the article. All the authors participated in the discussion of the final version of the article.
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human or animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kruglova, N.N., Galin, I.R. & Yegorova, N.A. The Content of Endogenous Hormones in Explants and Calluses of Lavandula angustifolia Mill. at the Initial Stages of an In Vitro Culture. Biol Bull Russ Acad Sci 51, 1221–1230 (2024). https://doi.org/10.1134/S1062359024607924
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359024607924