Abstract—
This paper is a revision of the original description of the karyotype of the sable Martes zibellina. It presents data on the mitotic metaphase chromosomes of the female and male of this species and a comparison of their sets with those of closely allied species in the genus Martes. Additionally, a description of the synaptonemal complex (SC) is provided in the early to middle prophase of meiosis in the sable spermatocytes. Comparative analysis confirmed the stability and similarity of the main cytogenetic parameters of sables and martens (2n = 38, NFa = 64–68, X is the average submetacentric and Y is the smallest meta-, submeta-, or acrocentrics). A slight polymorphism associated with the representation of one- or two-armed small elements of the diploid set of chromosomes is revealed. These characteristics are of little use for the development of the intraspecific taxonomy of the sable, but can be useful in determining phylogenetic relationships at the species and generic levels, as well as to identify the consequences of natural hybridization of allied species in the genus Martes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The sable Martes zibellina L., 1758, is a fur-bearing animal in the family of Mustelidae, inhabiting an enormous forested territory in Eurasia. In Russia, its range stretches from the Pre-Urals eastward to Kamchatka, from the northern boreal margin to Southern Primorye, and from the south of the boreal zone of Siberia to forest–steppes of Kazakhstan in Altai and the Northern Urals (Geptner et al., 1967). Considerable fluctuations in the prices for sable pelts are observed due to variations in their demand on international markets. According to OOO Soyuzpushina, the price for a pelt peaked at $268.5 at the International Fur Auction in St. Petersburg in 1987; the drop from $199 in 2012 to $43.2 in 2020 was recorded in the subsequent years.Footnote 1 The greatest value is attached to individuals that have fur with dark coloration, primarily arriving from Zabaikal’e (Barguzinskii Ridge) and from Yenisei; the animals from these locations account for more than half of the hunters’ harvest.
Based on the open sources, however, individuals with dark-colored fur also come onto the peltry markets from other parts of the sable range, including (in the amount from 1 to 10%) from the southeastern peripheries, such as insular populations of Sakhalin (Levenkova and Kashtanov, 2019). This suggests, on the one hand, the unity of the species, while, on the other, it points toward a mosaic of phenotypic variability across the entire range, which creates difficulties for subspecies taxonomy. Thus, in his summary, V.G. Monachov (2011) reports data that anywhere from two to 30 subspecies of sable were recognized in various sources. The intraspecific systematics of the sable also became complicated due to overharvesting for some periods of time and the subsequent large-scale reintroduction carried out in several steps. These primarily included specimens from Baikalia that have valuable fur. These measures, along with the hunting restrictions, led to an increase in the population size of the species, while its ability for significant natural migrations and favorable climatic factors contributed to regeneration of a continuous uninterrupted range and, furthermore, expansion of its boundaries (Safronov, 2016; Zakharov and Safronov, 2012; Kashtanov et al., 2022). The genetic analysis using the molecular markers discovered (under significant variability of allelic variants) a genetic affinity between populations from the central part of the range, which may be attributed to dispersal (spread) and mixing of the endemic sables and the offspring of the introduced species. The highest distinctiveness characterizes animals from the marginal (peripheral) habitats or habitats separated by the large natural (terrain) barriers (Pishchulina, 2013; Kashtanov et al., 2015a, 2022).
Despite the emergence of new approaches to study of the genetic structure of the species beginning in the mid-20th century, the karyotype still remains a universal character, both integral and differentiating, in the systematics of mammals to date (Orlov and Bulatova, 1983). Sable is among the first species, studies on the karyotype of which laid a foundation for the national karyosystematics. The first description of sable karyotypes (Orlov and Malygin, 1969) was published in the collection prepared for the 2nd All-Union Meeting on Mammals, comprising a series of communications on the cytogenetics of mammals in the country. The circumstances proved to be such that for the original description and subsequent investigations in the course of the development of cytogenetic techniques (Grafodatskii and Radzhabli, 1988; Grafodatskii et al., 1977), materials with respect to the sable karyotype were received from the fur-farm animals, rather than obtained directly from the natural populations. Thus, no data is available to date on the karyotypic characteristics of the subspecies. Comparative studies on karyotypes of sable and allied taxa, however, provide grounds for addressing the issues if not particularly of the intraspecific taxonomy, then of interspecific relations of linneon (macrospecies) Martes zibellina L.
Sable Subspecies and Their Place in the System
No uniform understanding exists to date about the systematics of the sable at the species and subspecies levels. At the former, this primarily concerns the taxonomic position of the marginal populations; whereas revision of the intraspecific forms should concern the species range as a whole. Thus, in their summary, V.G. Geptner et al. (1967) reported for the Soviet Union 17 subspecies divided into seven groups (large-size population); namely, the Tobol, Altai, Sayan, Yenisei, Zabaikal’e, Sakhalin, and Kamchatka groups. Separation of the two latter groups reflects a distinctiveness of the insular and semi-insular populations, which is genetically evidenced by discovering the unique tandem repeats in nuclear DNA in the individuals on the periphery of the range (Pishchulina, 2013; Kashtanov et al., 2015a, 2015b).
Similarly, 17 subspecies of sable are recognized in the reference work Mammal Species of the World (Wozencraft, 2005), though covering the entire range. Investigations of a large sampling based on the exterior characters, fur coloring, and cranial measurements, however, confirmed the occurrence of as little as four subspecies, namely, averini, sahalinensis, kamtschadalica, and zibellina (Monakhov et al., 1976, 2020, 2021). In their monograph, N.N. Bakeev et al. (2003) confirm the actual occurrence of as low as four sable subspecies, exhibiting rather trenchant morphological distinctions and inhabiting relatively isolated territories; specifically, the Altai, Tobol, Sakhalin, and Kamchatka sable, the same as listed earlier (Monakhov, 1976). Other researchers consider up to eight subspecies, that is, zibellina, yenisseensis, averini, princeps, schantaricus, sahaliensis, kamtchadalica, and brachyura within the contemporary boundaries of Russia (Aristov and Baryshnikov, 2001).
Molecular genetic analysis of mitochondrial and nuclear DNA opened new venues for revision of the species and intraspecific forms of the Russian sable and other species in the genus Martes (Kyle et al., 2003; Rozhnov et al., 2010, 2013; Ishida et al., 2013; Kinoshita et al., 2015; Kashtanov et al., 2015a, 2015b, 2018; etc.).
As noted above, reintroduction activities were held in the Soviet Union due to the overharvesting and dramatic drop in the size of the sable population. A total of 19 000 animals were released from 1901 to 1970; but the activities on sable dispersal were terminated in 1970 (Bobrov et al., 2008). The distinctiveness of the populations inhabiting different parts of the range was, however, revealed using the technique of nuclear DNA microsatellite analysis, despite the human intervention into the species structure (harvesting/hunting and reintroduction), as well as processes of migration recorded for the sable during various harvesting seasons (Pishchulina, 2013; Kashtanov et al., 2015b). This raises the possibility for revision of the species and intraspecific forms using the genetic methods.
Beyond the Russian borders, sable occurs in Kazakhstan, China, Mongolia, Korea, and Japan. The following subspecies are present in the northern Islands of Japan: M. z. brachyura in Hokkaido; the Japanese sable M. z. melampus on Honshu (Hondo), Shikoko, and Kyushu; M. z. tsuensis on Tsushima Island in the Korean Strait; and M. z. koreensis (sysnonym hamgyensis) on the Korean Peninsula (Geptner et al., 1967). In the western part of its range, the sable comes in contact with the European pine marten (sweet marten) Martes martes (an allied species), resulting in trapping by the hunters of interspecific hybrids or kidus.
The Nearctic realm hosts two species belonging to the genus Martes, that is, the fisher M. pennanti with three subspecies, and the American marten M. americana with 14 subspecies (Hall, 1981). A morphological and genetic affinity is observed between M. americana and a group of the Eurasian species (martes/zibellina/melampus/) in thte subgeneric characteristics of Martes. Members of this group occasionally used to be pooled into a single “superspecies” (Pavlinov and Lisovskii, 2012). Another American species is treated either as a subgenus of the genus Martes or as separate genus Pekania by appealing to the molecular genetic isolation (Pekania pennanti, ASM Mammal Diversity DatabaseFootnote 2).
Due to its valued fur and because of the dramatic drop in the population size, farm-breeding of sable in cages was launched in the early 1930s (Portnova, 1941), which made it possible to create the species production technology on fur farms (Kazakova et al., 1986). Currently, Russia has 11 fur farms for sable breeding with the total breeding stock of 45 200 females (Svodka …, 2021).
Thus, sables have been bred on Pushkinskii state fur farm (zverosovkhoz) in Moscow oblast since 1931. More than 100 animals were initially delivered. The animals were captured in the wild and had inhabited different ridges; specifically, the Barguzin, Amur, Yenisei, Altai, Minusinsk, and Tobol (Pavlov and Balieva, 1941). Sables from Zabaikal’e and Sakhalin were subsequently delivered (Kashtanov et al., 2016). Sables have been bred on Saltykovskii state fur farm of Moscow oblast since 1948 with the first 35 females and 20 males having been delivered from Raisino state fur farm of Moscow oblast. Unfortunately, the locations of capture of these animals could not be established (Kashtanov et al., 2020). The diversity of the initial capture locations of the sables, mixing, and time length of their breeding suggest that the genetic parameters in the individuals from different state fur farms can hardly be employed in building the intraspecific structure of the Martes spp.
Sable breeding was attempted in China (Monakhov and Li, 2013). Studies on sables are also conducted in Japan, the United States, and other countries. Peculiarities of morphology and biology of this species are relatively well-understood (Martynov, 1987; Monachov, 2011; Sergeev, 2016).
Taxonomic isolation of the genus Martes, which is represented by three or two subgenera and six to eight species, is generally acknowledged (Pavlinov, 2006; Geptner et al., 1967); discrepancies, however, exist in the interpretation of the species status and composition of subgenera. In his revision of Mustelidae, Geptner went by a broad interpretation of the genus Martes and, therefore, treated the taxonomic distinctiveness of the yellow-throated marten Martes flavigula and its vicariat, the Nilgiri marten, M. gwatkinsii, native to southern India (race of the yellow-throated marten), exclusively at the level of the subgenus Charronia (Geptner et al., 1967), whereas it appears conceivable to assign the yellow-throated marten to the separate genus Charronia (Aristov and Baryshnikov, 2001). The latter interpretation appears to be more plausible, since the karyotype of the yellow-throated marten (2n = 40; NFa = 68) (Fredga, 1966; Atlas of Mammalian Chromosomes, 2020) differs from that of other species in the genus Martes (Orlov and Bulatova, 1983). Assignment to a specialized genus (this time based on the molecular genetic data) was additionally proposed for the North American species M. pennanti (see above). In Russia, the genus Martes is represented by four species, three of which (M. zibellina, M. foina, and M. martes) are grouped into the subgenus Martes s.str. and one (M. flavigula) is classified into the subgenus (if not the genus) Charronia.
Analysis of the genetic markers revealed common patterns in Martes spp., that is, an enormous diversity of haplotypes, which is the maximum for continental populations of the sable, the European pine marten, and the American marten; the heterogeneity of their genotypic and phenotypic variability; and, at the same time, the distinctiveness of the populations based on their genetic markers, which is the most pronounced for insular populations on the margins of the ranges (Kyle et al., 2003; Kinoshita et al., 2015).
Note, however, that providing the use of the state-of-the-art molecular genetic techniques, precise mapping of the genes is difficult without bridging them to cytogenetic maps (Rubtsov and Karamysheva, 1999).
The issues of genetic relations and taxonomic distinctions between subspecies of the sable M. zibellina and the interspecific relationships within the subgenus Martes and between subgenera of the genus remain relevant and are still waiting to be answered using a set of novel and conventional approaches.
Cytogenetic Characteristics of Taxa of the Genus Martes
Researchers brought the sable genetics into steady focus beginning in the 1960s. The description of the karyotype became the first among the then-available technique-wise characteristics of the genome (Table 1). This was subsequently followed by the biochemical (largely immunochemical) (Belyev et al., 1980, 1984) and state-of-the-art molecular genetic studies (Pishchulina, 2013; Hosoda et al., 1999; Kurose et al., 1999; Inoue et al., 2010; Rozhnov et al., 2013; Kinoshita et al., 2015; Li et al., 2021; etc.).
Ehrlich (1949) was the first to mention the karyotype of a species in the genus Martes, when he discovered 19 chromosome pairs in somatic cells of the stone marten M. foina. Using contemporary techniques, Grafodatskii et al. (1982a) investigated the karyotype of a M. foina female (farm-bred), while R.I. Dzuev et al. (2013, 2020) practically echoed this description for a M. foina nehringi female and two males in the wild from two locations in the Caucasus (Table 1). The first mention of the diploid number (2n = 38) of the European pine marten dates back to 1967 (Fredga, 1967; Wurster and Benirschke, 1967), whereas a full description of the karyotype of the male species was reported in a paper by Grafodatskii et al. (1982a). A similar karyotype was discovered in three martens M. martes larenzi (2n = 38) captured in two locations on the northern macroslope of the Great Caucasus (Table 1) (Dzuev et al., 2013). The description of the karyotype of the American marten M. americana (Wurster and Benirschke, 1968) proved to be consistent with the data on the karyotype of the sable M. zibellinae (Orlov and Malygin, 1969).
Therefore, a conclusion was inferred with respect to the similarity and stability of karyotypes of Martes spp. and, accordingly, the lack of prospects for using cytogenetic parameters for the purposes of comparative taxonomic studies of species belonging to this genus (Grafodatskii et al., 1976, 1977).
Nevertheless, the very first comparative studies on chromosomes of the sable and allied species detected signs of polymorphism, the nature of which necessitated further cytogenetic investigations (Grafodatckii et al., 1977, 1982a, 1982b).
This paper presents generalization of the data on cytogenetics of the Russian sable and the results of comparison between its karyotype and the same of closely related species in the genus Martes; which is particularly relevant, since it appears impossible to find among the present-day web resources illustrative data supplementing descriptions of karyotypes of Martes published at the end of the past century.
MATERIALS AND METHODS
All the data on sable cytogenetics was obtained in our country from the breeding in Saltykovskii (six males and two females were studied) (Orlov and Malygin, 1969) and Pushkinskii (ten males) (Safronova et al., 2018) state fur farms in Moscow oblast, as well as the experimental farm at the Institute of Cytology and Genetics, Siberian Branch, USSR Academy of Sciences, and the Novosibirsk Institute of Biology (one male and two females) (Grafodatskii et al., 1977). Therefore, specific data are not available on geographic referencing of the investigated animals; there is a probability of mixed origin of these animals from the ancestors captured from the different Siberian populations.
This article reports refined data on the karyotype of a female and male of the sable from thte Saltykovskii state fur farm, where the sable’s karyotype was originally sketchily described with respect to the morphology of the chromosomes (Orlov and Malygin, 1969).
Somatic chromosome preparations were made of bone marrow by conventional techniques (Ford and Hamerton, 1956). Image editing tools of Photoshop CC (free trial version of Adobe Creative Cloud) were used to process the photographic images.
Testes of ten sexually mature males from the populations of the Pushkinskii state fur farm were used for analysis of the meiotic chromosomes. The technique for the analysis of synaptonemal complexes (SCs) was described earlier (Moses et al., 1977; Safronova et al., 2018). The lengths of the autosomal SC and sex bivalents of spermatocytes were measured using a Leica Application Suite V3 program on digital microphotography images. Numbering of the SC bivalents in the karyotype was done in the order of decreasing linear dimensions.
RESULTS AND DISCUSSION
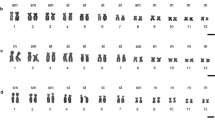
The diploid set of the female and male studied contains 38 chromosomes each (Fig. 1), including 18 pairs of autosomes and the sex chromosome, which are, based on the refined data, represented in the female by the fifth largest pair of metacentrics (Fig. 2a); the X in the male is the same as in the female; but the Y chromosome is the smallest acrocentric chromosome (Fig. 2b).
For ranking pairs of routinely stained elements, we compared the measurements of the chromosome arms made by us earlier on ten metaphase plates in a female and male of the sable with analogous measurements by the Photoshop CC ruler and, next, identified conditionally homologous pairs. According to classification by Levan et al. (Levan et al., 1964), the homologous pairs were distributed into three morphological groups based on the location of the centromere. Seven pairs of the meta-, seven pairs of the submeta-, and four pairs of acrocentrics were found in autosomes. The chromosome arm ratio was from 1 to 1.3 in the group of metacentrics, from 1.5 to 3.34 in submetacentrics, and higher than 3.4 in acrocentrics.
The largest proved to be a pair of submetacentric chromosomes; another submeta- and two pairs of metacentrics are similar to it in size, which distinguishes them from other chromosomes in the set. The smallest pair of metacentrics also stands out; the remaining autosomes smoothly decrease in the order of size (Figs. 1, 2). Of the acrocentrics, the largest one corresponds to the seventh (in the order of size) pair of autosomes; the rest are smaller than the acrocentrics. The Y chromosome is a small acrocentric similar in size to the 18th pair of the smallest metacentrics (Fig. 2b).
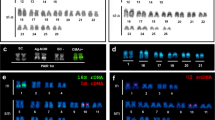
The primary parameters of the sable’s karyotype (2n and NF) were first defined in a brief communication with the female karyotype alone reported for illustrative purposes (Orlov and Malygin, 1969); but it was specified in the text body that the Y chromosome appeared as the smallest metacentric. In subsequent descriptions of the species karyotype, including with the differentially C- and G-stained chromosome, the Y chromosome was, however, identified as the smallest, completely C-positive acrocentric of the set (Grafodatskii and Radzhabli, 1988, Iwasa and Hosoda, 2002; Grafodatskii et al., 1977). This description of the Y chromosome has been confirmed in our revision of the data (Fig. 2b).
Importantly, the smallest acrocentric chromosome is heteromorphic; one or both homologs appear to bear secondary constriction (Fig. 2), which was first pointed out by Grafodatskii et al. (1977). Under the extensive spiralization, routine staining makes it resemble a two-arm element.
Altogether, the sable karyotypes reported herewith are similar to those described by Grafodatskii et al. (1977). The authors distributed the differentially stained (G-staining) autosomes into only two groups; specifically, the two-armed (matches our meta- and submetacentrics) and four pairs of one-arm acrocentrics. As noted above, inconsistencies in the descriptions can be attributed to interpretation of the chromosomes’ morphology, rather than variability of the karyotype.
We agree with the assumption put forth by Grafodatskii et al. (1978) with respect to conservatism of the X chromosome constitution as a large submetacentric. Although the authors reported data on the karyotypes of seven Mustelidae species, which did not embrace representatives of the genus Martes, morphologically similar X chromosomes were discovered by Grafodatskii et al. in other publications (1977, 1982a, 1982b, 1985; Grafodatskii and Radzhabli, 1988), as well as in studies of our Japanese colleagues (Tsuchiya, 1979; Obara, 1982, 1991; Iwasa and Hosoda, 2002).
The Japanese researchers provided a karyotype description of the species (M. z. brachyura) live-trapped in Hokkaido with permission from the Prefectural Government; the chromosomes were studied in fibroblast cells from lung tissue culture of two males (Iwasa and Hosoda, 2002). This publication presents comparative characteristic of differentially stained (using two fluorochromes, specifically, QM and CMA3) preparations of M. z. brachyura and its closely related species the Japanese marten Martes melampus melampus (male from Shikoku). Note that, in their monograph, Geptner et al. (1967) treat this species as one of the insular subspecies of sable M. z. melampus.
The karyotypes of all the foregoing animals proved to be similar (Obara, 1982, 1991; Iwasa and Hosoda, 2002) with few exceptions. In the karyotype of M. z. brachyura, the number of arms of autosomes (FN = 66) is the same as in the Siberian sables but differs from the Japanese sable (FN = 68) (Tsuchiya, 1979; Obara, 1982). The latter has in its karyotype the short arms of the chromosome in the small 14th pair. This variation does not affect the post-mating isolation mechanisms of these species (Iwasa and Hosoda, 2002).
Note that the analysis of mitochondrial and nuclear markers in sables from various regions of the Eurasian continent, Kamchatka Peninsula, insular populations of Kuril Chain, and Sakhalin and Hokkaido islands revealed both the affinity between the Russian and Japanese sables and the distinctiveness of haplotypes of the sables endemic to these islands (Ishida et al., 2013; Kinoshita et al., 2015).
A detailed account of the karyotype of the European pine marten and its comparison with the sable was given by Grafodatskii et al. (1982b). The authors examined a male from an experimental facility at the Novosibirsk Institute of Biology, but, unfortunately, failed to specify the place of its capture. Importantly, the affinity of the sable and European pine marten is reflected in the coincidence of nearly all elements of the diploid set of chromosomes, with one exception. The Y chromosome is the smallest acrocentrics in the sable, but the smallest two-arm element of the set in the European pine marten. The karyotype parameters are reported to be the same in M. martes lorenzi from the Great Caucasus (Dzuev, 2013). Additionally, an insignificant difference was recorded in the level and distribution of heterochromatin, as well as the position of nucleolus organizer regions detectable by С- and AgNOR-staining (Grafodatskii et al., 1977, 1982a, 1982b). Therefore, hybridization of these species appears perfectly natural in the Urals in the places where their ranges overlap.
Apparently, a relatively small level of heterochromatin in the karyotype of the sable correlates with a low content of DNA (82.2 ± 3.3% of human genome) per diploid cell among all the Mustelidae species investigated (Grafodatskii et al., 1977). The sable is more diverse than the European pine marten based on mitochondrial and microsatellite nuclear DNA (Pishchulina, 2013).
The SC number in the cell corresponds to the number of chromosome in the haploid set, while the relative lengths of the SC autosomes and the sex chromosome correlate with the relative lengths of the corresponding chromosomes in the mitotic karyotype (Demin et al., 1984; Bogdanov et al., 1996).
There is a well-defined trend of correlation with the mitotic karyotype at an early and middle prophase of meiosis of spermatocytes. The SC karyotype exhibits 18 bivalent elements of autosomes, gradually decreasing in the order of size, and the sex (X–Y) bivalent, corresponding to the ninth and tenth pair based on size. In the middle prophase, the X-axis length exceeds the length of the Y-chromosome axis threefold; while these axes “synapt” along their entire length (Fig. 3).
To date, we have knowledge of only one more description of SC in representatives of the family Mustelidae, namely, the American mink Neovison vison (Koykul and Basrur, 1995). In meiosis prophase, the sex complements (elements) behave in a similar way, which is not surprising, since the comparative analysis of karyotypes in several mustelid species showed their similarity with respect to the constitution of the X chromosome (Grafodatskii et al., 1976, 1985).
CONCLUSIONS
The limited data on characterization of karyotypes of Martes spp., which have been obtained over a period spanning more than half a century, beginning with the onset of their investigation, genuinely points toward a conservatism of the chromosome sets in species of this genus, considering the diploid numbers and homology based on G-staining.
We found that the sable either coincides with karyotypes of other species in the genus Martes s. str. (e.g., the European pine marten) or is rather close to them based on the cytogenetic parameters, specifically, 2n, NF, the shape of the X chromosome, and G- and C-staining, as well as the SC characteristics. Slight differences relate to two pairs of acrocentrics, as well as the Y chromosome, which may appear as the one- or two-arm elements (Table 1). These differences seem to result from the occurrence of secondary constrictions and the positions of centromeres. Note that, in contrast with representatives of other genera, in chromosomes of species in the genus Martes, there is little heterochromatin, accumulation or loss of which commonly causes chromosomal polymorphism.
Since the literature and our own data confirm the stability of karyotypes in the genus Martes, in our opinion, the use karyological characteristics for clarification of the sable intraspecific system is not particularly practical. It is worth noting, however, that our knowledge about the karyology of the sable across its range is fragmentary and rather incomplete. It is entirely possible that the observed polymorphism, which may serve to diagnose allied species, that is, the sable and European pine marten, does not relate to all of their populations. Nevertheless, these parameters can be applied to phyllogenetic tree-building at the species and generic levels. They can also be employed as an additional marker for identification of kidus during molecular genetic studies and interactions between the allied species in the genus Martes.
It is our hope that the present publication will be sought after both by contemporary studies that address issues with regard to phylogeny, systematics, and biodiversity of the Russian sable and contribute to solving practical problems associated with sable breeding and selection.
REFERENCES
Aristov, A.A. and Baryshnikov, G.F., Mlekopitayushchie fauny Rossii i sopredel’nykh territorii. Khishchnye i lastonogie (Mammals of the Fauna of Russia and Adjacent Territories. Carnivores and Pinnipeds), St. Petersburg, 2001, рр. 210–225.
Atlas of Mammalian Chromosomes, Graphodatsky, A.S., Perelman, P.L., and O’Brien, S.J., Eds., USA: Wiley-Blackwell, 2020, 2nd ed.
Bakeev, N.N., Monakhov, G.I., and Sinitsyn, A.A., Sobol’ (Sable), Vyatka, 2003, 2nd ed.
Belyaev, D.K., Baranov, O.K., Ternovskaya, Yu.G., and Ternovskii, D.V., Comparative immunochemical study of serum proteins in Mustelidae (Carnivora), Zool. Zh., 1980, vol. 59, no. 2, pp. 254–260.
Belyaev, D.K., Baranov, O.K., Fomicheva, I.I., Smirnykh, S.I., Ternovskii, D.V., and Ternovskaya, Yu.G., Interspecies antigenic variability of serum proteins in the family Mustelidae (Carnivora), Zool. Zh., 1984, vol. 63, no. 6, pp. 912–922.
Benirschke, K. and Yang, E., Chromosomes of the fisher (Martes pennanti), Mamm. Chromosomes Newslett., 1966, no. 21, p. 150.
Bobrov, V.V., Varshavskii, A.A., and Khlyap, L.A., Chuzherodnye vidy mlekopitayushchikh v ekosistemakh Rossii (Alien Species of Mammals in the Ecosystems of Russia), Moscow: KMK, 2008, рр. 123–126.
Bogdanov, Yu.F., Grishaeva, T.M., Kolomiets, O.L., and Fedotova, Yu.S., Cytogenetic patterns of synapsis of meiotic chromosomes in animals and plants, Genetika, 1996, vol. 32, no. 11, pp. 1471–1493.
Demin, Yu.S., Safronova, L.D., Cherepanova, L.V., and Safronov, V.A., Study of synaptonemal complexes in mammals. Message 1. The nature and mechanism of formation of centric fusions of chromosomes (Robertsonian translocations), Genetika, 1984, vol. 20, no. 9, pp. 1499–1506.
Dzuev, R.I., Sukhomesova, M.V., Sharibova, A.Kh., and Cheprakova, A.A., Chromosome set of the Caucasian pine marten (Martes martes lorenzi Ogn., 1926) in the North Caucasus, Izv. Gorsk. Gos. Agr. Univ., 2013, vol. 50, no. 3, pp. 312–315.
Dzuev, R.I., Sabanova, R.K., Evgazhukova, A.A., Irugova, E.Z., and Dzuev, A.R., Chromosome set, distribution, abundance and biotopic confinement of the stone marten (Martes foina nehringi Satunin, 1905) in the North Caucasus, Polevoi Zh. Biol., 2020, vol. 2, no. 2, pp. 132–142. https://doi.org/10.18413/2658-3453-2020-2-2-132-142
Ehrlich, I., Uber chromomenzahl, hodenzyklen und brunft bei Martes foina Erxl, Rev. Suisse Zool. (Geneve), 1949, vol. 56, no. 34, pp. 621–626.
Ford, C.E. and Hamerton, J.L., A colchicine hypotonic citrate squash sequences for mammalian chromosomes, Stain. Technol., 1956, vol. 31, pp. 247–251.
Fredga, K., Chromosome studies in six species of Mustelidae and one of Procyonidae, Mamm. Chromosomes Newslett., 1966, no. 21, p. 145.
Fredga, K., Comparative chromosome studies of the family Mustelidae, Heredite, 1967, vol. 57, no. 4, p. 295.
Geptner, V.G., Naumov, N.P., Yurgenson, P.B., Cludskii, A.A., Chirkova, A.F., and Bannikov, A.G., Mlekopitayushchie Sovetskogo Soyuza (Mammals of the Soviet Union), Moscow, 1967, vol. 2, part 1, pp. 507–553.
Grafodatskii, A.S. and Radzhabli, S.I., Khromosomy sel’skokhozyaistvennykh i laboratornykh zhivotnykh. Atlas (Chromosomes of Farm and Laboratory Animals. Atlas), Novosibirsk: Nauka, 1988, рр. 108–109.
Grafodatskii, A.S., Volobuev, V.T., Ternovskii, D.V., and Radzhabli, S.I., G-differential staining of chromosomes of seven mustelid species (Mustelidae, Carnivora), Zool. Zh., 1976, vol. 55, no. 11, pp. 1704–1709.
Grafodatskii, A.S., Ternovskaya, Yu.G., Ternovskii, D.V., and Radzhabli, S.I., G- and C-staining of chromosomes and the amount of DNA in sable, Tsitol. Genet., 1977, vol. 10, no. 6, pp. 483–490.
Grafodatskii, A.S., Ternovskaya, Yu.G., and Ternovskii, D.V., Differential staining of chromosomes in the stone marten Martes foina (Carnivora, Mustelidae), Zool. Zh., 1982a, vol. 61, no. 10, pp. 1607–1608.
Grafodatskii, A.S., Ternovskaya, Yu.G., and Ternovskii, D.V., Differential staining of the pine marten (Martes martes) chromosomes, Zool. Zh., 1982b, vol. 61, no. 2, pp. 313–314.
Grafodatskii, A.S., Lushnikova, T.P., Romashchenko, A.A., and Radzhabli, S.I., Distribution of structural heterochromatin and repetitive DNA sequences on the chromosomes of a number of marten species (Carnivora, Mustelidae), Genetika, 1985, vol. 21, pp. 147–152.
Graphodatsky, A.S., Yang, F., Perelman, P.L., O’Brien, P.C., Serdukova, N.A., Milne, B.S., Biltueva, L.S., Fu, B., Vorobieva, N.V., Kawada, S.I., Robinson, T.J., and Ferguson-Smith, M.A., Comparative molecular cytogenetic studies in the order Carnivora: mapping chromosomal rearrangements onto the phylogenetic tree, Cytogenet. Genome Res., 2002, vol. 96, nos. 1–4, pp. 137–145. PMID: 12438790.https://doi.org/10.1159/000063032
Hall, E.R., The Mammals of North America, New York: Tor, 1981, vol. 2, pp. 981–987.
Hosoda, T., Suzuki, H., Iwasa, M.A., Hayashida, H., Watanabe, S., Tatara, M., and Tsuchiya, K., Genetic relationships within and between two Japanese species, the Japanese marten Martes melampus and the sable M. zibellina, based on variation of mitochondrial DNA and nuclear ribosomal DNA, Mamm. Study, 1999, vol. 24, pp. 25–33.
Inoue, T., Murakami, T., Abramov, A.V., and Masuda, R., Mitochondrial DNA control region variations in the sable Martes zibellina of Hokkaido Island and the Eurasian Continent, compared with the Japanese marten M. melampus, Mamm. Study, 2010, vol. 35, pp. 145–155.
Ishida, K., Sato, J.J., Kinoshita, G., Hosoda, T., Kryukov, A.P., and Suzuki, H., Evolutionary history of the sable (Martes zibellina brachyura) on Hokkaido inferred from mitochondrial Cytb and nuclear Mc1r and Tcf25 gene sequences, Acta Theriol., 2013, vol. 58, pp. 13–24. https://doi.org/10.1007/s13364-012-0103-z
Iwasa, M.A. and Hosoda, T., A note on the karyotype of the sable, Martes zibellina brachyura, in Hokkaido, Japan, Mamm. Study, 2002, vol. 27, pp. 83–86.
Kashtanov, S.N., Svishcheva, G.R., Pishchulina, S.L., Lazebnyi, O.E., Meshcherskii, I.G., Simakin, L.V., and Rozhnov, V.V., Geographical structure of the sable (Martes zibellina L.) gene pool on the basis of microsatellite loci analysis, Russ. J. Genet., 2015a, vol. 51, no. 1, pp. 69–79.
Kashtanov, S.N., Svishcheva, G.R., Lazebnyi, O.E., Kolobkov, D.S., Pishchulina, S.L., Meshcherskii, I.G., and Rozhnov, V.V., Influence of anthropogenous factors on the genetic variety of the sable (Martes zibellina L.), Mol. Biol. (Moscow), 2015b, vol. 49, no. 3, pp. 449–454.
Kashtanov, S.N., Sulimova, G.V., Shevyrkov, V.L., and Svishcheva, G.R., Breeding of the Russian sable: stages of industrial domestication and genetic variability, Russ. J. Genet., 2016, vol. 52, no. 9, pp. 889–899.
Kashtanov, S.N., Stolpovsky, Yu.A., Meshchersky, I.G., et al., Taxonomic status and genetic identification of Altai sable (Martes zibellina averini Bazhanov, 1943), Russ. J. Genet., 2018, vol. 54, no. 11, pp. 1327–1337. https://doi.org/10.1134/S1022795418110078
Kashtanov, S.N., Kirilushkin, K.I., and Fedorova, O.I., Saltykovskaya serebristaya, a new breeding achievement in fur farming, Vet., Zootekh. Biotekhnol., 2020, no. 9, pp. 85–91.
Kashtanov, S.N., Zakharov, E.S., Begletsov, O.A., Svishcheva, G.R., Rychkov, S.Yu., Filimonov, P.A., Onokhov, A.A., Levenkova, E.S., Meshcherskii, I.G., and Rozhnov, V.V., Expansion of the sable (Martes zibellina L.) from the north of the Central Siberian Plateau into tundra ecosystems, Russ. J. Genet., 2022, vol. 58, no. 8, pp. 955–966.
Kazakova, G.P., Snytko, E.G., Gladilov, Yu.I., Pavlyuchenko, S.V., Kulichkov, A.B., Sergeev, E.G., and Aulova, S.V., Tekhnologiya proizvodstva shkurok sobolya (nastavlenie) (Technology for the Production of Sable Skins (Manual)), NIIPZK, 1986.
Kinoshita, G., Sato, J.J., Meschersky, I.G., Pishchulina, S.L., Simakin, L.V., Rozhnov, V.V., Malyarchuk, B.A., Derenko, M.V., Denisova, G.A., Frisman, L.V., Kryukov, A.P., Hosoda, T., and Suzuki, H., Colonization history of the sable Martes zibellina (Mammalia, Carnivora) on the marginal peninsula and islands of Northeastern Eurasia, J. Mamm., 2015, vol. 96, no. 1, pp. 172–184. https://doi.org/10.1093/jmamma/gyu021
Koykul, W. and Basrur, P.K., The XY pair of the mink (Mustela vision) during different periods of testicular activity, Hereditas, 1995, vol. 122, no. 2, pp. 169–176.
Kurose, N., Masuda, R., Siriarooart, B., and Yoshida, M.C., Intraspecific variation of mitochondrial cytochrome b gene sequences of the Japanese marten Martes melampus and the sable Martes zibellina (Mustelidae, Carnivora, Mammalia) in Japan, Zool. Sci., 1999, vol. 16, pp. 693–700.
Kyle, C.J., Davison, A., and Strobeck, C., Genetic structure of European pine martens (Martes martes), and evidence for introgression with M. americana in England, Conserv. Genet., 2003, vol. 4, pp. 179–188.
Levan, A., Fredga, K., and Sandberrg, A.A., Nomenclature for centromeric position on chromosomes, Hereditas, 1964, vol. 52, pp. 201–220.
Levenkova, E.S. and Kashtanov, S.N., Phenotypic and genotypic variability of the Russian sable Martes zibellina L., in Genetika – fundamental’naya osnova innovatsii v meditsine i selektsii: Mat. VIII mezhd. nauch.-prakt. konf. (Proc. VIII Int. Sci.-Pract. Conf. “Genetics—A Fundamental Basis for Innovations in Medicine and Breeding”), Rostov-on-Don, 2019, pp. 168–169.
Li, B., Lu, J., Monakhov, V., Kang, H., Xu, Y., An, B., Ghani, M.U., Li, M., Peng, W., and Ma, X., Phylogeography of subspecies of the sable (Martes zibellina L.) based on mitochondrial genomes: implications for evolutionary history, Mamm. Biol., 2021. https://doi.org/10.1007/s 42991-020-00092-0
Martynov, V.F., Sobol’: Bibliograficheskii ukazatel’ 1586–1985 gg. (Sable: A Bibliographic Index 1586–1985), Novosibirsk: VASKhNIL VNIIBTZh, 1987.
Monachov, V.G., Martes zibellina (Carnivora: Mustelidae), Mamm. Species, 2011, vol. 43, no. 876, pp. 75–86. https://doi.org/10.1644/876.1
Monakhov, G.I., Geographical variability and taxonomic structure of the sable fauna of the USSR, Tr. VNII Okhot. Khoz. Zverovod., 1976, no. 26, pp. 54–86.
Monakhov, V.G. and Li, B., The current state, protection and use of resources of the sable Martes zibellina in Russia and China, Vestn. Okhotoved., 2013, vol. 10, no. 2, pp. 192–197.
Monakhov, V.G., Species specificity of the structure of the frontal part of the skull in sable (Martes sibellina) and pine marten (Martes martes), Zool. Zh., 2020, vol. 99, no. 11, рр. 1298–1306.https://doi.org/10.31857/S0044513420080073
Monakhov, V.G., Ranyuk, M.N., and Modorov, M.V., Population structure of sable in the Baikal Mountain Land: analysis of genetic and phenotypic traits, Russ. J. Ecol., 2021, vol. 52, no. 2, pp. 155–164.
Moses, M.J., Slatton, G.H., Gambling, T.M., and Starmer, C.F., Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus griseus). III. Quantitative evaluation, Chromosoma (Berl.), 1977, vol. 60, no. 4, pp. 345–375.
Nie, W., Wang, J., O’Brien, P.C., Fu, B., Ying, T., Ferguson-Smith, M.A., and Yang, F., The genome phylogeny of domestic cat, red panda and five mustelid species revealed by comparative chromosome painting and G-banding, Chromosome Res., 2002, vol. 10, no. 3, pp. 209–222. PMID: 12067210. https://doi.org/10.1023/a:1015292005631
Nie, W., Wang, J., Su, W., Wang, D., Tanomtong, A., Perelman, P.L., Graphodatsky, A.S., and Yang, F., Chromosomal rearrangements and karyotype evolution in carnivores revealed by chromosome painting, Heredity (Edinb.), 2012, vol. 108, no. 1, pp. 17–27. PMID: 22086079, PMCID: PMC3238119.https://doi.org/10.1038/hdy.2011.107
Obara, Y., C- and G-banded karyotypes of the Japanese marten, Martes melampus melampus, Chromosome Inf. Service, 1982, no. 33, pp. 21–23.
Obara, Y., Karyosystematics of the mustelid carnivores of Japan, Honyurui Kagaku, 1991, vol. 30, pp. 197–220.
Orlov, V.N. and Bulatova, N.Sh., Sravnitel’naya tsitogenetika i kariosistematika mlekopitayushchikh (Comparative Cytogenetics and Karyosystematics of Mammals), Moscow: Nauka, 1983.
Orlov, V.N. and Malygin, V.M., Chromosomal set of sable, Martes zibellina L., in II Vses. Sov. po mlekopitayushchim “Mlekopitayushchie: evolyutsiya, kariologiya, taksonomiya, faunistika,” Tezisy dokladov (II All-Union Meet. on Mammals “Mammals: Evolution, Karyology, Taxonomy, and Faunistics,” Abstracts of Papers), Novosibirsk: Sib. Otd. Akad. Nauk SSSR, 1969, р. 22.
Pavlinov, I.Ya., Sistematika sovremennykh mlekopitayushchikh (Systematics of Modern Mammals), Moscow: Zool. Muz. Mosk. Gos. Univ., 2006.
Pavlinov, I.Ya. and Lisovskii, A.A., Mlekopitayushchie Rossii: sistematiko-geograficheskii spravochnik (Mammals of Russia: Systematic and Geographical Reference Book), Moscow: KMK, 2012.
Pavlov, M.K. and Balieva, I.V., Breeding work in sable farming, Krolikovod. Zverovod., 1941, no. 6, pp. 15–19.
Pishchulina, S.L., Interaction between pine marten and sable populations in the sympatry zone: a genetic aspect, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Moscow, 2013.
Portnova, A.T., Experience of the sable farm of the Pushkino fur farm, Krolikovod. Zverovod., 1941, no. 6, pp. 20–22.
Rozhnov, V.V., Meschersky, I.G., Pishchulina, S.L., and Simakin, L.V., Genetic analysis of sable (Martes zibellina) and pine marten (M. martes) populations in sympatric part of distribution area in the Northern Urals., Russ. J. Genet., 2010, vol. 46, no. 4, pp. 488–492.
Rozhnov, V.V., Pishchulina, S.L., Meschersky, I.G., Simakin, L.V., Lazebny, O.E., and Kashtanov, S.N., Genetic structure of sable (Martes zibellina L.) in Eurasia—analysis of the mitochondrial lineages distribution, Russ. J. Genet., 2013, vol. 49, no. 2, pp. 220–227.
Rubtsov, N.B. and Karamysheva, T.V., Article 5: Multicolor nature of modern cytogenetics, or Multicolor FISH today, Vavilov. Vestn. VOGiS, 1999, no. 11, pp. 1–16.
Safronov, V.M., Climate change and mammals of Yakutia, Zool. Zh., 2016, vol. 95, no. 12, pp. 1459–1474. https://doi.org/10.7868/S004451341612014X
Safronova, L.D., Cherepanova, E.V., Malygin, V.M., and Sergeev, E.G., Atlas sinaptonemnykh kompleksov (SK-kariotipov) nekotorykh vidov mlekopitayushchikh (Atlas of Synaptonemal Complexes (SC-Karyotypes) of Some Mammalian Species), Moscow: KMK, 2018, рр. 11–12.
Sergeev, E.G., Sobol’. Bibliograficheskii ukazatel’ 1986–2014 gg. Metodicheskoe posobie (Sable. Bibliographic Index 1986–2014 Toolkit), Moscow: Nauchnaya biblioteka. 2016.
Svodka NATs. Pokazateli vosproizvodstva kletochnykh pushnykh zverei v RF po sostoyaniyu na 01.07.2021 g. (operativnye dannye) (NAC Summary. Reproduction Indices of Caged Fur Animals in the Russian Federation as of July 1, 2021 (Operational Data)), 2021 (manuscript).
Tsuchiya, K., A contribution to the chromosome study in Japanese mammals, Proc. Jpn. Acad., B, 1979, vol. 55, pp. 191–195.
Wozencraft, W.C., Order Carnivora. Mammal Species of the World: A Taxonomic and Geographic Reference, Wilson, D.E. and Reeder, D.M., Eds., Baltimore, Maryland: Johns Hopkins Univ. Press, 2005, 3rd ed, рр. 532–628.
Wurster, D.H. and Benirschke, K., Chromosome numbers in thirty species of carnivores, Mamm. Chromosomes Newslett., 1967, no. 8, p. 195.
Wurster, D.H. and Benirschke, K., Comparative cytogenetic studies in the order Carnivora, Chromosoma, 1968, vol. 24, no. 3, pp. 336–382.
Zakharov, E.S. and Safronov, V.M., Ecology of sable (Martes zibelline L.) in western Yakutia, Vestn. Tomsk. Gos. Univ., Biol., 2012, vol. 1, no. 17, pp. 73–84.
ACKNOWLEDGMENTS
We are grateful to N.S. Bulatova for her helpful remarks and proofing microphotographs of the sable karyotype.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors
Additional information
Dedication to Viktor Nikolaevich Orlov, one of the founders of the Russian school of karyology in zoological studies, in honor of his birthday.
Translated by E. Kuznetsova
Rights and permissions
About this article
Cite this article
Malygin, V.M., Safronova, L.D., Sergeev, E.G. et al. Taxonomic Assessment of the Karyological Characteristics of the Sable (Martes zibellina) and Other Representatives of the Genus Martes (Carnivora: Mammalia). Biol Bull Russ Acad Sci 50, 416–425 (2023). https://doi.org/10.1134/S1062359023700188
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359023700188