Abstract
The effect of light-emitting diode (LED) lamps with five different spectra on L. sativa plants (variety Aficion) grown in protected soil conditions was studied. Warm white light (WW) was used as the control (the ratio of blue, green, and red portions in the spectrum was 14 : 48 : 38%). The maximum values of the leaf size and the fresh weight of the aerial parts were found in the RGB group (blue–green–red ratio 32 : 19 : 49%), and the highest values of the dry weight and ash content were in the FS group (blue–green–red ratio 27 : 11 : 62%). The stomatal size and density on the abaxial side of the leaf was the highest in SB plants (blue–green–red ratio 33 : 41 : 38%). This was the most effective combination of parameters in terms of leaf architectonics. An increase in the portion of red light in the spectrum with a decrease in the portion of green light had a positive effect on the development of L. sativa plants, while an increase in the portion of green light in the spectrum positively affected the formation of the stomatal apparatus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The intensity and spectral composition of light are factors that regulate both growth and photosynthetic reactions in the whole plant system (Protasova, 1987; Frąszczak and Kula-Maximenko, 2021). Understanding the processes of signal transmission and the formation of an adaptive response will make it possible to develop mechanisms for controlling the development potential of plants. Interest in the subject increases with the cultivation of crops. One of them is the lettuce Lactuca sativa L. This plant contains polyunsaturated fatty acids, which are important for human health (Kaur et al., 2014). The main fatty acids in lettuce are α-linolenic acid and linoleic acid, accounting for 60 and 20% of the total fatty acids, respectively (Le Guedard et al., 2008). In addition, the leaves contain elements such as Na, K, Ca, Mg, Zn, and Fe (Kawashima and Soares, 2003; Mou, 2005; Koudela and Petríková, 2008; Baslam et al., 2013); vitamin C, E, and B9 (Kim et al., 2016); carotenoids (Mou, 2005), etc. The presence of fiber in lettuce plants when eaten helps reduce the risk of cardiovascular disease, diabetes, and colon cancer (Kim et al., 2016; Shimokawa et al., 2014).
Studies of the light effect and the intensity of the spectra on different varieties of lettuce began at the end of the last century. To date, the mechanisms of light exposure have been studied in more than 40 varieties. It was determined that, depending on the variety, the reaction of plants to a different spectrum was not always the same—the synthesis of substances or growth parameters changed (Hunter and Burritt, 2004; Caldwell and Britz, 2006; Stutte et al., 2009; Shimokawa et al., 2014; Bantis et al., 2016).
When studying the effect of red light (R, 660 nm) on the development of lettuce seedlings, a strong elongation of plants was revealed. The addition of blue photons (B, 435–470 nm) to the red spectrum led to minimal elongation of the hypocotyls and leaf petioles (Hoenecke et al., 1992). The inhibitory effect on the growth of lettuce shoots using blue light and the stimulating developmental processes in red light was later confirmed (Hunter and Burritt, 2004). With constant exposure, an increase in the proportion of blue light promotes a change in the photosynthetic characteristics and growth due to the stimulation of morphological and physiological responses (Wang et al., 2016). Blue LEDs (440 nm) regulate metabolic pathways leading to increased concentrations of antioxidants in lettuce leaf tissue (Stutte et al., 2009).
Ultraviolet has a certain effect on the development of plants. Supplemental UV irradiation has been shown to increase the concentration of carotenoids and chlorophyll in green leaf lettuce while simultaneously decreasing the levels of these compounds in red leaf lettuce (Caldwell and Britz, 2006). The addition of 1% UV led to an increase in the total weight, root/shoot ratio, and total phenol content (Han et al., 2017).
A combination of light spectra of different wavelengths has been studied. Thus, the alternation of red and blue light significantly accelerated the growth of lettuce plants and increased the accumulation of fresh mass by 1.6 times than with simultaneous irradiation and by almost two times under fluorescent lighting (Shimokawa et al., 2014). Similar results have been obtained when using four lighting modes with different intervals of glowing red (R) and blue (B) light (Chen et al., 2017). R/B (8 h) and R/B (1 h) spectra were shown to result in higher yields, while R/B (4 h) and R/B (2 h) gave a higher nutritional value compared to plants grown under constant RB light (Chen et al., 2017).
The study of the effect of green light on the growth of lettuce plants is considered in several works (Kim et al., 2004a, 2004b, 2004c, 2005, 2006; Johkan et al., 2012; Mishanov et al., 2015). The authors used green LEDs with different wavelengths (G510, G520, G530) and intensities (photon flux density in the visible spectrum (PPF) of 100 and 300). The development of plants cultivated under G510 at PPF 300 was the most intensive (Johkan et al., 2012). Changing the proportion of green light in the spectrum can affect the morphometric parameters in lettuce plants (Kim et al., 2004a, 2004b, 2004c; Mishanov et al., 2015).
Despite various studies on the effect of spectra on the development of lettuce, the formation of adaptive responses in plants remains not fully understood. In addition, the optimization of lighting modes to obtain the maximum morphometric indicators of plants while maintaining product quality is relevant. The purpose of this work is to reveal the morphophysiological response of the Aficion lettuce plant variety (Lactuca sativa) during their development under the conditions of the use of LED sources of polychrome spectra.
MATERIALS AND METHODS
In this work we used the Aficion variety of lettuce (Lactuca sativa). The seeds for the experiment were provided by the Federal State Unitary Enterprise, Surazhevskii Far East Greenhouse Complex. For cultivation of plants, we used plastic containers with a volume of 10 cm3 and soil with the following content of elements: nitrogen, 160–240 mg/L; phosphorus, 145–215 mg/L; potassium, 180–290 mg/L; magnesium, 135 mg/L; humic substances, 35 mg/L; and the pH of the aqueous solution, 5.5–7 (LLC Terra Master, Novosibirsk).
Three days after sowing, the plastic containers (KV9M 0.5 L, Jetta LLC, Russia) with germinated seeds were placed in the boxes of the experimental setup located at the Laser Center of the Institute of Automation and Control Processes, Far East Branch, Russian Academy of Sciences (LC IACP FEB RAS). The installation consisted of five boxes covered inside with a reflective material, in the upper part of which there were light-emitting diode (LED) lamps with different illumination spectra (Fig. 1, Table 1). Specialized light sources were designed and manufactured at IACP FEB RAS. The light sources used matrices with a set of Chanzon (China) LEDs with a power of 3 W and a current of 700 mA each. A lamp with a spectrum of warm white light with a color temperature of 3500K (WW) was used as the control. PPF was 150 µmol/s m2. To create the same level of illumination, the radiation power of each lamp was selected using light drivers (EUM-150S105DG, China). The illumination level was monitored with a TKA-Spektr spectrophotometer (NTP TKA, Russia). In the boxes, the temperature was maintained at 21°C during the day and 18°C at night, with air humidity of 75% and a 16/8 light regime. The duration of the experiments was 35 days. The experiment was carried out in triplicate.
At the end of the experiment, 20 plants from each box were measured: number of leaves, length and width of the first three leaves in the rosette, and the wet and dry mass of the aerial part of the plant and roots. To determine the percentage of ash (ash content), the total sample of all the studied plants was used in each experiment. After drying in air-drying cabinet (SNOL-3,5.5.3,5/3,5-I5, Russia), the samples were ground in a laboratory grain mill (LZM-1, Russia) and ashed in a muffle furnace (EKPS-10, Russia) at 450°C for 8 h.
The study of leaf stomata was carried out on preparations prepared according to generally accepted methods with some modifications in relation to this object (Khrolenko et al., 2012). Casts of the epidermis were viewed and photographed under an Axioskop-40 microscope using an AxioCam HRc built-in video camera (Zeiss, Germany). The length of the guard cells of the stomata and their number per conventional unit of area (1 cm2) on both sides of the leaf blade were determined in 50 repetitions for each specimen grown under different light conditions. The counting was carried out on micrographs obtained from preparations of casts of the leaf epidermis using the Axio Vision program version 4.8.3. (for registration, processing, and analysis of images). The area for counting the number of stomata was limited by the area of the circle S = πr2 with radius r = 178.46 µm. Under the given conditions, the values were n × 103/cm2. The microscopic studies were done using equipment of the Instrumental Center for Biotechnology and Gene Engineering, Federal Scientific Center of the East Asia Terrestrial Biodiversity, FEB RAS.
All results obtained were statistically processed using the Statistica package, version 13.3. The diagrams are built on the basis of the arithmetic mean values of the measured parameters with the standard error. Comparison of the average data for plants of different variants with the control was carried out in pairs using Student’s t-test. The critical significance level was taken equal to 0.05.
RESULTS
The appearance of plants and the values of the morphometric parameters of plants are given in Table 2 and Figs. 2 and 3. When analyzing the results obtained, a tendency to an increase in the size and number of leaves in rosettes in plants of the RGB group was revealed. The smallest value of the number of leaves was noted in the SB+ group. The first leaf was significantly longer in plants of the RGB, FS, and SB+ groups, and wider and longer in the SB, RGB, and FS groups (Fig. 3). There are no significant differences in the length of the second and third sheets. Significant differences in the width of the second leaf were found in plants of the RGB and FS groups compared to the control (Fig. 3). The width of the third leaf was significantly smaller in plants of the SB+ group (Fig. 3).
The maximum values of fresh weight indicators of the aerial part were noted in plants of the RGB group (Table 2), the minimum values were observed in plants of the SB+ and control (WW) groups. At the same time, the raw weight of the roots is greater in control plants; however, no significant differences from plants of other groups were found. Plants of the FS group were characterized by close to the maximum values of the wet weight of the aerial parts, and the maximum values of the dry weight of the aerial parts and roots and ashes. The minimum values of the parameters were noted in plants of the SB+ and WW groups (control).
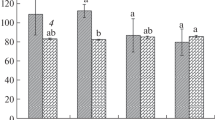
It has been found that the leaves of L. sativa have a dorsoventral, amphistomatous, and anomocytic type of stomata. The large-celled lower epidermis is single-layered, with sinuous anticlinal walls (Fig. 4). The quantitative indicators of the stomatal apparatus of lettuce leaves grown under different modes of polychromatic LED lighting are presented in Fig. 5.
Changes in the quantitative parameters of the stomata of the lower epidermis of L. sativa, grown under different LED lighting conditions: (a) number of stomata per unit leaf area (103/cm2); (b) length of guard cells of leaf stomata, µm; ▫, average value; ◻, mean value ± SE; I, mean value ± 1.96 SE. Lighting options: WW, warm white; SB is the solar spectrum; RGB, red–green–blue; FS, red–blue, SB+, modified solar spectrum.
DISCUSSION
The spectra of light at which the highest values of the studied morphometric parameters were observed in plants (number, length and width of leaves, wet and dry masses of the aerial parts and roots) contain the largest proportion of red light (49% for the RGB spectrum, 62% for FS) and the smallest proportion of green light (19% for the RGB spectrum, 11% for FS). The minimum values of the morphometric parameters were noted in plants of groups SB+ and WW (control). The shape of the spectrum of the SB+ lamp contributed to the elongation of plant leaves, which in turn affected the minimum accumulation of mass in L. sativa plants and the lower percentage of ash obtained from this experimental sample. In the SB+ and WW spectra, the shares of red light are less than those of RGB and FS and are 34% for SB+ and 38% for WW, while the shares of green light are higher at 35 and 48%, respectively. Thus, for growing lettuce plants of the Aficion variety, the proportion of green in the spectrum should probably be up to 20% and the proportion of red should be twice as much.
Similar results were obtained earlier (Kim et al., 2004a, 2004b, 2004c; Mishanov et al., 2015). Thus, when studying the influence of two spectra, it was revealed that a change in the ratio of green and red light in the spectrum of the emitter towards a decrease in green leads to an increase in the accumulation of dry and organic substances in L. sativa plants (Mishanov et al., 2015). American scientists (Kim et al., 2004a, 2004b, 2004c) when growing “Waldmann’s Green” L. sativa plants under red, blue, and green LEDs found that 24% green light in the spectrum resulted in higher wet and dry weights and greater leaf area than plants grown under cool white fluorescent lights (CWF, 51% green) or fluorescent blue (0% green) light. An excess (86%) of green light in the spectrum resulted in the smallest mass of plants. Later, the same authors found that light sources with more than 50% green in the spectrum cause a decrease in the growth rate of plants, while combinations including up to 24% green accelerate growth (Kim et al., 2005, 2006).
The narrowest leaves were noted in the control group (WW). In this spectrum, the proportion of blue light is minimal and amounts to 14%, while in all other spectra its proportion is higher, 26–32% (Table 1). Previously, when studying the influence of blue compared to high intensity red light (500 and 1000 mmol/s m2) revealed that the change in the proportion of blue light (from 0 to 6%) contributed to an increase in the leaf area. By this fact the authors explained the increase size of cell by 3.1 times and the frequency of cell division by 1.6 times (Dougher and Bugbee, 2004).
The spectral composition of the light affects photosynthesis and stomata conductance. Thus, it has been shown that the photosynthesis rate in the leaf and stomatal conductance were higher in plants grown under white fluorescent lights (CWF) than in plants grown under red LEDs with or without additional blue light (Yorio et al., 2001).
In our experiment, when studying the stomatal apparatus of leaves, it was revealed that the total surface density of the distribution of stomata per unit area of the leaf ranged from 12 000/cm2 (SB+) up to 19 000/cm2 (SB). These values fall within the range characteristic of sciophytic plants, which are typical representatives of the undergrowth. This is also found in mesophytic and economically valuable plant species, as we have shown earlier (Nakonechnaya et al., 2022).
In this work, there is a statistically significant (p < 0.01) increase in the surface density of stomata distribution on the abaxial side of the leaves in the SB, SB+, and FS variants (Fig. 5а). In plants of the RGB group, the change in this indicator is not statistically significant compared to the control. Similar results were obtained by Chinese authors. When cultivating potato microplants under FL (Fluorescent white light), there was an increase in the distribution density of stomata on the abaxial side of the leaf (Ma et al., 2015). The leaves of lettuce plants (Lactuca sativa L.), grown at a ratio of the red and blue spectra R/B = 1, the surface density of stomata distribution increased significantly with a slight decrease in the size of the stomata themselves and, as a result, these plants had the highest photosynthesis rate (Wang et al., 2016).
The size of the stomatal guard cells in the SB+ and FS groups corresponded to the control (differences are not significant) or were slightly higher than that. An interesting fact is that the largest stomata in the experiment were observed in samples of the SB variant (differences from the control are significant p < 0.01) (Fig. 5b) at the highest density of stomata distribution on the leaf. This, contrary to what was expected, does not confirm the regularity known to plants—the presence of a negative correlation between the length of stomatal guard cells and their number (Jones et al., 1987). However, the combination of parameters of the stomatal apparatus, as revealed in the SB group of plants, is the most effective in terms of the architectonics of the photosynthetic leaf.
The resistance to diffusion of gas into the leaf is the sum of the stomatal and mesophilic resistances. The stomatal resistance is determined by factors such as the degree of opening of the stomata, the number of stomata per unit leaf area, etc. An increase in the surface distribution density of stomata should allow plants under conditions of sufficient moisture to increase the conductivity of leaf gas exchange and thus avoid a limitation of photosynthesis (Mokronosov and Gavrilenko, 1992). There is no clear evidence in the literature of a correlation between certain spectra and the parameters of plant stomata. Thus, Japanese authors found that, under the influence of the blue spectrum, the surface density of the distribution of stomata of microplants of different grape varieties increases, and a change in the size of the guard cells of stomata under this spectrum revealed varietal specificity (Poudel et al., 2008). At the same time, Chinese authors showed that the highest stomata distribution density on the leaf and the largest stomata in cotton microplants were treated with red and blue spectra (Li et al., 2010).
CONCLUSIONS
When studying the effect of five spectra of LED lamps on plant morphology of the Aficion varieties of L. sativa, grown in closed soil conditions, it was revealed that the proportion of light of a certain wavelength, as well as the ratio of the fractions of wavelengths in the spectrum of lamps, can affect the development of plants. To get developed L. sativa plants, in the spectrum of illumination, the share of green light should not exceed 20%, the share of red should be 50–60%, and the share of blue should be about 30%. An increase in the proportion of green and a decrease in the proportion of blue light in the spectrum causes plants to stretch. As a result of this study, it was found that the influence of the spectral composition of light SB+, SB, and FS stimulated the formation of stomata. A regular decrease in the length of the guard cells of stomata was revealed in the same variants, with the exception of SB, in which the largest stomata were observed with the maximum density of their distribution on the leaf. Thus, the data obtained suggest that plants of the SB+, SB, and FS variants have the lowest stomatal conductance and, accordingly, a potentially higher level of photosynthesis.
REFERENCES
Bantis, F., Ouzounis, T., and Radoglou, K., Artificial led lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success, Sci. Hortic., 2016, vol. 198, pp. 277–283.
Baslam, M., Morales, F., Garmendia, I., and Goicoechea, N., Nutritional quality of outer and inner leaves of green and red pigmented lettuces (Lactuca sativa L.) consumed as salads, Sci. Hortic., 2013, vol. 151, pp. 103–111.
Caldwell, C.R. and Britz, S.J., Effect of supplemental ultraviolet radiation on the carotenoid and chlorophyll composition of green house-grown leaf lettuce (Lactuca sativa L.) cultivars, J. Food Compos. Anal., 2006, vol. 19, nos. 6–7, pp. 637–644.
Chen, X.L., Yang, Q.C., Song, W.P., Wang, L.C., Guo, W.Z., and Xue, X.Z., Growth and nutritional properties of lettuce affected by different alternating intervals of red and blue led irradiation, Sci. Hortic., 2017, vol. 223, pp. 44–52.
Dougher, T.A. and Bugbee, B., Long-term blue light effects on the histology of lettuce and soybean leaves and stems, J. Am. Soc. Hortic. Sci., 2004, vol. 129, no. 4, pp. 467–472.
Frąszczak, B. and Kula-Maximenko, M., The preferences of different cultivars of lettuce seedlings (Lactuca sativa L.) for the spectral composition of light, Agronomy, 2021, vol. 11, no. 6, p. 1211.
Le Guedard, M., Schraauwers, B., Larrieu, I., and Bessoule, J.-J., Development of a biomarker for metal bioavailability: the lettuce fatty acid composition, Environ. Toxicol. Chem., 2008, vol. 27, no. 5, pp. 1147–1151.
Han, T., Vaganov, V., Cao, S., Li, Q., Ling, L., Cheng, X., Peng, L., Zhang, C., Yakovlev, A.N., Zhong, Y., and Tu, M., Improving “color rendering” of led lighting for the growth of lettuce, Sci. Rep., 2017, vol. 7, p. 45944.
Hoenecke, M.E., Bula, R.J., and Tibbitts, T.W., Importance of “blue” photon levels for lettuce seedlings grown under red-light-emitting diodes, HortScience, 1992, vol. 27, no. 5, pp. 427–430.
Hunter, D.C. and Burritt, D.J., Light quality influences adventitious shoot production from cotyledon explants of lettuce (Lactuca sativa L.), In Vitro Cell. Dev. Biol., Plant, 2004, vol. 40, no. 2, pp. 215–220.
Johkan, M., Shoji, K., Goto, F., Hahida, S.N., and Yoshihara, T., Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa, Environ. Exp. Bot., 2012, vol. 75, pp. 128–133.
Jones, H.G., Farquar, G.D., and Cowan, I.R., Breeding for stomatal characters, in Stomatal Function, Zeiger, E., Farquhar, G.D., and Cowan, I.R., Eds., Stanford, CA: Stanford Univ. Press, 1987, pp. 431–443.
Kaur, N., Chugh, V., and Gupta, A.K., Essential fatty acids as functional components of foods—a review, J. Food Sci. Technol., 2014, vol. 51, no. 10, pp. 2289–2303.
Kawashima, L.M. and Soares, L.M.V., Mineral profile of raw and cooked leafy vegetables consumed in southern Brazil, J. Food Compos. Anal., 2003, vol. 16, no. 5, pp. 605–611.
Khrolenko, Yu.A., Burundukova, O.L., Burkovskaya, E.V., and Zhuravlev, Yu.N., Mesophyll structure and chloroplast density in Panax ginseng leaves from the Sikhote-Alin Mts, Acta Biol. Cracov., Ser. Bot., 2012, vol. 54, no. 2, pp. 54–60.
Kim, H.H., Goins, G.D., Wheeler, R.M., and Sager, J.C., A comparison of growth and photosynthetic characteristics of lettuce grown under red and blue light-emitting diodes (LEDs) with and without supplemental green leds, Acta Hort., 2004a, vol. 659, pp. 467–475.
Kim, H.H., Goins, G.D., Wheeler, R.M., and Sager, J.C., Green-light supplementation for enhanced lettuce growth under red- and bluelight-emitting diodes, HortScience, 2004b, vol. 39, pp. 1617–1622.
Kim, H.H., Goins, G.D., Wheeler, R.M., and Sager, J.C., Stomatal conductance of lettuce grown under or exposed to different light qualities, Ann. Bot. (Lond.), 2004c, vol. 94, pp. 691–697.
Kim, H.H., Wheeler, R.M., Sager, J.C., Gains, G.D., and Naikane, J.H., Evaluation of lettuce growth using supplemental green light with red and blue light-emitting diodes in a controlled environment-a review of research at Kennedy Space Center, in V International Symposium on Artificial Lighting in Horticulture, 2005, vol. 711, pp. 111–120.
Kim, H.H., Wheeler, R.M., Sager, J.C., Goins, G.D., and Norikane, J.H., Evaluation of lettuce growth using supplemental green light with red and blue light-emitting diodes in a controlled environment—a review of research at Kennedy Space Center, Acta Hort., 2006, vol. 711, pp. 111–119.
Kim, M.J., Moon, Y., Tou, J.C., Mou, B., and Waterland, N.L., Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.), J. Food Compos. Anal., 2016, vol. 49, pp. 19–34.
Koudela, M. and Petríková, K., Nutrients content and yield in selected cultivars of leaf lettuce (Lactuca sativa L. var. crispa), Hortic.Sci, 2008, vol. 35, no. 3, pp. 99–106.
Li, H., Xu, Z., and Tang, C., Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro, PCTOC, 2010, vol. 103, no. 2, pp. 155–163.
Ma, X., Wang, Y., Liu, M., Xu, J., and Xu, Z., Effects of green and red lights on the growth and morphogenesis of potato (Solanum tuberosum L.) plantlets in vitro, Sci. Hortic., 2015, vol. 190, pp. 104–109.
Mishanov, A.P., Markova, A.E., Rakut’ko, S.A., Brovtsin, V.N., and Rakut’ko, E.N., Influence of the ratio of the shares of green and red radiation on the biometric indicators of lettuce, Sb. Nauchn. Tr. IAEP, 2015, no. 87, pp. 264–271.
Mokronosov, A.T. and Gavrilenko, V.F., Fotosintez. Fiziologo-ekologicheskie i biokhimicheskie aspekty (Photosynthesis. Physiological-Ecological and Biochemical Aspects), Moscow: Mosk. Gos. Univ., 1992.
Mou, B., Genetic variation of beta-carotene and lutein contents in lettuce, J. Am. Soc. Hortic. Sci., 2005, vol. 130, no. 6, pp. 870–876.
Nakonechnaya, O.V., Kholin, A.S., Subbotin, E.P., Grishchenko, O.V., Burkovskaya, E.V., Khrolenko, Yu.A., Burdukovskii, M.L., Kul’chin, Yu.N., and Zhuravlev, Yu.N., Development of tomato plants under illumination of different spectral composition, Russ. J. Plant Physiol., 2022, vol. 69, no. 5.
Poudel, P.R., Kataoka, I., and Mochioka, R., Effect of red- and blue-light-emitting diodes on growth and morphogenesis of grapes, Plant Cell, Tiss. Organ Cult., 2008, vol. 92, no. 2, pp. 147–153.
Protasova, N.N., Light culture as a way to identify the potential productivity of plants, Fiziol. Rast., 1987, vol. 34, no. 4, pp. 812–822.
Shimokawa, A., Tonooka, Y., Matsumoto, M., Ara, H., Suzuki, H., Yamauchi, N., and Shigyo, M., Effect of alternating red and blue light irradiation generated by light emitting diodes on the growth of leaf lettuce, bioRxiv, 2014, p. 003103.
Stutte, G.W., Edney, S., and Skerritt, T., Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes, HortScience, 2009, vol. 44, no. 1, pp. 79–82.
Wang, J., Lu, W., Tong, Y., and Yang, Q., Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light, Front. Plant Sci., 2016, vol. 7, pp. 64–73.
Yorio, N.C., Goins, G.D., Kagie, H.R., Wheeler, R.M., and Sager, J.C., Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation, HortScience, 2001, vol. 36, no. 2, pp. 380–383.
Funding
This work was carried out within the framework of the state assignment of the Ministry of Science and Higher Education of the Russian Federation (project mo. 121031000144-5) and supported by grant no. 20-16-00016 from the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Nakonechnaya, O.V., Kholin, A.S., Subbotin, E.P. et al. The Influence of LED Lights of Different Spectra on the Development of Lactuca sativa. Biol Bull Russ Acad Sci 50, 371–378 (2023). https://doi.org/10.1134/S1062359023700176
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359023700176