Abstract
The reproductive strategies of reptiles vary according to many factors. In lizards, intraspecific and interspecific competition, and environmental and seasonal factors affect their reproductive biology. This study aimed to conduct a comparative analysis of testis sizes in the sympatrically coexisting species, Podarcis muralis and Podarcis tauricus, located in Kofçaz, Kırklareli, Turkey, considering both intra-species and inter-species variations. Changes in body sizes and testis sizes according to months were monitored. As a result, it was determined that in both species, there was a positive correlation between body size and testis size, and that in P. tauricus, both body and testis sizes were higher compared to P. muralis, showing a significant difference. It was also observed that in the P. muralis species, the testis size reached its peak value in April (mean daily temperature 9.9°C), while in the P. tauricus species, it reached the highest value in May (mean daily temperature 15.5°C). It was found that the testis size in P. muralis reached its lowest value in August (mean daily temperature 23.8°C), while in P. tauricus, it was lowest in September (mean daily temperature 20.2°C). The fact that testis size is high in different months in these two sympatric species suggests that their active breeding periods differ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Reproductive biology and reproductive behaviors can significantly differ over temporal and spatial scales in response to various ecological and environmental conditions (Castilla and Bauwens, 2000; Lukanov and Tzankov, 2016). Studies on lizard ecology and evolution require a fundamental understanding of their reproductive and life history traits, which is also essential for their conservation status (Cree and Hare, 2016). The diversity in reproductive and life history traits among lizard species, which are components of reproductive biology, is partially explained by the physical environment (Benabib, 1994). There have been numerous studies on various aspects of testis in relation to lizard species, including their size, volume, sperm production, histological structure, seasonal changes, and relationships with body sizes (Bostic, 1966; Boretto and Ibargüengoytía, 2006; Cardone et al., 2008; Todd, 2008; Kahrl and Cox, 2015; Sagonas et al., 2019; Pizarro et al., 2022).
Sperm morphology and testis size exhibit significant variability within species (Kahrl and Cox, 2017) and among species of lizards (Uller et al., 2010), but the extent of variation among populations remains unknown (Kahrl and Cox, 2017). Increased testis mass can be associated with elevated sperm production and may be indicative of a mating strategy involving multiple matings with one or many females (Naretto et al., 2016). Body size serves as an important signal of mate quality in many species (Andersson, 1994) and can provide advantages to animals involved in agonistic interactions (Keogh et al., 2013). To better understand the evolutionary and environmental determinants of reproductive diversity, it is essential to investigate closely related taxa, sympatric taxa, and allopatric ones (Rheubert et al., 2020). Sympatric conspecifics can differ in terms of breeding timing, size, age of maturity, or clutch size (Ballinger, 1973; Barbault, 1976; Tinkle and Dunham, 1986; James, 1991). Moreover, sympatry can lead to differences in inter-species interactions, pre- and post-mating competition traits, or reproductive strategies (Naretto et al., 2016). Studies on the reproductive biology of sympatric lizards have been conducted (Lin and Nelson, 1981; Taylor, 2004; Carretero et al., 2006; Ramirez-Pinilla et al., 2009; Naretto et al., 2016; Kahrl and Cox, 2017). Differences in secondary sexual characteristics, relative testis mass, and sperm component length have been reported between allopatric and sympatric Salvator sp. lizards (Naretto et al., 2016). Despite numerous studies examining the morphology of various components of the reproductive system, there are few studies that have investigated sympatric squamates (Rheubert et al., 2020). Determining the extrinsic and intrinsic interactions in the reproductive biology of sympatric species is important for monitoring and conserving these species. Therefore, this study focused on Podarcis muralis (Laurenti, 1768) and Podarcis tauricus (Pallas, 1814), which are sympatrically distributed in Kofçaz, Kırklareli, Thrace region, Türkiye.
This study aimed to conduct a comparative examination of testis sizes (testis weight, length, width, volume) and body sizes (body weight and snout-vent length) of the sympatrically living species Podarcis muralis and Podarcis tauricus, both within and between the species, across different months.
MATERIALS AND METHODS
Study Areas and Data Collection
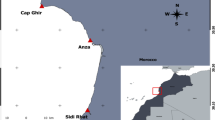
We collected 44 male lizards of P. muralis and 35 male P. tauricus from Kırklareli/Kofçaz (Fig. 1). The lizards were captured by hand between April and September 2023. The mean daily temperatures for the months (April–September) are as follows: 9.9, 15.5, 19.0, 27.0, 23.8, 20.2°C, respectively (Kofçaz, Meteoroloji Genel Müdürlüğü [General Directorate of Meteorology]). The samples obtained from previous studies related to these species were also used.
Morphological Measurements and Indexes
We conducted measurements on all specimens, including snout-vent length (SVL), body weight (BW), and the month of capture. The body measurements were taken with a digital caliper accurate to 0.01 mm, and weights were recorded using a digital scale with a precision of 0.01 g. For the male individuals, measurements were focused on the right testicle, including testicular width, testicular length, and testicular weight, which was measured using a sensitive scale accurate to 0.1 mg. The testicle’s volume was approximated as an ellipsoid using the formula: V = (4/3) πab2, where “a” represents half of the testicle’s length, and “b” represents half of the testicle’s width, as described by Mayhew in 1963.
Statistical Analyses
All the data collected from the specimens were analyzed using the SPSS software (version 26). To assess the normality of the data, the Kolmogorov–Smirnov Test was employed. For comparisons between species, the Student’s t-test was utilized for parametric data, while the Mann–Whitney U Test was applied for nonparametric data. Scatterplots and boxplots were generated using R Studio.
RESULTS
All the descriptive statistics for the measurements taken from a total of 44 P. muralis and 37 P. tauricus samples are provided in detail in Table 1.
When the individuals of the two species were compared, a statistically significant difference was observed in BW (Mann–Whitney test; Z: –2.190, p: 0.029) and SVL (Mann–Whitney test; Z: –2.944, p: 0.003) measurements. It was determined that Podarcis tauricus was both heavier and larger than Podarcis muralis.
Statistically significant differences were found in testicular weight (t-test; t: 2.469, p: 0.016), testicular width (Mann–Whitney test; Z: –2.257, p: 0.024), and testicular volume (t-test; t: 2.929, p: 0.004) measurements, while there was no significant difference in testicular length (t-test; t: 1.895, p: 0.062). Similar to morphological measurements, individuals of the Podarcis tauricus species had larger testicular measurements compared to those of the Podarcis muralis species.
While in the Podarcis muralis species, SVL and testicular volume did not show a significant correlation (r: 0.207, p: 0.177), in the Podarcis tauricus species, a significant positive correlation was observed (r: 0.479, p: 0.003). It was determined that as SVL increased in Podarcis tauricus, testicular volume also increased (Fig. 2).
When examining BW and testicular volume, both Podarcis muralis (r: 0.300, p: 0.048) and Podarcis tauricus (r: 0.497, p: 0.002) exhibited positive correlations. It was observed that in both species, as body weight increased, testicular volume also increased (Fig. 3).
When examining BW and testicular weight, both Podarcis muralis (r: 0.417, p: 0.005) and Podarcis tauricus (r: 0.485, p: 0.002) exhibited positive correlations (Fig. 4).
In both species, there were significant differences in testis volumes between the months of June and July (P. muralis p: 0.008; P. tauricus p: 0.003). It was observed that in both Podarcis muralis and Podarcis tauricus, testis volumes decreased after the end of the breeding season in June (Fig. 5). In Podarcis muralis, a gradual decline was observed from April, while in Podarcis tauricus, there was a decrease after reaching its highest level in May following a lower point in April.
DISCUSSION
The snout-vent length of mature individuals of the P. muralis species was reported to be 54 cm (Sacchi et al., 2012). However, the data we obtained indicates that the reproductive activity and sexual maturity of this species start from 50 cm. For the P. tauricus species, it was reported that mature individuals were larger than 53 cm (Altunışık et al., 2016). Significant differences were found in the SVL and BW values between the two populations showing sympatric distribution, with P. tauricus being larger than P. muralis.
Previous studies reported a positive correlation between body size and testis size (Aleksic and Ljubisavljevic, 2001; Taylor, 2004; Todd, 2008; Ramírez-Pinilla et al., 2009; Sagonas et al., 2019). In our study, we also found positive correlations between body size and testis size in both species. Testicular weight, testicular width, and testicular volume values showed significant differences between the two sympatric species, with P. tauricus having higher values. In sympatry, interspecific interactions can lead to convergence in traits related to pre- and post-copula competition or divergence in reproductive strategies (Naretto et al., 2016).
It was noted that testis mass and testis volume exhibited significant variations between months (Sagonas et al., 2019) and were related to precipitation and temperature (Ramírez-Pinilla et al., 2009). In both species emerging from hibernation, P. muralis had the highest testis volumes in April (mean daily temperature 9.9°C), while P. tauricus reached its peak in May (mean daily temperature 15.5°C). The lowest testis volumes were observed in P. muralis in August (mean daily temperature 23.8°C) and in P. tauricus in September (mean daily temperature 20.2°C). It was reported that in P. muralis, the heaviest testis mass was observed in April, while the lightest was in July (Aleksic and Ljubisavljevic, 2001). Additionally, in the case of Phoenicolacerta kulzeri, it was reported that in the hottest months, July and August, testis volume decreased in 40% of males (Rizk and Nassar, 2015). After reaching peak testis volume, both species show a decline until the month of August. The males were found to be reproductive from August to December, with the maximum testis volume in both Ctenotus robustus and C. taeniolatus species occurring in early spring, while the minimum testis volume was recorded in early autumn (Taylor, 2004). Previous studies reported a decrease in testis size in lizard species after reaching peak levels (Pianka and Parker, 1975; Ortiz et al., 2001). According to our data, there was a slight increase in P. muralis during the September period. In Anolis trinitatis, it was reported that the fat body data increased after August, following a decrease in testis, indicating an increase in fat levels a few months after the decrease (Licht and Gorman, 1970). Given the correlation between body size and testis volume, it can be suggested that the increase in fat before hibernation in September results in an increase in testis volume.
Reproductive activity in lizards may be controlled by complex interactions involving phylogenetic, physiological, and environmental factors (Angelini et al., 1976, 1978; Saint Girons, 1984; Carretero, 2006). It was reported that P. tauricus was active during the reproductive period at a temperature of 18°C (Fischer et al., 2019), while P. muralis was active at 18.1°C (Eroğlu et al., 2018). According to the testis volume, it can be suggested that P. tauricus is in the reproductive period at 15.5°C, and P. muralis at 9.9°C, indicating potential differences in sympatric settings. Differences in some reproductive characteristics among populations of Salvator merianae and S. rufescens were reported based on social contexts (sympatry, allopatry, social contexts), suggesting that they may be subjected to different selective pressures caused not only by the presence of competing species but also by the presence of mates and rivals (Naretto et al., 2016).
The gradual decline in the testis volume of the Podarcis muralis species, as opposed to the fluctuating decline in the Podarcis tauricus species, may reflect differences in reproductive strategies between the two sympatric species. Additionally, the peak in testis size occurring in April for P. muralis and in May for P. tauricus under different seasonal conditions suggests that their active breeding periods differ.
REFERENCES
Aleksic, I. and Ljubisavljevic, K., Reproductive cycle in the common wall lizard (Podarcis muralis) from Belgrade, Arch. Biol. Sci., 2001, vol. 53, pp 73–81.
Altunışık, A., Kalaycı, T.E., Uysal, İ., Tosunoğlu, M., and Özdemir, N., Age, adult survival rate, and adult life expectancy of a Podarcis tauricus population (Reptilia: Lacertidae) from Saros Bay, Turkey, Russ. J. Herpetol., 2016, vol. 23, no. 4, pp. 278–282.
Andersson, M., Sexual Selection, Princeton, NJ, USA: Princeton University Press, 1994.
Angelini, F., Picariello, O., and Botte, V., Influence of photoperiod and temperature on the testicular activity of the lizard, Lacerta s. sicula Raf, Ital. J. Zool., 1976, vol. 43, pp. 111–123. https://doi.org/10.1080/11250007609434890
Angelini, F., D’uva, V., Picariello, O., and Ciarcia, G., Effects of mammalian gonadotrophs and testosterone on the male sexual cycle of the lizard (Lacerta s. sicula Raf.) During the autumn spermatogenesis, Monitore Zoologico Italiano, 1978, vol. 12, pp. 117–141. https://doi.org/10.1080/00269786.1978.10736313
Ballinger, R.E., Comparative demography of two viviparous iguanid lizard (Sceloporus jarrovi and Sceloporus poinsetti), Ecology, 1973, vol. 54, no. 2, pp. 269–283. https://doi.org/10.2307/1934336
Barbault, R., Population dynamics and reproduction patterns of three African skinks, Copeia, 1976, no. 3, pp. 483–490. https://doi.org/10.2307/1443363
Benabib, M., Reproduction and lipid utilization of tropical populations of Sceloporus variabilis, Herpetol. Monogr., 1994, vol. 8, pp. 160–180. https://doi.org/10.2307/1467079
Boretto, J. and Ibargüengoytía, N., Asynchronous spermatogenesis and biennial female cycle of the viviparous lizard Phymaturus antofagastensis (Liolaemidae): reproductive responses to high altitudes and temperate climate of Catamarca, Argentina, Amphibia–Reptilia, 2006, vol. 27, no. 1, pp. 25–36. https://doi.org/10.1163/156853806776052119
Bostic, D.L., A preliminary report of reproduction in the teiid lizard, Cnemidophorus hyperythrus beldingi, Herpetologica, 1966, vol. 22, no. 2, pp. 81–90.
Cardone, A., Comitato, R., and Angelini, F., Spermatogenesis, epididymis morphology and plasma sex steroid secretion in the male lizard Podarcis sicula exposed to diuron, Environ. Res., 2008, vol. 108, no. 2, pp. 214–223. https://doi.org/10.1016/j.envres.2008.07.011
Carretero, M.A., Reproductive cycles in Mediterranean lacertids: plasticity and constraints, in Reproductive Cycles in Mediterranean Lacertids, 2006, pp. 1000–1022. https://doi.org/10.1400/73922
Carretero, M., Ribeiro, R., Barbosa, D., Sá-Sousa, P., and Harris, D.J., Spermatogenesis in two Iberian Podarcis lizards: relationships with male traits, Anim. Biol., 2006, vol. 56, no. 1, pp. 1–12. https://doi.org/10.1163/157075606775904759
Castilla, A.M. and Bauwens, D., Reproductive characteristics of the lacertid lizard Podarcis atrata, Copeia, 2000, vol. 2000, no. 3, pp. 748–756. https://doi.org/10.1643/0045-8511(2000)000[0748:RCOTLL]2.0.CO;2
Cree, A. and Hare, K.M., Reproduction and life history of New Zealand lizards, in New Zealand Lizards, 2016, pp. 169–206. https://doi.org/10.1007/978-3-319-41674-8_7
Eroğlu, A.İ., Bülbül, U., Kurnaz, M., and Odabaş, Y., Age and growth of the common wall lizard, Podarcis muralis (Laurenti, 1768), Anim. Biol., 2018, vol. 68, no. 2 pp. 147–159. https://doi.org/10.1163/15707563-17000019
Fischer, D., Babická, K., Fischerová, J., Lerch, Z., Mikátová, B., Reiter, A., and Rehák, I., Discovery of the Podarcis tauricus population in the Czech Republic (Squamata: Lacertidae), Acta Soc. Zool. Bohem., 2019, vol. 83, pp. 239–254.
James, C.D., Population dynamics, demography, and life history of sympatric scincid lizards (Ctenotus) in central Australia, Herpetologica, 1991, vol. 47, no. 2, pp. 194–210.
Kahrl, A.F. and Cox, R.M., Diet affects ejaculate traits in a lizard with condition-dependent fertilization success, Behav. Ecol., 2015, vol. 26, no. 6, pp. 1502–1511. https://doi.org/10.1093/beheco/arv105
Kahrl, A.F., and Cox, R.M., Consistent differences in sperm morphology and testis size between native and introduced populations of three Anolis lizard species, J. Herpetol., 2017, vol. 51, no. 4, pp. 532–537. https://doi.org/10.1670/16-184
Keogh, J.S., Umbers, K.D., Wilson, E., Stapley, J., and Whiting, M.J., Influence of alternate reproductive tactics and pre- and postcopulatory sexual selection on paternity and offspring performance in a lizard, Behav. Ecol. Sociobiol., 2013, vol. 67, pp. 629–638. https://doi.org/10.1007/s00265-013-1482-0
Licht, P. and Gorman, G. C., Reproductive and Fat Cycles in Caribbean Anolis Lizards, Berkeley: University of California Press, 1970.
Lin, J.Y. and Nelson, C.E., Comparative reproductive biology of two sympatric tropical lizards Chamaeleo jacksonii Boulenger and Chamaeleo hoehnelii Steindachner (Sauria: Chamaeleonidae), Amphibia–Reptilia, 1981, vol. 3, no. 4, pp. 287–311. https://doi.org/10.1163/156853881X00393
Lukanov, S. and Tzankov, N., Life history, age and normal development of the Balkan–Anatolian crested newt (Triturus ivanbureschi Arntzen and Wielstra, 2013) from Sofia district, North-West. J. Zool., 2016, vol. 12, no. 1, pp. 22–32.
Mayhew, W.W., Reproduction in the granite spiny lizard, Sceloporus orcutti, Copeia, 1963, pp. 144–152. https://doi.org/10.2307/1441282
Naretto, S., Blengini, C.S., Cardozo, G., and Chiaraviglio, M., Pre- and postcopulatory traits of Salvator male lizards in allopatry and sympatry, Scientifica, 2016, vol. 2016. https://doi.org/10.1155/2016/8176267
Ortiz, M.F., De Oca, A.N.M., and Ugarte, I.H.S., Diet and reproductive biology of the viviparous lizard Sceloporus torquatus torquatus (Squamata: Phrynosomatidae), J. Herpetol., 2001, vol. 35, no. 1, pp. 104–112. https://doi.org/10.2307/1566029
Pianka, E.R. and Parker, W.S., Ecology of horned lizards: a review with special reference to Phrynosoma platyrhinos, Copeia, 1975, vol. 1975, no. 1, pp. 141–162. https://doi.org/10.2307/1442418
Pizarro, J.E., Laspiur, A., Acosta, J.C., Blanco, G.M., and Boretto, J.M., High reproductive effort in a vulnerable lizard from high altitudes in Argentina: reproductive biology and sexual dimorphism in Phymaturus extrilidus, An. Acad. Bras. Ciênc., 2022, vol. 94, no. 4. https://doi.org/10.1590/0001-3765202220210179
Ramírez-Pinilla, M.P., Calderón-Espinosa, M.L., Flores-Villela, O., Muñoz-Alonso, A., and de la Cruz, F.R.M., Reproductive activity of three sympatric viviparous lizards at Omiltemi, Guerrero, Sierra Madre del Sur, Mexico, J. Herpetol., 2009, vol. 43, no. 3, pp. 409–420.
Rheubert, J., Pasternak, M.A., Ely, M., Siegel, D.S., T-rauth, S.E., Gribbins, K.M., and Sever, D.M., Seasonal histology and ultrastructure of the urogenital system in two sympatric lizards, J. Zool., 2020, vol. 310, no. 4, pp. 273–286. https://doi.org/10.1111/jzo.12748
Rizk, K. and Nassar, F., Male reproduction cycle of Kulzer’s Rock Lizard, Phoenicolacerta kulzeri (Müller & Wettstein, 1932), in Lebanon (Reptilia: Lacertidae), Zool. Middle East, 2015, vol. 61, no. 4, pp. 318–323. https://doi.org/10.1080/09397140.2015.1101920
Sacchi, R., Pellitteri-Rosa, D., Capelli, A., Ghitti, M., Di Paoli, A., Bellati, A., Scali, S., Galeotti, P., and Fasola, M., Studying the reproductive biology of the common wall lizard using ultrasonography, J. Zool., 2012, vol. 287, no. 4, pp. 301–310. https://doi.org/10.1111/j.1469-7998.2012.00917.x
Sagonas, K., Pafilis, P., Lymberakis, P., and Valakos, E.D., Sexual maturation and reproduction of the Balkan green lizard Lacerta trilineata specimens in mainland and island populations from Greece, North-West. J. Zool., 2019, vol. 15, no. 1, pp. 55–61.
Saint Girons, H., Les cycles sexuels des lézards mâles et leurs rapports avec le climat et les cycles reproducteurs des femelles, Ann. Sci. Nat. (Zool. Biol. Anim.), 1984, vol. 6, no. 4.
Taylor, J.E., Reproduction in sympatric lizards: comparison of two species of Ctenotus (Scincidae) in south-eastern Australia, Aust. J. Zool., 2004, vol. 52, no. 6, pp. 649–666.
Tinkle, D.W. and Dunham, A.E., Comparative life histories of two syntopic sceloporine lizards, Copeia, 1986, vol. 1986, no. 1, pp. 1–18. https://doi.org/10.2307/1444882
Todd, A.C., Using testis size to predict the mating systems of New Zealand geckos, N. Z. J. Zool., 2008, vol. 35, no. 2, pp. 103–114. https://doi.org/10.1080/03014220809510107
Uller, T., Stuart-Fox, D., and Olsson, M., Evolution of primary sexual characters in reptiles, in The Evolution of Primary Sexual Characters in Animals, UK: Oxford University Press, 2010.
Funding
This work was supported by Çanakkale Onsekiz Mart University, The Scientific Research Coordination Unit, Project no. FBA-2023-4348.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
For the conducted studies, the necessary permissions were obtained from the Ethics Committee of Animal Experiments of Çanakkale Onsekiz Mart University (decision no. 2023/05-01).
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baycan, B., Kurtul, D., Boran, B. et al. Testicular Morphologies of Two Sympatric Podarcis Species. Biol Bull Russ Acad Sci 51, 1106–1112 (2024). https://doi.org/10.1134/S106235902360592X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106235902360592X