Abstract
In this study, the effects of different concentrations of carbohydrates on the phenoloxidase activity (PO) and development of Ephestia kuehniella larvae were investigated. For this purpose, 10 artificial diets-containing different carbonhydrates (sucrose or arabinose) were prepared. According to results, development and immunity are related to each other. A negative correlation was determined between the pupa mass, the amount of pupal lipid and the amount of pupal crude protein, and PO activity for all carbohydrate types. One of the remarkable results of the study is that the larvae fed on the 5A:P diet failed to reach the pupal stage. Diets-containing arabinose apart from 1A:P diet were consumed less than sucrose-containing diets. The PO activities of the larvae differ according to the dietary nutritional imbalance and the type of carbohydrates of diet. Except for 1A:3P and 1A:5P diets, the larvae fed on diets-containing arabinose generally have higher PO activities. The results show that arabinose is toxic to this species or that the carbohydrate is not usable by the larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Diet quality and quantity are key determinants of insect development and immunity (Klemola et al., 2007; Mason et al., 2014; Barbehenn et al., 2015; Borzoui et al., 2017). Nutrient balance, protein, carbohydrate, secondary metabolites, and water are among the factors determining the diet quality (Kraus et al., 2019). Proteins have many physiological functions such as structural support in animals, growth, transport of other compounds, signal transduction, and immunity (Matson, 1980). Carbohydrates are the first source of energy and carbon in most organisms. They also participate in the structure of DNA, RNA, and ATP. Also, they serve as respiratory fuel, provide the carbon basis in molecular synthesis, and constitute building materials for the insect cuticle (Kraus et al., 2019). Not all sugars are usable by all insects and some monosaccharides can be toxic because they compete with other essential sugars (e.g.,mannose blocks glucose pathway in bees) (Kraus et al., 2019). For example, arabinose is a carbohydrate that is toxic to many insect species, inhibits the growth of larvae, and causes death (Rockstein, 1978; Hu et al., 2010). The digestive capability for carbohydrates also varies among insect species.
Insects balance their nutrient intake by changing their consumption or their physiology when their diet is unbalanced (Kraus et al., 2019). Failure to reach the nutrient target in insects affects the physiology, reproduction, and development of the individuals (Borzoui et al., 2017). Unbalanced diets also affect the immune system of individuals (Sivrikaya et al., 2020).
Invertebrates have developed an innate immune system because they lack the adaptive immune system features (e.g. T cells) (Sharrock and Sun, 2020). The innate immune system also contains many specific and non-specific responses. One of the important elements of these reactions is the phenoloxidase enzyme (PO). This enzyme is responsible for the activation of melanogenesis in invertebrates. Also, the phenoloxidase enzyme has many functions: Sclerotization of insect cuticle (Asano et al., 2019); encapsulation and melanization of foreign organism (Sadawarte et al., 2019; Schwier et al., 2021) and wound healing (Ankola et al., 2018).
Nutrition and immunity are related to each other (Cotter et al., 2011; Kangassalo et al., 2018). Immune functions are costly, and immune investment is usually dependent on the individual’s condition and resource availability (Green, 2021). Lee et al. (2008) stated that protein quality does not affect phenoloxidase activity. However, there are also studies in the literature that suggest that protein-poor diets suppress immunity (Srygley et al., 2009; Brunner et al., 2014). So, deficiency of nutrients can also reduce immune responses (Vogelweith et al., 2015). In addition to the individual effects of nutrients, the rate of macronutrients in the diet is one of the parameters affecting the development and immunity (Cotter et al., 2011; Garvey et al., 2020). Because larvae need a diverse array of nutrient for their development.
In this research, for this purpose, Mediterrarian floor pest Ephestia kuehniella larvae were used. E. kuehniella causes intense damage to the storage products, such as flour, almond, date and cereals and causes weight loss on storage products (Nouri et al., 2019). In addition, due to pests, feces, and netting material released by the pests, quality product losses occur (Xu, 2010). Therefore, determining the factors affecting the development of this species is also economically important. Although there are many studies in the literature on the effect of protein and balanced diets on immunity. We know little about the effect of toxic and growth-inhibiting carbohydrates on immunity. Carbohydrate-based feeding protects Aedes aepypti individuals against arboviral infections (Almire et al., 2021). Dietary carbohydrate affects phenoloxidase activity (Green, 2021). In addition, there is no study on whether carbohydrates considered toxic are effective in phenoloxidase activity. In order to fill this gap in the literature, the effect of dietary carbohiydarate type on phenoloxidase activity was investigated.
MATERIALS AND METHODS
Insect and Diets
Ephestia kuehniella cultures were prepared in the laboratory following the suggestions by Bulut and Kılınç (1987) in 2017–2018 years. Artificial diets are based on a wheat germ and supplemented by casein. In this study, a total of 10 artificial diets were prepared by Yamamoto (1969). The content of Yamamoto’s artificial diet is wheat germ, casein as the protein, saccharose or arabinose as the carbohydrate, torula yeast, vitamin mixture, salt mixture, cholesterol, sorbic acid, methyl paraben, linseed oil, agar, and water (Table 1). Sucrose (S) or arabinose (A) was used as a carbohydrate source, and the carbohydrate source in the artificial food was added according to the diet. Five of these diets contain sucrose as an additional carbohydrate source, while others contain arabinose. Diets containing sucrose were named S, and diets containing arabinose were named A. The protein ratio was also named P. The dietary protein and carbohydrate ratios of diets are as follows: S:P, 3S:P, 5S:P, S:3P, S: 5P, 1A:P, 3A:P, 5A:P, 1A:3P, 1A:5P.
Larval Growth Performance
Trials were performed in 2 groups. In the 1st experimental group, the growth performance of larvae (pupa mass, pupa lipid amount and pupa crude protein amount) were measured. 20 larvae were used for each diet. Individuals in the same larval stage were used in feeding experiments. All weighings were measured on a scale with an accuracy of 0.001. The larvae, the food given and the remaining food were weighed every other day. Then each one was placed into a separate plastic cup with a cover. The diets were weighed before being fed to the larvae. Diets were changed every other day. The remaining food was packed and dried at 50°C and weighed after it reached the constant weight. This procedure was repeated until all of the larvae entered the pupal stage (Lee et al., 2002).
The total lipid amount was calculated by the a modified procedure from Loveridge (1973) (Loveridge, 1973; Simpson and Raubenheimer, 2001). Stored lipid of each pupa was removed by treatment with chloroform. The pupae placed in lidded containers were closed by adding chloroform. The chloroform of the pupae treated for 24 h in the shaker was removed and new chloroform was added. This process was repeated 3 times. At the end of the third chloroform extraction, the pupae were re-dried and re-weighed to calculate their lipid amount.
Lipid-removed pupae were used in the pupa crude protein analysis. For this process, pupa crude protein analysis was performed using the Thermo Scientific Flash 2000 series-NCS analyzer device, according to the Dumas method (Yi et al., 2013). Crude protein was calculated by multiplying the amount of nitrogen by the constant 6.25 (Oonincx et al., 2015).
Phenoloxidase Activity
A second experimental group was established to determine the specific phenoloxidase activity (PO). A sample of haemolymph was collected from each larva for the quantification of immune parameters. In this experimental group, larvae were fed collectively in each food group. Four days after ecdysis to the final larval stadium, haemolymph was collected from individuals by piercing the final proleg with a sterile needle. Haemolymph was collected in Eppendorf tubes and frozen at –27°C until needed. PO activity and the amount of protein were measured according to Lee et al. (2008). For the PO activity assay, 100 μL of 10 mM l-Dopa (substrate) was added to 100 μL of ice-cold phospate-buffered saline haemolymph (PBS, pH 7.4) and the absorbance of the mixture was measured at 492 nm on a Versamax tunable microplate reader after 20 min of incubation at 25°C. PO activity is expressed as PO units, in which one unit represents the amount of enzyme required to increase the absorbance by 0.001 min–1. Haemolymph protein content was quantified by Bradford’s method (Bradford, 1976). Triplicate samples were used for examining PO activity and protein level.
Statistical Analysis
The amount of food consumption by each larva fed on each artificial diet, growth performance parameters, larval duration and PO activity were analyzed statistically using SPSS 17 version. A normality test was performed to determine whether the variables were normally distributed. In order to determine the differences between the groups, ANOVA and TUKEY tests were performed in the data with a normal distribution. Pupa mass does not show normal distribution. Kruskal–Wallis H test was performed to determine whether there are differences between the groups. Mann–Whitney U test was used to determine the difference between non-normally distributed data. Correlation testing was performed to determine whether there was a correlation between the amount of food consumed, the amount of pupae protein, the amount of pupae lipid, pupae dry weight, and PO activity. A regression analysis was performed after the relationship was determined.
RESULTS
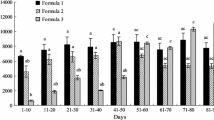
The type of carbohydrate affects the amount of food consumption and phenoloxidase activity of the larvae Figs. (1 and 5). The food consumption amount in diets containing sucrose is higher than the food consumption amount of diets containing arabinose. Only the food consumption amount of 1A:1P diet is different from the food consumption amounts of diets containing arabinose (Fig. 1). The least food consumption is in the diet containing 5A:P. Larvae fed on this diet could not complete the last larval stage and could not pupae. When the diets containing arabinose were examined among themselves, it was determined that both the carbohydrate amount and protein amount of the diet affected the food consumption amount of the larvae negatively (respectively r: –0.35, p < 0.01; r: –0.5, p < 0.01). The food consumption is tightly relevant to protein levels.
For the diets containing sucrose, the amount of food consumption increased with the increase in the amount of protein in the diet (r: 0.89, p < 0.01). The increase in the amount of carbohydrates of diet decreased the amount of consumption as in arabinose (r: –0.67, p < 0.01). So, arabinose has negative effects on food consumption, but sucrose has fewer effect.
One of the striking results of the study is that the larvae fed on the 5A:P diet could not complete the last larval stage and could not pupae. The larvae were fed until the 4th day of the last larval stage, but they could not be pupae. Pupa mass differs from each other in other diets (Kruskal–Wallis, chi Square: 64.94, p < 0.01). However, there is no definite difference between carbohydrate types and pupa mass (Fig. 2).
In sucrose-containing diets, pupa mass increases as the amount of carbohydrates in the diet increases (r: 0.279, p < 0.01). No relationship was found between the amount of protein in the diet and the pupa mass (p > 0.05). In diets containing arabinose, no correlation was found between the amount of carbohydrate and the pupa mass (p > 0.5). But there is a negative decommunization between the ratio of carbohydrates and proteins and the dry weight of the pupa (p < 0.01, r: –0.34). A negative relationship was determined between the amount of consumption and the pupa mass (r: –0.50, p < 0.01).
Pupal lipid and pupal protein amounts also differ between diets (respectively ANOVA, F: 68.87, p < 0.01; F: 68.28, p < 0.01). The highest amount of pupal lipid was determined in 1S:3P and 1A:3P diets (Fig. 3). In diets containing sucrose, neither the carbohydrate amount of the diet nor the protein amount affect the pupal lipid amount (p > 0.01). The factor affecting pupal lipid amount in this diet is pupal dry weight (r: 0.8, p < 0.01). In diets containing arabinose, while the amount of carbohydrate does not affect the amount of pupal lipid, it has a weak effect on the amount of protein (r: 0.25, p < 0.05). A negative correlation was determined between the amount of consumption and the amount of pupal lipid (r: –0.62, p < 0.01).
It is noteworthy that the lowest amount of pupal protein was determined in the 1S:5P, 3S:1P and 1A:1P diets, while the highest protein amount was determined in the 1A:3P diet (Fig. 4). The increase of protein content in the sucrose-containing diets causes a decrease in the pupal protein content (r: –0.22, p < 0.05). However, the increase in the carbohydrate amount of the diet did not affect the pupal protein amount (p > 0.05). In diets containing arabinose, the amount of pupal protein decreases with the increase in the amount of carbohydrates in the diet (r: –0.27, p < 0.05). An increase in the amount of protein of the diet causes an increase in the amount of pupal protein (r: 0.38; p < 0.01).
Another remarkable result of the study is that the phenoloxidase activities of the larvae fed on diets containing sucrose were low. Larvae fed on diets containing arabinose have high phenoloxidase activities (Fig. 5). In diets containing arabinose, the increase in the amount of carbohydrates of the diet increases the activity of phenoloxidase (r: 0.8, p < 0.01). The increase in the amount of protein of the diet caused a decrease in phenoloxidase activity (r: –0.7, p < 0.01). The increase of food consumption decreased the phenoloxidase activity (r: 0.35, p < 0.01). Larvae with high phenoloxidase activity have low pupal weights, pupal lipid amounts and pupal protein amounts (respectively r: –0.49, p < 0.01; r: –0.36, p < 0.01; r: –0.52, p < 0.01). In the diets containing sucrose, unlike the diets containing arabinose, the amount of carbohydrates did not affect the phenoloxidase activity of larvae (p > 0.05). The amount of protein in the diet causes an increase in phenoloxidase activity of larvae (r: 0.79, p < 0.01). This result also differs from diets containing arabinose. Another a different result is that the increase in the amount of consumption increases the activity of phenoloxidase for larvae (r: 0.46, p < 0.01). The increase in phenoloxidase activity causes a decrease in pupal weight, pupal lipid and protein content (respectively r: –0.33, p < 0.01; r: –0.4, p < 0.01; r: –0.31, p < 0.01).
DISCUSSION
The larval growth performance and phenoloxidase activities are related to each other. The quality of nutrients consumed by herbivores directly affects the development, fecundity, survival, and immune activities of individuals (Klemola et al., 2007). E. kuehniella larvae consumed all diets containing arabinose other than besides sucrose, except for 5A:P diet. In the literature, sucrose is more preferred by many insect species as well so (Corneilus et al., 1996; Bluthgen and Fiedler, 2004; Juma et al., 2013). Because arabinose are not useful or toxic carbohydrate for some insect species (Hu et al., 2010; Mercimek et al., 2010). Carbohydrates that have toxic effects cause changes in the insect cuticula, dry the cuticula or block the spiracles and cause them to suffocate (Puterka et al., 2003). Mehmetoğlu and Başhan (1996) suggested that some pentoses had a toxic effect. Also, Rockstein (1978) stated that arabinose inhibited larval development of Tenebrio molitor and Diatraea grandiosella and increased larval development time of Callosobruchus chinensis and mortality. E. kuehniella larvae can develop by tolerating arabinose up to a certain concentration. However, at the 5A:P concentration, the larvae could not complete their development. So, this is an indication that E. kuehniella larvae have an arabinose metabolic pathway. Therefore, high concentrations of arabinose may be toxic to this species. L-arabinose is one of the components of biopolymers such as cereal fiber and pectin in a variety of crops, corn, wheat, barley, rye, and oats, 30–40% produced in its linked form and up to 75% in the “hairy region” of beet pectin.
Toxic sugars may also be a feeding deterrent (Hu et al., 2010). Decreased consumption of larvae with the increase in the amount of arabinose in the diet supports this idea. Although sucrose is a more consumed carbohydrate than arabinose, as the concentration of sucrose in the diet increases, the amount of consumption decreases. Because, species have a specific nutritional need and a certain concentration range that they can tolerate and develop. The optimum concentration range for most species is very narrow. Also, chemoreceptors on the labrum, labella, tarsi, and cibarium likely dictate the biochemical decision-making process that result in the distinct distribution of sugar and blood meals (Airs et al., 2019). Sucrose stimulates feeding by chemoreceptors, while arabinose can act as a feeding deterrent.
Pupa mass increased with the increase of ratio of sucrose in the diet. This may be the indicative of the use of carbohydrates in the body, in other words, sucrose is non-toxic. This result also supports the result of Harvey (1974). Harvey (1974) stated that there is a direct relationship between larval growth rate and adult weight with the increase in the ratio of some sugars in the diet. Airs et al. (2019) stated that sugar availability is linked with fitness. Pupal mass is associated with fitness. The prolongation of the development period and the decrease of the pupal mass affect the fitness negatively (Freitak et al., 2007). The decrease in pupal mass with the increase in arabinose amount may also be an indication that arabinose negatively affects fitness. There is a decrease in thing of consumption as the concentration increases can be the insect’s nutrient balancing mechanism. Because insects can tolerate nutrient imbalances of diet by increasing consumption or reducing consumption (Klemola et al., 2007). The high consumption of 1S:3P and 1S:5P diets proves this idea. The larvae may be more likely to consume diets than to meet the carbohydrate requirement.
In diets containing arabinose, the increase in the amount of consumption and the decrease in the amount of pupal lipid can be interpreted in two different ways. Although arabinose was consumed, it could not be used by the larvae. In particular, hunger regulation mechanisms such as physiological adaptations to sugar intake can reduce the amount of lipid stored. Stress starvation can trigger early pupation and migration. Lipid metabolism is controlled by the hormone Adipokinetic hormone (AKH). AKH stimulates lipolysis and causes lipids to be delivered to the hemolymph (Zhang et al., 2019). As the amount of arabinose in the diet increases, the amount of pupal protein decreases. This indicates that the ingested carbohydrate cannot be converted.
The phenoloxidase activity is a factor associated with the developmental parameters of larvae and the type of carbohydrate. In our study, it was determined that the PO activity of larvae fed on sucrose-containing diets, except for 1S:5P, was lower than the PO activity of larvae fed on arabinose-containing diets. Gonzalez-Santoyo and Cordoba-Aguilar (2011) stated that if the phenoloxidase production is nutrient-dependent, individuals in good condition have high phenoloxidase activities. However, this statement contradicts our results. The immune systems of animals are triggered in situations (such as heavy metal, starvation) that may cause stress (Klemola et al., 2007; Zuk and Stoehr, 2002). Arabinose, which is a toxic carbohydrate, prevents the development of larvae. In addition, since larvae could not use arabinose effectively, the situation may have been evaluated as hunger by individuals. The increase in phenoloxidase activity with the increase in the amount of arabinose in the diet sheds light on the idea that the enzyme is involved in detoxification in addition to the immunity. Wu et al. (2015) study also supports this idea. Wu et al. (2015) suggested that phenoloxidase may be involved in the detoxification of phenolic substances.
The PO activity of the larvae fed on the 3A:P diet is higher than the PO activity of the larvae fed on the 5A:P diet. The 5A:P diet contains the highest amount of arabinose in all diets. According to life-history theory, all animals represent an “optimization” of tradeoffs between growth, survival, reproduction, and immunity. Animals should optimally use their energies and nutrients to develop, to survive, to mature, and most importantly to reproduce (Zuk and Stoehr, 2002). Meanwhile, activation of immunity may affect other physiological (Vogelweith et al., 2015). The larvae fed on the 5A:P diet, which has the highest amount of arabinose with toxic effects, may have less PO activity due to the tendency of larvae to reduce the cost of immunization by spending the existing nutrients on their physiological activities. Also, to increase the utilization of nutrients, larvae may have reduced the cost of efforts for immunity. Because the real goal is to survive.
For larvae fed on sucrose-containing diets, there was a positive correlation between protein concentration of the diet and PO activity. Changes in protein and carbohydrate intake from diets significantly affect immune properties. Protein acts in many physiological reactions in the body, such as synthesis of enzymes, growth, reproduction. Therefore, an increase in the amount of protein amount in of the diet can provide the use of proteins for immunity. Because, immunity is costly, and nutrients can be used optimally if there are not enough nutrients in the diet. This may result in a low production of the PO enzyme when the amount of protein is low. In our study, a negative correlation was found between pupae dry weight, pupa lipid, pupa crude protein, and PO activity for both carbohydrate types. Pupae dry weight, pupal lipid, and the pupa crude protein are indicative of fecundity (Klemola et al., 2007). According to the life-history theory, nutrients are used optimally. One of the most important goals of animals is to ensure the continuity of their generations. Therefore, they kept their immune systems at a certain level in the case of food shortages. Thus, they may have spent their nutrient to increase the pupa dry weight, lipid, and protein content.
As a conclusion, E. kuehniella larvae can tolerate arabinose to a certain concentration. However, as the concentration increases, the larvae cannot tolerate arabinose. The highest phenoloxidase activity occurred in a diet containing 3 times more arabinose compared to protein. This result suggests that arabinose triggers the immune response of larvae. Also, excess arabinose in the diet inhibits the development of larvae. Furthermore, E. kuehniella larvae are trying to regulate the immune system activities related to the nutrient content in the diets they have consumed.
REFERENCES
Airs, P.M., Kudrna, K.E., and Bartholomay, L.C., Impact of sugar composition on meal distribution, longevity, and insecticide toxicity in Aedes aegypti, Acta Trop., 2019, vol. 191, pp. 221–227.
Almire, F., Terhzaz, S., Terry, S., McFarlane, M., Gestuveo, R.J., Szemiel, A.M., Varjak, M., McDonald, A., Kohl, A., and Pondeville, E., Sugar feeding protects against arboviral infection by enhancing gut immunity in the mosquito vector Aedes aegypti, PLoS Pathog., 2021. https://doi.org/10.1371/journal.ppat.1009870
Ankola, K., Kumar, S.S., Mondel, P. and Puttaraju, H.P., Age dependent regulation of phenoloxidase activity through single and multiple wounding in silkworm, Bombyx mori (Lepidoptera: Bombycidae), Entomol. News, 2018, vol. 127, no. 5, pp. 401–406.
Asano, T., Seto, Y., Hashimoto, K. and Kurushima, H., Mini-review an insect-specific system for terrestrialization: laccase-mediated cuticle formation, Insect Biochem. Mol. Biol., 2019, vol. 108, pp. 61–70.
Barbehenn, V.R., Knister, J., Marsik, F., Miller, J.C. and Nham, W., Nutrients are assimilated efficiently by Lymantria dispar caterpillars from the mature leaves of trees in the Salicaceae, Physiol. Entomol., 2015, vol. 40, pp. 72–81.
Bluthgen, N. and Fiedler, K., Preferences for sugars and amino acids and their conditionality in a diverse nectar-feeding ant community, J. Anim. Ecol., 2004, vol. 73, pp. 155–166.
Borzoui, E., Naseri, B., and Nouri-Ganbalani, G., Effects of food quality on biology and physiological traits of Sitotroga cerealella (Lepidoptera: Gelechiidae), J. Econ. Entomol., 2017, vol. 110, no. 1, pp. 266–273.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Brunner, F., Schmid-Hempel, P., and Barribeau, S.M., Protein-poor diet reduces host-specific immune gene expression in Bombus terrestris, Arch. Proc. R. Soc., B: Biol. Sci., 2014, vol. 281, p. 20140128.
Bulut, H. and Kılınçer, N., Yumurta parazitoiti Trichogramma spp. (Hym: Trichogrammatidae) un güvesi (Ephestia kuehniella Zell. (Lepidoptera: Pyralidae) yumurta üretimi ve konukçu parazitoid ilişkileri, in Proceedings of the First Turkish National Congress of Entomology, October 13–16, 1987, Türkiye Entomoloji Derneği, İzmir, Turkey, 1987.
Cornelius, M.L., Grace, J.K. and Yates, J.R., Acceptability of different sugars and oils to three tropical ant species (Hymen., Formicidae), J. Pest Sci., 1996, vol. 69, pp. 41– 43.
Cotter, S.C., Simpson, S.J., Raubenheimer, D. and Wilson, K., Macronutrient balance mediates trade-offs between immune function and life history traits, Funct. Ecol., 2011, vol. 25, pp. 186–198.
Freitak, D., Wheat, C.W., Heckel, D.G., and Vogel, H., Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni, BMC Biol., 2007, vol. 5, no. 56, pp. 1–13.
Garvey, M., Bredlau, J., Kester, K., Creighton, C., and Kaplan, I., Toxin or medication? Immunotherapeutic effects of nicotine on a specialist caterpillar, Funct. Ecol., 2020, vol. 35, pp. 614–626.
Gonzales-Santoyo, I. and Aguilar, A.C., Phenoloxidase: a key component of the insect immune system, Entomol. Exp. Appl., 2012, vol., 142, pp. 1–16.
Green, K.K., The effects of host plant species and larval density on immune function in the polyphagous moth Spodoptera littoralis, Ecol. Evol., 2021, vol. 11, pp. 10090–10097.
Harvey, G.T., Nutritional studies of eastern spruce budworm (Lepidoptera: Tortricidae). I. Soluble sugars, Can. Entomol., 1974, vol. 106, pp. 353–365.
Hu, J.S., Gelman, D.B., Salvucci, M.E., Chen, Y.P., and Blackburn, M.B., Insecticidal activity of some reducing sugars against the sweet potato whitefly, Bemisia tabaci, biotype B, J. Insect Sci., 2010, vol. 10, pp. 1–22.
Iwanaga, S. and Lee, B.L., Recent advances in the innate immunity of invertebrate animals, Int. J. Biochem. Mol. Biol., 2005, vol. 38, pp. 128–150.
Juma, G., Thiongo, M., Dutaur, L., Rharrabe, K., Marion-Poll, F., Le, R.B., Magoma, G., Silvain, J.F., and Calatayud, P.A., Two sugar isomers influence host plant acceptance by a cereal caterpillar pest, Bull. Entomol. Res., 2013, vol. 103, pp. 20–28.
Kangassalo, K., Valtonen, T.M., Sorvari, J., Kecko, S., Polkki, M., Krams, I., Krama, T., and Rantala, M.J., Independent and interactive effects of immune activation and larval diet on adult immune function, growth and development in the greater wax moth (Galleria mellonella), J. Evol. Biol., 2018, vol. 10, pp. 1485–1497.
Klemola, N., Klemola, T., Rantala, M.J., and Ruuhola, T., Natural host-plant quality affects immune defence of an insect herbivore, Entomol. Exp. Appl., 2007, vol. 123, pp. 167–176.
Kraus, S., Monchanin, C., Gomez-Moracho, T., and Lihoreau, M., in Encyclopedia of Animal Cognition and Behavior, Vonk, J. and Shackelford, T., Eds., Cham: Springer, 2019. https://doi.org/10.1007/978-3-319-47829-6_1137-1
Lee, K.P., Behmer, S.T., Simpson, S.J., and Raubenheimer, D., A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval), J. Insect Physiol., 2002, vol. 48, pp. 655–665.
Lee, K.P., Simpson, J.S., and Wilson, K., Dietary protein-quality influences melanization and immune function in an insect, Funct. Ecol., 2008, vol. 22, pp. 1052–1061.
Locker, E.S., Adema, C.M., Zhang, S.M., and Kepler, T.B., Invertebrate immune systems—not homogeneous, not simple, not well understood, Immunol. Rev., 2004, vol. 198, pp. 10–24.
Loveridge, J.P., Age and the changes in water and fat content of adult laboratory-reared Locusta migratoria migratorioides, Rhod. J. Agric. Res., 1973, vol. 11, pp. 131–143.
Mason, A.P., Smilanich, M.A., and Singer, S.M., Reduced consumption of protein-rich foods follows immune challenge in a polyphagous caterpillar, J. Exp. Biol., 2014, pp. 2250–2260.
Mattson, W.J., Herbivory in relation to plant nitrogen content, Ann. Rev. Ecol. Syst., 1980, vol. 11, pp. 119–161.
Mehmetoğlu, R. and Başhan, M., Melanogryllus desertus Pall. (Orthoptera: Gryllidae)’nin Karbohidrat Ihtiyaçları, Turk. J. Biol., 1996, vol. 23, pp. 91–100.
Mercimek, S., Özalp, P., and Coşkun, M., The effect of some sugar supplemented diet with various sucrose ratios on the life cycle and egg productivity of the adult females of Pimpla turionellae L. (Hymenoptera: Ichneumonidae), J. Entomol. Res. Soc., 2010, vol. 12, no. 1, pp. 1–7.
Muller, K., Vogelweith, F., Thiery, D., Moret, Y., and Moreau, J., Immune benefits from alternative host plants could maintain polyphagy in a phytophagous insect, Oecologia, 2014, vol. 177, pp. 467–475.
Nouri, B., Fotouhi, K., Mohtasebi, S.S., Nasiri, A., and Goldansaaz, S.H., Detection of different densities of Ephestia kuehniella pest on white flour at different larvae instar by an electronic nose system, J. Stored Prod. Res., 2019, vol. 84, p. 101522.
Oonincx, D.G.A.B., Broekhoven, S.V., Huis, A.V., Joop, J.A., and Loon, V., Feed conversion, survival and development, and composition of four ınsect species on diets composed of food by-products, PLoS One, 2015, pp. 1–20.
Puterka, G.J., Glenn, D.M., Sekutowski, D.G., Unruh, T.R., and Jones, S.K., Particle film, Surround WP, effects on glassy-winged sharpshooter behavior and its utility as a barrier to sharpshooter infestation in grapes, Plant Health Programme, 2003. https://doi.org/10.1094/PHP-2003-0321-RS
Rockstein, M., Biochemistry of Insects, New York: Academic Press, 1978.
Sharrock, J and Sun, J.C., Innate ımmunological memory: from plants to animals, Curr. Opin. Immunol., 2020, vol. 62, pp. 69–78.
Sadawarte, A.K., Nachane, M.N., Moharil, M.P., and Satpute, N.S., Phenoloxidase activity in hemolymph of naïve and HaNPV infected larvae of Helicoverpa armigera (HB), its characterization and inhibition, J. Pharmacogn. Phytochem., 2019, vol. 8, no, 1, pp. 1756–1763.
Schwier, N., Zhang, K., Nakamura, S., and Furukawa, S., Larvae of the tachinid fly, Drino inconspicuoides (Diptera: Tachinidae), suppress melanization in host lepidopteran insects, J. Asia-Pac. Entomol., 2021, vol. 24, no. 4, pp. 1050–1054.
Simpson, S.J. and Raubenheimer, D., The geometric analysis of nutrient–allelochemical interactions: a case study using locusts, Ecology, 2001, vol. 82, pp. 422–439.
Sivrikaya, R., Altun, N., and Faiz, Ö., Diet-mediated modulation on the development and phenoloxidase activity in the Alder leaf beetle larvae, Agelastica alni (L., 1758) (Coleoptera: Chrysomelidae), Turk. J. Entomol., 2020, vol. 44, no. 2, pp. 193–202.
Srygley, R.B., Lorch, P.D., Simpson, S.J., and Sword, G.A., Immediate protein dietary effects on movement and the generalised immunocompetence of migrating mormon crickets Anabrus simplex (Orthoptera: Tettigoniidae), Ecol. Entomol., 2009, vol. 34, pp. 663–668.
Vogelweith, F., Thiery, D., Quaglietti, B., Moret, Y., and Moreau, J., Host plant variation plastically impacts different traits the immune system of a phytophagous, Funct. Ecol., 2011, vol. 25, pp. 1241–1247.
Vogelweith, F., Thiery, D., Moret, Y., and Memoreau, J., Food-mediated modulation of immunity in a phytophagous insect: An effect of nutrition rather than parasitic contamination, J. Insect Physiol., 2015, vol. 77, pp. 55–61.
Wu, K., Zhang J., Zhu, S., Shao, Q., Clark, C.D., Liu, Y., and Ling, E., Plant phenolics are detoxified by prophenoloxidase in the insect gut, Sci. Rep., 2015, vol. 5, p. 16823.
Yamamoto, R.T., Mass rearing of Tobacco Hornworm. II. Larval rearing and pupation, J. Econ. Entomol., 1969, vol. 62, pp. 1427–1431.
Yi, L., Lakemonda, C.M.M., Sagisb, L.M.C., Eisner-Schadlerc, V., Van Huisd, A., and Van Boekela, M.A.J.S., Extraction and characterization of protein fractions from five insect species, Food Chem., 2013, vol. 141, pp. 3341–3348.
Xu, J., Reproductive behaviour of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae), PhD Thesis, Massey University, New Zealand, 2010.
Zhang, D.W., Xiao, Z.J., Zeng, B.P., Li, K., and Tang, Y.L., Insect behavior and physiological adaptation mechanisms under starvation stress, Front. Physiol., 2019, vol. 10, pp. 1–8.
Zuk, M. and Stoehr, A.M., Immune defense and host life history, Am. Nat., 2002, vol. 160, pp. 9–22.
ACKNOWLEDGMENTS
This study was conducted as a master thesis at Recep Tayyip Erdoğan University. Furthermore, it was presented as an oral presentation at the Iseep-2017 VIII. International Symposium on Ecology and Environmental Problems, Çanakkale, Turkey, and it was published as a summary abstract in the proceedings book. Opinions, findings, conclusions, and recommendations expressed in this publication are all of the author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest. Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Ebru Korkmaz, Altun, N. & Faiz, Ö. Effects of Diet on Phenoloxidase Activity and Development of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) Larvae. Biol Bull Russ Acad Sci 49 (Suppl 1), S189–S197 (2022). https://doi.org/10.1134/S106235902213009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106235902213009X