Abstract
The relationships between the yellowhammer (Еmberiza citrinella) and the pine bunting (Еmberiza leucocephalos) represent quite a rare case of mass hybridization in a broad sympatry zone. The hybrid population was monitored from 2008 to 2019 in a model area in the Altai Mountains, Russia, with the purpose to identify the potential pre- and postcopulatory isolation mechanisms in these species. The area selected as the model one is the only place in the vast secondary contact zone of the two species distinguished by the maximum hybridization level and a high proportion of birds with phenotypes of both parental species in approximately equal proportions. During the observation period, the proportion of phenotypic hybrids increased from 32 to 58%. Nevertheless, the population retains some precopulatory isolation mechanisms that are manifested in the positive mating assortativity and partial biotopic segregation between the species. On par with individuals featuring parental phenotypes, hybrids participate in breeding and successfully bring out nestlings: the phenotypic composition of feeding birds does not differ from the phenotypic composition of the population as a whole. However, the life span of hybrids is shorter; this applies even to birds featuring parental species phenotypes with slightly expressed characters indicating their hybrid origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Hybrid zones are often called natural evolution laboratories (Hewitt, 1988): studies conducted in such zones make it possible to test various hypotheses explaining the ways and mechanisms of the formation of new species. The key milestone in the speciation process is the rejection of individuals to heterospecific mating (Coine and Orr, 2004). Therefore, the identification the reasons of hybridization (be it a disturbance of native habitats, a decline in population, or an incorrect imprint of the sexual partner’s appearance) makes it possible to understand the nature of reproductive isolation between closely related species (Grant and Grant, 1997).
One of the fundamental postulates in the biological species concept is the assumption that hybrids between forms of the near-species rank feature a lower viability. This assumption inevitably arises from the fact that coadapted gene complexes of the parental forms are destroyed in hybrids (Mayr, 1968). The Dobzhansky–Muller incompatibility model is used as a rationale for that: according to it, alleles that have not passed the test for compatibility with each other are accumulated in isolated populations (for more detail, see (Rubtsov, 2015)), and the probability of a decline in fitness increases in potential hybrids as the square of time that has elapsed since the geographical isolation of the population (Orr, 1995). The Dobzhansky–Muller model is simple and logical, and modern researchers often use it as evidence of the lower viability in hybrids, even though its authors have formulated it as a hypothesis (Dobzhansky, 2010) that, similarly to any hypothesis, requires empirical verification. The goals of this study were as follows: (1) test the assumption that fitness declines in hybrids; and (2) assess the effectiveness of precopulatory isolation mechanisms (both biotopic and ethological ones).
Among the various examples of natural hybridization in birds (Panov, 1989; McCarthy, 2006; Price, 2008), two extreme variants occur most frequently: (1) random hybridization between significantly divergent species in a broad sympatry zone and (2) mass hybridization between sister forms in a narrow secondary contact zone. More or less regular hybridization in a broad contact zone represents an intermediate type and occurs much more rarely. The natural hybridization case described in this study belongs to this rare type. Apparently, such cases represent examples of young hybrid zones; the situation in such a zone is unstable and develops dynamically towards one of the two above-described extreme variants, which explains their relative rarity (Price, 2008). Our multiyear studies of the phenotypic composition of populations in the secondary contact zone of two bunting species confirm this point of view.
The zone of secondary contact and hybridization between yellowhammer (Еmberiza citrinella) and pine bunting (Е. leucocephalos) stretches for 2500 km from the Urals Mountains to Baikal Lake. Furthermore, its area continuously increases due to the eastward expansion of the yellowhammer to Transbaikalia (Panov et al., 2003) and westward expansion of the pine bunting to the Kama River region (Rubtsov and Tarasov, 2017). Analysis of the literature shows that the mutual expansion of the two species has continued throughout the entire 20th century. At the end of the 19th century, the western boundary of the distribution range of the pine bunting had passed east of Omsk, while the secondary contact zone of the two species was presumably located between the northwestern foothills of the Altai Mountains and Kuznetsk Alatau (Rubtsov and Tarasov, 2017). In Irkutsk oblast, the yellowhammer was first registered in the 1920s and became a common nestling species in the middle of the 20th century (Panov et al., 2003).
Several subzones differing in the phenotypic composition of their populations can be distinguished within the modern boundaries of the sympatry zone. Near the western (eastern part of Kurgan oblast) and eastern (Irkutsk oblast, Baikal region) boundaries of the sympatry zone, both parental species are common and form mixed settlements where they are represented in approximately equal proportions, while the proportion of phenotypic hybrids is 25–30% (Panov et al., 2003; Rubtsov and Tarasov, 2017). In the central subzone (Novosibirsk and Kemerovo oblasts, Altai krai, and the Republic of Khakassia), the pine bunting is rare or completely absent, while the proportion of phenotypic hybrids ranges from 30 to 60% (Panov et al., 2003, 2007). Apparently, this situation formed in this region relatively recently. In 1967, the pine bunting had predominated in the mixed bunting population of the Novosibirsk Akademgorodok, and the proportion of phenotypic hybrids did not exceed 10%. Thirty years later, in 1997, the pine bunting was completely absent there, while the share of phenotypic hybrids had reached 60% (Panov et al., 2003). A “classical” narrow (about 150 km wide) hybrid zone is located in the southern part of the sympatry zone in mountainous regions of the Altai Mountains. “Pure” yellowhammer populations are localized in the northwest up to the Seminskii Pass: although they are located within the sympatry zone, the share of phenotypic hybrids there does not exceed 10–15%. In the southeast (Republic of Tyva and Mongolia), the allopatric section of the pine bunting distribution range is located, and the phenotypically pure population closest to the Altai hybrid zone is located in the Chui steppe. In the central part of this hybrid zone (Ongudai district), both parental species are represented in equal proportions, while the share of phenotypic hybrids is some 50% (Panov et al., 2007).
Even in areas with the maximum proportion of phenotypic hybrids between the two bunting species, the proportion of individuals belonging to the parental species and having no phenotypic characters indicating their hybrid origin remains rather high in the populations. This indicates the existence of some precopulatory isolation mechanisms that hinder hybridization. One such mechanism is partial biotopic segregation. In mountainous areas, the pine bunting inhabits primarily sparse larch forests on slopes and hilltops, while the yellowhammer inhabits floodplain forests and terraces of river valleys (Panov et al., 2003). In plain areas, the yellowhammer gives preference to birch–aspen groves and forest edges, while the pine bunting inhabits overgrowing cleared and burnt areas with abundant deadwood and sparse grass cover (Rubtsov and Tarasov, 2017).
MATERIALS AND METHODS
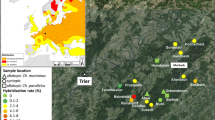
To assess the hybrid fitness and effectiveness of precopulatory isolation mechanisms between the yellowhammer and the pine bunting, a model area was established near the village of Khabarovka, Ongudai district, Altai Republic (50.73° N, 86.32° E). This area is located in the middle of the Altai hybrid zone where, as noted above, the maximum proportion of phenotypic hybrids is observed amid a fairly high abundance of both parental species. Such a situation does not exist anywhere else throughout the vast sympatry zone of the two bunting species. Observations were conducted in 2008–2019 on an annual basis from May 20 to June 20. In different years, from 40 to 60 singing male buntings were registered in the model area about 0.5 km2 in size (Fig. 1). Every year, individual sites of singing males within the model area were mapped using GPS; their phenotypes were described; and their songs were recorded using a Sennheizer K6/ME67 directional shotgun microphone with a Zeppelin windscreen and a Marantz PMD661 digital recorder (recording parameters: 16 bit, 44.1 kHz). Each year, sonograms were produced for each male in the model area, and its repertoire (i.e., set of individual song variants, see below) was determined. Sonograms were produced using the SpectraLAB V4.32 software with a frequency resolution of 43.066 Hz and a time resolution of 5.8 ms. Some birds were captured with Ecotone cobwebs 2.5 m high and 3 m long with a mesh size of 16 mm by luring them with recorded songs (sound trap method). The captured birds were marked with standard aluminum rings and photographed, their coloration patterns were described in detail, standard morphometric measurements were taken, and blood samples were collected for genetic analysis. During the observation period, 277 males were registered in the model area; 124 of them were captured. One hundred nine males were included in the life expectancy analysis: only captured birds were used, and males registered in the model area only once in the first or last years of observation were excluded from the analysis. This analysis technique results in a slight overestimation of the life expectancy (some of the birds who have actually lived for only one year are intentionally excluded from the analysis), but it should not affect the relative life spans of birds with different phenotypes in the hybrid population.
Distribution of singing male buntings within the model area in 2015: (1) yellowhammers, (2) pine buntings, (3) white hybrids (white hybrid + leucocephalos hybrid), and (4) yellow hybrids (yellow hybrid + citrinella hybrid). The dotted lines mark the zones selected for the identification of biotopic preferences of the two bunting species: (A) floodplain forests and the Ursul River terrace; and (B) larch forests on hilltops.
The yellowhammer and the pine bunting are similar in body size and proportion; but they have clearly manifested differences in the mating coloration of males (Panov et al., 2003). Male pine buntings are distinguished by the absence of yellow tones in the body plumage and the presence of a chestnut color in the head plumage (throat and eyebrow); by contrast, the yellowhammer has no chestnut colors, while the yellow color is well developed in it. Hybrids combine these characters independently of each other, and each of these characters varies quantitatively. Three coloration characters were used to describe the phenotypes of captured males; each of these characters was ranked on an eight-point scale (Table 1), and then each male was subsumed under one of the eight categories (Table 2). The “pure citrinella” and “semi-citrinella,” as well as “pure leucocephalos” and “semi-leucocephalos” phenotypic classes could only be distinguished for captured birds; therefore, in data arrays collected using binocular observations, they were consolidated into the citrinella and leucocephalos phenotypic classes, respectively. Female buntings feature the same differences as males, but they are manifested to a lesser extent. Furthermore, the presence of the chestnut color in the head plumage is not a reliable character since it is not present in all female pine buntings. Therefore, the species of females was determined based on only one character: the degree of development of the yellow color in the plumage (bright yellow corresponds to yellowhammers, pale yellow to hybrids, and white to pine buntings).
The song of yellowhammers and pine buntings is a short warble lasting for 2–3 s and consisting of a series of homotypic signals and one or two end notes. Six to 15 signals consisting of one, two, or three notes constitute a homotopic series. The repertoire of one male usually includes two or three versions of the song differing in the form and relative arrangement of notes in signals of the homotypic series. The diversity of song variants is very high (Fig. 2), but different males often use similar (in some cases, almost identical) song variants (Rubtsov, 2007). However, our studies in the model population have shown that the song repertoire of a given male does not change throughout his life, and the probability of two, let alone three, matching song variants in different males is extremely low. The set of individual song variants in combination with the phenotype and the presence/absence of the ring make it possible to determine accurately the individual affiliation of each male in the model area in different years, both for phenotypic hybrids and for birds featuring phenotypes of parental species.
Statistical data processing was performed in Statistica 10; the χ2 criteria were used to compare the samples. The validity of statistical hypotheses was assessed at a significance level of 5%.
RESULTS
Changes in the Phenotypic Composition of the Population from 2010 to 2019
Over ten years of observation, the phenotypic composition of birds nesting in the model area changed significantly (Fig. 3): the proportion of phenotypic hybrids almost doubled (from 32 to 58%), while the proportion of parental phenotypes, respectively, decreased. Concurrently, the proportion between yellowhammers and pine buntings remains approximately equal (samples dated 2010, 2015, and 2019 were compared for phenotypic classes yellowhammer—hybrids—pine bunting: χ2 = 10.6, df = 4, p = 0.03). The phenotypic composition of hybrids has also changed significantly: the proportion of white hybrids increased from 4 to 30% (samples dated 2010, 2015, and 2019 were compared for phenotypic classes white hybrids—other hybrids: χ2 = 6.6, df = 2, p = 0.04); while the ratio between the remaining three hybrid phenotypic classes did not change significantly. Therefore, the proportion of phenotypic hybrids has increased from 30 to 60% solely due to the increase in the number of white hybrids.
Year-to-year changes in the phenotypic composition of nesting buntings in the model area. The numbers below the graphs represent years; the numbers above the graphs represent the sample size in each of these years. Phenotypic classes (see Table 2 for descriptions): (CT) citrinella, (CP) “pure citrinella” (yellowhammers without phenotypic characters indicating hybridization), (CS) “semi-citrinella” (yellowhammers with characters indicating their hybrid origin), (CH) citrinella hybrid, (YH) yellow hybrid, (WH) white hybrid, (LH) leucocephalos hybrid, (LC) leucocephalos, (LP) “pure leucocephalos” (pine buntings without phenotypic characters indicating hybridization), and (LS) “semi-leucocephalos” (pine buntings with characters indicating their hybrid origin).
Life Expectancy Estimates for Phenotypically Pure Individuals and Hybrids
For the sample as a whole, the distribution of bird sightings in different years corresponds to a geometric distribution (Fig. 4). To construct the distribution, birds registered in the first and last years of observation were excluded from the sample (since it is not clear how long they actually lived). The geometric distribution shows the number of unsuccessful attempts to be made before the occurrence of a certain event that occurs with a given probability: the number of shots before the first hit, the number of coin tosses before the first “tails,” etc. (Borovikov, V.P. and Borovikov, I.P., 1998). In this case, an “attempt” is a search for a given male in the model area in each subsequent year of observation, while an “event” is its disappearance due to death or relocation to a new nesting site outside the model area.
Geometric distribution of the numbers of male bunting registrations in different years within the model area. Bars represent the observed distribution; the black line represents the theoretical distribution; geometric distribution parameter: survival (i.e., probability of registering the male within the model area in the next year) s = 0.534. Correlation between the observed distribution and the theoretical distribution: χ2 = 0.809, df = 3, p = 0.847 (n = 96).
To analyze the relative life span, the sample was divided into four classes based on the number of registrations of ringed males in different years: one, two, three, and four or more years. Based on male phenotypes, the sample was divided into five classes: phenotypically pure citrinella and leucocephalos, semi-citrinella, semi-leucocephalos, and hybrids. After checking the citrinella and leucocephalos samples for the absence of statistically significant differences, they were consolidated into a single sample: “phenotypically pure individuals”; while semi-citrinella and semi-leucocephalos were put into the “almost pure individuals” sample. To increase the power of the test, the number of degrees of freedom was reduced by combining samples based on the number of bird sightings in different years. The above four classes were consolidated into two classes: (1) birds registered one or two times and (2) birds registered three or more times. The results obtained are presented in Table 3. Verification of the statistical significance of differences between the samples: (1) citrinella—leucocephalos: χ2 = 0.21 (df = 1), p = 0.89; (2) “pure”—hybrids: χ2 = 5.48 (df = 1), p = 0.02; (3) “pure”—“almost pure”: χ2 = 6.64 (df = 1), p = 0.01; and (4) “almost pure”—hybrids: χ2 = 1.01 (df = 1), p = 0.32. Therefore, the proportion of birds registered for more than two years in a row is statistically significantly higher for phenotypically pure individuals than for hybrids; while the duration of stay in the model area is shorter for “almost pure” individuals than for pure ones and does not differ from the values obtained for hybrids. In all three samples, the distribution corresponds to the geometric one; the distribution parameters are as follows: (1) “pure”: s = 63%, x = 2.73, and σ = 3.15; (2) “almost pure”: s = 36%, x = 1.56, and σ = 0.37; and (3) hybrids: s = 45%, x = 1.83, and σ = 1.60, where s is the survival rate (i.e., the probability that the bird returns to the nesting site the next year), x is the average life expectancy, and σ is the standard deviation.
The method used to estimate the relative life expectancy for hybrids brings adequate results provided that the phenotypic composition of the population in different years is stable. However, in our case, the proportion of hybrids increased significantly during the observation period (see above), which can cause erroneous results: a significant part of phenotypic hybrids was registered at the end of the observation period, and these birds did not have the possibility to live for a long time. The fact that the proportion of hybrids has increased solely due to an increase in the phenotypic class “white hybrids” can be used to check whether changes in the phenotypic composition of the population do not distort the relative life expectancy estimates produced for birds of different phenotypic classes. The “white hybrids” (n = 17) and “other hybrids” (n = 35) samples do not differ in the number of male sightings in the model area: χ2 = 0.09 (df = 1), p = 0.77. Therefore, the observed changes in the phenotypic composition of the population over the years do not significantly affect the relative life expectancy estimates.
The disappearance of a bird from the model area can be caused not only by its death, but also by its relocation to another nesting site outside the model area, which inevitably results in an underestimation of life expectancy. It is logical to assume that birds change their nesting sites after a bad nesting experience, and it is likely that young birds nesting for the first time should do this more often. The probability of relocation to another nesting site was estimated based on the monitoring data collected in the model area. Of the 277 males registered over all observation years, 111 birds were registered for two or more years in a row. Most of them returned to their former nesting sites; only eight birds (7.2%) relocated to new nesting sites located away from their previous sites, but still within the model area. Of these eight birds, five were registered for more than two years in a row; three of them changed their nesting sites in the second year after settling in the model area, and the other two birds changed nesting sites several times. These data are consistent with our assumption: first, the proportion of birds that change their nesting sites is relatively small (less than 10%) and, second, birds that change their nesting sites do so either after the first nesting experience or several times during their life.
Biotopic Segregation
The model area is located in a forest–steppe landscape on a gentle hilly north-facing slope of a mountain. South-facing slopes of the hills are steppe sites occupied by low-growing herbaceous vegetation of the succulent type. North-facing slopes are occupied by light sparse larch forests with dense undergrowth formed by pea (acacia) shrubs. At the foot of the hills, edges of larch forests are formed by high (more than 2.5 m) pea shrubs, bird cherry, and dense herbaceous meadow-type vegetation. On hilltops, shrubs are lower, up to 1.5 m high; in addition to pea shrubs, meadowsweets and cotoneaster occur there. In the Ursul River floodplain, birch, bird cherry, and individual larches grow; terrace slopes are occupied by tall pea shrubs and herbaceous meadow-type vegetation (mainly graminoids).
To identify biotopic preferences of the studied species within the model area, the most contrasting habitats were selected: the Ursul River floodplain and hilltops (Fig. 1). The consolidated data for all observation years clearly show that the yellowhammer selects for nesting more wetted areas with tall shrubs and dense grass cover, while the pine bunting is more xerophilous. As for hybrids, there are no significant differences between the two biotopes, neither in their total share nor in the ratio between phenotypic classes of hybrids (Fig. 5). Comparison of samples: (1) all phenotypes: χ2 = 19.6, df = 5, p = 0.0015, and (2) parental phenotypes—hybrids: χ2 = 1.37, df = 1, p = 0.24.
Phenotypic composition of nesting male buntings in different habitats within the model area in 2010–2019. CT, LC, CH, YH, WH, and LH are phenotypic classes (see Table 2 for descriptions).
Composition of Couples
Over the entire observation period (2008–2019), the phenotypic composition of 68 couples was determined (Table 4). As noted above, female yellowhammers and pine buntings were identified by the presence or absence of the yellow cover in the plumage; binocular observations make it possible to diagnose this feature reliably. Females featuring only a yellow tone in their plumage were considered hybrids. The data provided in Table 4 indicate that phenotype-based species identification is less reliable for females in comparison with males: the proportion of birds featuring hybrid phenotypes is much smaller among females. This is why the phenotypes of females are given in quotation marks: “yellow,” “white,” and “hybrid.” Still, a clear positive mating assortativity trend is observed: the proportion of conspecific pairs and hybrid—hybrid pairs is significantly higher than the values expected for random mating (given in parentheses). The differences are statistically significant: χ2 = 16.8, df = 4, p = 0.002.
Participation of Hybrids in Breeding
Buntings nest on the ground and are very shy in the period when they fledge nestlings. Most of the nests discovered after frightening away the sitting female were subsequently ravaged by predators. Therefore, it was impossible to collect the amount of data sufficient to assess the reproductive success of nesting couples. The fact that hybrids were repeatedly spotted with food in their nibs and with fledglings indicates that they are fertile and successful in choosing sexual partners and bringing out nestlings. To find out whether the phenotype of the male affects the breeding success, the phenotypic composition of feeding males was compared with the composition of birds in the entire model population for all years of observation. The results are provided in Table 5; the differences are not statistically significant: χ2 = 4.1, df = 5, p = 0.53.
DISCUSSION
As stated above, the model area was selected to study reproductive isolation mechanisms in the two bunting species for a reason: the Ongudai district in the Altai Republic is the only place in the vast secondary contact area within the sympatry zone of the two species where the proportion of phenotypic hybrids is maximum, while the parental species are represented in equal proportions. The results of this study indicate that precopulatory isolation mechanisms, both ecological (biotopic segregation) and behavioral (positive mating assortativity) ones, are partially preserved there. However, the hybrids are apparently fully fertile: the phenotypic composition of birds that successfully bring out nestlings does not differ from the phenotypic composition of the population as a whole. This result in a gradual increase in the hybridization level: over ten years of observation, the proportion of phenotypic hybrids increased from 30 to 60%. Unfortunately, the data obtained are insufficient to draw a firm conclusion whether the observed phenomenon reflects directed changes or random fluctuations in the phenotypic composition. However, our earlier studies indicate that the hybridization level gradually increases in the central part of the sympatry zone; as a result, the pine bunting abundance decreases, including its complete disappearance in some populations (Panov et al., 2003). Therefore, the first assumption seems to be more logical.
It must be noted that postcopulatory reproductive isolation mechanisms exist as well; they are manifested in a shorter life span of hybrids compared to individuals featuring parental phenotypes. This conclusion, however, encounters some methodological difficulties that have to be discussed in more detail. As stated above, the disappearance of a bird from the model area can be caused both by its death and its relocation to a new nesting site after an unsuccessful nesting experience. It is logical to assume that young birds nesting for the first time should relocate more often. But if there were many such cases, then the observed distribution would differ from the theoretically expected one with the predominance of individuals registered only in the first year of observation. The consistency between the real distribution and the expected one suggests that, in most cases, birds do not appear in the model area in the next year due to their death, not due to their relocation to new nesting sites. Analysis of the literature also confirms this assumption: it was demonstrated on various passerine species that birds change their nesting site more often after an unsuccessful nesting experience in the previous year (Haas, 1998; Hoover, 2003; Shitikov et al., 2012; Shitikov et al., 2017). Furthermore, the loyalty of a bird to its nesting site (philopatry) increases with the number of broods successfully brought out on this site (Hoover, 2003). It must be admitted though that the above arguments are very unreliable and based on speculative assumptions. It is obvious that the studied sample includes birds that did not die, but relocated to nesting sites outside the model area, and the proportion of such individuals cannot be estimated even approximately. However, this is not a problem because, for the purposes of this study, the life span of birds per se is of no importance: the goal is to assess the relative life duration of hybrids in comparison with their parental species. A comparative life expectancy analysis is valid only if the degree of philopatry is the same in birds belonging to different phenotypic classes in a hybrid population. Let us examine a possible reason why the degree of philopatry in hybrid males may be lower than that in birds belonging to the parental species.
It can be assumed that hybrid males featuring a “normal” viability compared to individuals of their parental species have difficulties with the formation of mating couples because their phenotypes are rare and unusual. Hybrid males stay single more often and, therefore, tend to change nesting sites more frequently. But the data obtained make it possible to reject this assumption confidently since individuals featuring parental phenotypes with slightly expressed characters indicating their hybrid origin (“almost pure” ones) also have a shorter life span. These include the presence of the yellow color on the wing fold in male pine buntings and brown spots or thin “moustache” on the sides of the throat in yellowhammers. There is no reason to suggest that such characters significantly reduce the attractiveness of “almost pure” individuals in the hybrid population resulting in their inability to form a couple and subsequent relocation to a new nesting site.
CONCLUSIONS
Overall, the obtained data are consistent with the Dobzhansky–Muller model: in hybrid zones, alleles of genes that are different in individuals belonging to different parental species are mixed and tested for compatibility, and some combinations of alleles can adversely affect the fitness of hybrids.
The attempt to estimate the level of divergence between the yellowhammer and the pine bunting encountered significant difficulties: allopatric populations of these species virtually do not differ in mitochondrial DNA, which is often used in phylogeny inference. This can either indicate their extremely close evolutionary relationship or be a result of the mitochondrial DNA exchange due to their long-time hybridization (Irwin et al., 2009). The phylogeny of the studied species reconstructed on the basis of their songs and morphological characters using cladistic techniques (Pavlinov, 2005) indicates that they may not be sister species (Rubtsov and Opaev, 2012): the yellowhammer is evolutionarily closer to the cirl bunting (Emberiza cirlus); while the pine bunting is closer to the white-capped bunting (E. stewarti). These facts support the second hypothesis, which inevitably leads to the conclusion that hybridization between the yellowhammer and the pine buntings has a long history; apparently, this history consists of several cycles involving their geographic isolation with the subsequent establishment of sympatry and a gradual increase in hybridization (Rubtsov, 2010). However, despite intense hybridization in the broad sympatry zone resulting in a significant fusion of genomes of the two bunting species, both of them retain their morphological identity. This indicates the presence of some mechanism ensuring the stability of the species-specific phenotype. The reduced viability of hybrids demonstrated in this study may act as such a mechanism.
REFERENCES
Borovikov, V.P. and Borovikov, I.P., STATISTICA—statisticheskii analiz i obrabotka dannykh v srede Windows (Statistica—Statistical Analysis and Data Processing in the Windows Environment), Moscow: Inform.-Izdat. Dom Filin, 1998.
Coyne, J.A. and Orr, H.A., Speciation, Sunderland, MA: Sinauer Associates, 2004.
Dobzhanskii, F.G., Genetika i proiskhozhdenie vidov (Genetics and Origin of Species), Moscow: Inst. Komp. Issled., Nauchno-Issled. Tsentr Regul. Khaotich. Dinam., 2010.
Grant, P.R. and Grant, B.R., Hybridization, sexual imprinting, and mate choice, Am. Nat., 1997, vol. 149, no. 1, pp. 1–28.
Haas, C.A., Effects of prior nesting success on site fidelity and breeding dispersal: an experimental approach, Auk, 1998, vol. 115, no. 4, pp. 929–936.
Hewitt, G.M., Hybrid zones—natural laboratories for evolutionary studies, Trends Ecol. Evol., 1988, vol. 3, no. 7, pp. 158–167.
Hoover, J.P., Decision rules for site fidelity in a migratory bird, the prothonotary warbler, Ecology, 2003, vol. 84, no. 2, pp. 416–430.
Irwin, D.E., Rubtsov, A.S., and Panov, E.N., Mitochondrial introgression and replacement between yellowhammers (Emberiza citrinella) and pine buntings (Emberiza leucocephalos) (Aves: Passeriformes), Biol. J. Linn. Soc., 2009, vol. 98, no. 2, pp. 422–438.
Mayr, E., Animal Species and Evolution, Cambridge, MA: Harvard Univ. Press, 1963.
McCarthy E.M., Handbook of Avian Hybrids of the World, Oxford: Oxford Univ. Press, 2006.
Orr, H.A., The population genetics of speciation: the evolution of hybrid incompatibilities, Genetics, 1995, vol. 139, no. 4, pp. 1805–1813.
Panov, E.N., Gibridizatsiya i etologicheskaya izolyatsiya u ptits (Hybridization and Ethological Isolation in Birds), Moscow: Nauka, 1989.
Panov, E.N., Rubtsov, A.S., and Monzikov, D.G., Relationships between two species of buntings (the yellowhammer Emberiza citrinella and the pine bunting E. leucocephalos) hybridizing in the overlap zones of their ranges, Zool. Zh., 2003, vol. 82, no. 4, pp. 470–484.
Panov, E.N., Rubtsov, A.S., and Mordkovich, M.V., New data on the relationship between two species of buntings (Emberiza citrinella, E. leucocephalos) hybridizing in areas of overlapping of their ranges, Zool. Zh., 2007, vol. 86, no. 11, pp. 1362–1378.
Pavlinov, I.Ya., Vvedenie v sovremennuyu filogenetiku (kladogeneticheskii aspekt) (Introduction to Modern Phylogenetics (Cladogenetic Aspect)), Moscow: Tovar. Nauchn. Izd. KMK, 2005.
Price, T.D., Speciation in Birds, Greenwood Village, CO: Roberts and Company, 2008.
Rubtsov, A.S., Song variability of the yellowhammer (Emberiza citrinella) and pine bunting (Emberiza leucocephala) as an indicator of population structure and evolutionary history of species, Zool. Zh., 2007, vol. 86, no. 7, pp. 863–876.
Rubtsov, A.S., The evolutionary role of hybridization in birds on the example of the yellowhammer (Emberiza citrinella) and pine bunting (E. leucocephalos), in Trudy mezhdunarodnoi nauchnoi konferentsii “Charl’z Darvin i sovremennaya biologiya” (21–23 sentyabrya 2009 g., Sankt-Peterburg) (Proc. Int. Sci. Conf. “Charles Darwin and Modern Biology,” September 21–23, 2009, St. Petersburg), St. Petersburg: Nestor-Istoriya, 2010, pp. 260–271.
Rubtsov, A.S., Reproductive isolation and the concept of species in birds, Zool. Zh., 2015, vol. 94, no. 7, pp. 816–831.
Rubtsov, A.S. and Opaev, A.S., Phylogeny reconstruction of the yellowhammer (Emberiza citrinella) and pine bunting (Emberiza leucocephala) based on song and morphological characters, Biol. Bull. (Moscow), 2012, vol. 39, no. 9, pp. 715–728.
Rubtsov, A.S. and Tarasov, V.V., Relations between the yellowhammer (Emberiza citrinella) and the pine bunting (Emberiza leucocephalos) in the forested steppe of the Trans-Urals, Biol. Bull. (Moscow), 2017, vol. 44, no. 9, pp. 1059–1072.
Shitikov, D., Fedotova, S., Gagieva, V., Fedchuk, D., Dubkova, E., and Vaytina, T., Breeding-site fidelity and dispersal in isolated populations of three migratory passerines, Ornis Fenn., 2012, vol. 89, no. 1, pp. 53–62.
Shitikov, D.A., Vaytina, T.M., Makarova, T.V., Fedotova, S.E., Krasnykh, N.A., and Yurchenko, Yu.Yu., Breeding success affects the apparent survival of grassland passerines, Biol. Bull. (Moscow), 2017, vol. 44, no. 9, pp. 1046–1055.
Funding
This study was supported by the Russian Foundation for Basic Research, project nos. 14-04-01259, 17-04-00903, and 18-04-00770.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The author declares that he has no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by L. Emeliyanov
Rights and permissions
About this article
Cite this article
Rubtsov, A.S. Composition of Couples, Biotopic Preferences, and Relative Life Duration of Birds in a Hybrid Yellowhammer (Еmberiza citrinella) and Pine Bunting (E. leucocephalos) Population (Passeriformes, Emberizidae) in the Altai Mountains. Biol Bull Russ Acad Sci 49, 1186–1196 (2022). https://doi.org/10.1134/S1062359022080180
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022080180