Abstract—
The mesonephros ultrastructure has been studied for 12 specimens of sexually mature Carassius gibelio Bloch 1782 living in the freshwater Finogenov Pond and in the middle reaches of the Khara River (salinity of 6‰). Both reservoirs belong to the basin of the Volga River. A slight increase in the water salinity up to 6‰ primarily caused changes in the quantitative characteristics of leukocyte mitochondria and in all types of epithelial cells, as well as specialized types of inclusions in eosinophils, macrophages, proximal tubules type I, and distal tubules of the nephron. There were also changes in the nuclear structures of some types of interstitial cells and epitheliocytes. In the nephron tubules, epithelial cells of smaller sizes were registered, in the epithelial cells of the tubules, there was a more developed smooth endoplasmic reticulum, as well as a shorter brush border of the proximal tubule cells. As the water salinity increased, the area of the renal corpuscles, of glomerular capillaries, and of podocytes decreased, the thickness of the basement membrane and mass transfer in the renal corpuscles and tubules changed as well. Cytological rearrangements during the transition of the stenohaline freshwater species to brackish water testified to the high adaptive capacity of the cellular structures of the mesonephros.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Changes in the conditions of the aquatic environment predetermine the phenotypic variability (while maintaining the genotype), which ensures the adaptation of the organism to the load of a heterogeneous environment, including an increase in the salinity of water courses (Mazzarella et al., 2015; Komoroske et al., 2016; Sunde et al., 2018; Verhille et al., 2016).
Most studies aim to study the compensatory morphological rearrangements in the gills and kidneys, which are the basic organs performing osmoregulatory function of migrating salmon fish with regard to the habitat change (Folmar and Dickhoff, 1980; Maksimovich et al., 2000). Structural rearrangements of fish gill cells have also been studied under an artificial increase in the water salinity in laboratory conditions (Yang et al., 2017). Comprehensive studies of the features of the ultrastructure of cells that form the fish kidney tissues (both laboratory-reared animals and individuals from natural populations) changing under various water salinity are still missing.
This study aims to describe the fine structure of the cells forming the kidney interstitium and nephrons in Carassius gibelio Bloch 1782 inhabiting streams with varying salinity.
MATERIALS AND METHODS
Sexually mature diploid females of Carassius gibelio Bloch 1782, aged 5+ and 6+, were used in the studies. Samples were obtained in summer (July–August) in the freshwater Finogenov Pond, which is the source of the Khara River (area no. 1) and in the middle reaches of the Khara River (area no. 2), which is the largest tributary of Lake El’ton, characterized by a unique salinity regime (Burkova, 2011; Zinchenko et al., 2017; Gusakov, 2019) (Fig. 1). The total mineralization of area no. 2 is 6‰. The Khara River is a slow-flowing watercourse with an asymmetric valley. The mineralization is mainly represented by chloride–sodium–calcium and sulfate ions (Gusakov, 2019). The mixing of the riverine fresh waters with the saline waters of the lake at the river mouth results in a smooth salinity gradient in the tributaries of Lake El’ton (Burkova, 2011; Gusakov, 2019). Such conditions made it possible to sample individuals from local sub-populations living at various water salinities. A total of 29 specimens were tested in this study. The body length and weight were measured, scales were taken to determine the age, and the blood was taken from the tail vein to determine ploidy. Blood smears were prepared according to the standard procedure. Then the fish was dissected, the sex was determined, and small tissue fragments were excised from the middle part of the mesonephros and fixed according to the standard technique for electron microscopy (Timakova et al., 2014). In the laboratory, blood smears were analyzed under a MICMED-6 microscope and the area of erythrocytes and of their nuclei was measured. The presence/absence of ploidy for each fish specimen was based on at least 14 erythrocytes analyzed. According to the results of biological and cytometric analyses, twelve specimens of Prussian carp (equal size, same sex) were selected; according to the morphometric parameters of the peripheral blood erythrocytes, they corresponded to diploid individuals (Sezaki et al., 1977; Mezhzherin and Lisetskii, 2004). The first group were six specimens from the freshwater Finogenov Pond (body length 18.6 ± 0.32 cm, weight 298 ± 5.77 g). The second group were another six specimens caught in the middle course of the Khara River (body length 18.3 ± 0.42 cm, weight 297 ± 5.36 g).
Ultrathin sections were prepared on a Leica EM UC7 microtome and analyzed under a JEM 1011 microscope (Timakova et al., 2014). Digital photographs were obtained from each section. In the photographs, the area of the renal corpuscle and the lumen of the capillaries, cells, and their nuclei, heterochromatin, organelles, and inclusions were measured using the program J Micro Visionv 1.2.7. The linear dimensions of the nuclear pores, tubules of the smooth endoplasmic reticulum, epitheliocytes, basement membrane, cavity of the renal corpuscle, zone of endocytosis of epitheliocytes, and brush border of the proximal tubules were measured as well. The number of mitochondria, nucleoli, and inclusions was counted.
In order to analyze the efficiency of mass transfer through the wall of epitheliocytes, the efficiency of the diffusion flow, based on the transformed equation of Fick’s laws of diffusion, was calculated as
where M1 is the average amount of a substance passing through the wall of the tubule in the first group of Prussian carp; M2 is the average amount of a substance passing through the wall of the tubule in the second group of Prussian carp; A1 is the average diffusion area through which the substance is transferred in the tubules of the first group of Prussian carp, µm2; A2 is the average area of diffusion through which the substance is transferred in the tubules of the second group of Prussian carp, μm2; h1 is the average wall thickness of the tubule of the first group of Prussian carp, µm; h2 is the average wall thickness of the tubule of the second group of Prussian carp, µm.
During statistical processing, the mean values and their standard errors (M ± m) were calculated. Compliance with the normal distribution was assessed using the Shapiro–Wilk’s (W) test. Two-tailed Student’s t-test was applied to assess the significance of differences under the condition of a normal distribution of the sample data. If these conditions were not met, the Mann–Whitney U-test was applied. The critical level of significance was set as P ≤ 0.05. Statistical analysis was performed using the STATISTICA 10 software, StatSoft Inc. (2011).
RESULTS
The cells of the interstitium mesonephros (lymphocytes, macrophages, neutrophils, eosinophils, and the cells with radially arranged vesicles), as well as the structures that form the nephron, have a single structural plan in the groups of Prussian carp considered.
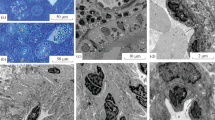
Lymphocytes are the cells most commonly found in the mesonephros interstitium of the individuals studied. Lymphocytes are the smallest among leukocytes, and the area of their cells and nuclei differs slightly in Prussian carp of the first and second groups. The nucleus occupies almost the entire volume of the cell. There are no statistically significant differences in the number of nucleoli and the width of the nuclear pores. The nucleus heterochromatin is more condensed in the lymphocytes of individuals of the second group compared to those of the first group. In the second group, folded membranes of the lymphocyte nuclei are found; unlike those of the first group, these membranes are smooth. The cytoplasm contains mitochondria. In the first group, only one mitochondrion is found on the lymphocyte sections, while the number of mitochondria reaches four on the cell sections of the second group. In the second group, the area of mitochondria of lymphocytes significantly exceeds this indicator of the first group (Table 1; Figs. 2a, 2b).
Ultrastructure of agranulocytes of Prussian carp: (a) lymphocyte (group 1); (b) lymphocyte (group 2); (c) plasma cell (group 1); (d) plasma cell (group 2); (e) macrophage (group 1); (f) macrophage (group 2); HC, heterochromatin; CC, cell center; L, lysosome; M, mitochondria; C, cytoplasm; RER, rough endoplasmic reticulum; EC, euchromatin; Ph, phagosome; N, nucleus; NP, nuclear pore.
Regardless of the habitat of the Prussian carp, plasma cells have an oval shape with an eccentrically located rounded nucleus. In the second group, heterochromatin of the cell nucleus is more condensed than that of the first group. The cell cytoplasm contains mitochondria, lysosomes, and rough endoplasmic reticulum, which is more developed in the cells of the second group compared to the first one. In the second group, mitochondria and lysosomes of a larger area on the sections of plasma cells are found in comparison with subcellular structures of the first group. No statistically significant differences are found for the nucleus area; the number of nucleoli, mitochondria, or lysosomes; or for the width of nuclear pores (Table 1; Figs. 2c, 2d).
In the groups of Prussian carp considered, macrophages are large oval cells, with an eccentrically located nucleus containing a large number of nucleoli (from five to ten). Lumpy heterochromatin is more condensed in the nuclei of individuals of the second group; it is localized to a greater extent on the periphery of the nucleus with a break in the nuclear pores. The width of the nuclear pores is greater in the cells of individuals of the second group compared to those of the first group. The cytoplasm contains single cisternae of the rough endoplasmic reticulum, mitochondria, and phagosomes (on average, six), the area of the latter on the cell sections in the second group exceeds more than twofold this indicator in the first group (Table 1; Figs. 2e, 2f).
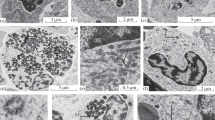
Neutrophils are round cells with an acentric nucleus. The areas of cells and nuclei of Prussian carp of the first and second groups differ slightly. In the mesonephros interstitium, cells with a stab nucleus dominate. Lumpy heterochromatin, more condensed in the nuclei of the second group, is located mainly along the nuclear membrane with a break in the nuclear pores. The width of the nuclear pores of neutrophils of the second group significantly exceeds this indicator of the first group. In the cell cytoplasm, mitochondria, tubules of the rough endoplasmic reticulum, vesicles, and electron-dense specific granules filling the cytoplasm are found. The area of mitochondria in the neutrophils of the second group is significantly smaller than that of the first group. The size and number of granules in neutrophils of both groups of Prussian carp differ insignificantly (Table 2; Figs. 3c, 3d).
Ultrastructure of granulocytes and cells with radially arranged vesicles of Prussian carp: (a) eosinophil (group 1); (b) eosinophil (group 2); (c) neutrophil (group 1); (d) neutrophil cell (group 2); (e) cell with radially arranged vesicles (group 1); (f) cell with radially arranged vesicles (group 2); V, vesicle; G, granule; HC, heterochromatin; CC, cell center; M, mitochondria; SG, specific granules; C, cytoplasm; EC, euchromatin; N, nucleus; NP, nuclear pore.
Eosinophils are round cells with an average area of 63–64 µm2 with an eccentrically located nucleus (8–10 µm2). In the second group, the mesonephros is characterized by the presence of more mature eosinophils, as evidenced by different shapes of the cell nucleus. In the first group, rounded nuclei are found on sections of most cells; in the second group, they are rod-shaped. In contrast to neutrophils, nucleoli in the eosinophil nuclei are rare in both groups of Prussian carp. It should be noted that the width of nuclear pores is significantly greater in the cells of the second group of Prussian carp compared to the first one. The cytoplasm of most eosinophils of Prussian carp of the first group contains four mitochondria, while that of the second group has 7–11 mitochondria. These differences are significant. The area of mitochondria in the cells of the second group is significantly smaller than this indicator of the first group. Rounded electron-dense specific granules filling the cytoplasm are a characteristic feature of eosinophils. There is a more than twofold statistically significant excess in the second group, and the number of specific granules exceeds that of the first group more than twofold, but their size is smaller (Table 2; Figs. 3a, 3b).
The cells with radially arranged vesicles are round-shape and have an eccentrically located round or bean-shaped nucleus. In the mesonephros interstitium, a greater number of cells with a bean-shaped nucleus are noted in the second group; these nuclei contain more densely packed heterochromatin compared to that of the first group. The nuclear pores in the cell nuclei of the second group are wider compared to that of the first group, and this difference is statistically significant. The cytoplasm of the cells contains 10–13 vesicles and mitochondria, and the latter are larger in the cells of the second group comparing to those of the first group (Table 2; Figs. 3e, 3f).
The renal corpuscle is one of the main elements of the filtration apparatus of the kidney, which has a single structural plan in the groups of Prussian carp considered. In the second group, the area of the renal corpuscle is 83% less than that of the first group (statistically significant difference). The wall of the renal corpuscle consists of two layers (parietal and visceral), separated by a 2- to 3-µm thick lumen. The visceral layer of the renal corpuscle is formed by podocytes, the area of which differs slightly in the groups considered. In podocytes and capillary endotheliocytes, the basal membrane of the visceral layer of the renal corpuscle is similar; its thickness is significantly greater in the cells of individuals of the second group compared to that of the first one. The area of endotheliocytes differs slightly in the first and second groups. In the first group, the area of capillaries of the glomerulus is significantly less than that of the second group (Table 3; Figs. 4a, 4b). Mass transfer through the capillary wall into the Bowman’s cavity capsules are twice as large in the first group compared with the second one.
Ultrastructure of the renal corpuscle and epitheliocytes of the proximal tubule of Prussian carp: (a) renal corpuscle (group 1); (b) renal corpuscle (group 2); (c) epitheliocytes of the proximal tubule type I (group 1); (d) basal part of the epithelial cell of the proximal tubule of type I (group 1); (e) zone of endocytosis of the proximal tubule of type I (group 1); (f) epitheliocytes of the proximal tubule of type I (group 2); (g) basal part of the epitheliocyte of the proximal tubule of type I (group 2); (h) zone of endocytosis of the proximal tubule of type I (group 2); (i) epitheliocytes of the proximal tubule of type I (group 1); (j) apical part of epitheliocytes of the proximal tubule of type II (group 1); (k) basal part of epitheliocytes of the proximal tubule of type II (group 1); (l) epitheliocytes of the proximal tubule of type II (group 2); BM, basement membrane; V, vesicle; HC, heterochromatin; SER, smooth endoplasmic reticulum; EZ, endocytosis zone; Cap, capillary; M, mitochondria; MV, microvilli; BC, body cavity; PC, podocyte of the visceral layer of Bowman’s capsule; SG, secretory granules; TR, tubular reticulum; BB, brush border; EN, endotheliocyte; FE, flat epithelium of the parietal lobe of Bowman’s capsule; EC, euchromatin; N, nucleus; Nu, nucleolus; NP, nuclear pore.
The epitheliocytes of the proximal tubule of the considered groups of Prussian carp are characterized by the typical structure of the cells of this part of the nephron. Conventionally, two types of epitheliocytes (I and II) may be distinguished in regard to the ultrastructure of epitheliocytes of the proximal tubule.
The beginning of the proximal tubule is formed by epithelial cells of type I. These are elongated, pyramidal cells, 23.4 ± 0.7 μm in height (the first group) or 21.2 ± 0.3 μm (the second group), located on the basal membrane and tightly adjacent to each other. The thickness of the basement membrane is significantly greater in the tubules of the second group compared to the first one (Table 3; Figs. 4c–4f). Mass transfer through the wall of the tubule formed by epitheliocytes of type I in the first group exceeds 1.6 times that in the second one.
The nuclei of epitheliocytes are rounded, located in the basal part of the cells. In the second group, the nuclei of epitheliocytes carry a smaller amount of more densely packed heterochromatin. They have a greater number of nucleoli and a greater width of nuclear pores compared to these indicators in the nuclei of the first group.
The granular cytoplasm contains mitochondria, and their number and area in the epitheliocytes of the second group is greater than that of the first one. Strands of the plasma membrane stretch from the basal part; these strands then pass into the system of tubules of the smooth endoplasmic reticulum. It should be noted that a smooth endoplasmic reticulum with wider cisternae is noted in the epitheliocytes of the second group compared to the first group. SER is localized mostly in the basal part of the cell, almost parallel to the basement membrane. In the epithelial cells of the first group, this organoid is oriented along the cell axis. There are also lysosomes and large electron-dense secretory granules characteristic of this area of the nephron. The number and area of secretory granules are significantly greater in the cells of the second group of Prussian carp compared to the first one. In the apical part of the cells, there is a well-developed zone of endocytosis on the margin of the brush border. The brush border is the highest in epithelial cells of this type. The length of the brush border of tubules in the second group is significantly less than that in the first group (Table 3; Figs. 4c–4h).
The epitheliocytes of type II are the cells that are structurally similar to the type I cells, but are smaller than those in cell height. The height and area of epitheliocytes of type I are less than those of type II; on the contrary, the thickness of the basal membrane is significantly greater in type I compared to type II (Table 3; Fig. 4l). Mass transfer through the wall of the tubule formed by epitheliocytes of type II was 1.16 times greater in the first group of Prussian carp than in the second one. The nuclei of epitheliocytes of type II are rounded, located in the basal part of the cells. The cells of the second group are characterized by larger nuclei with a smaller amount of heterochromatin, a greater width of nuclear pores, and a greater number of nucleoli compared to those of the first group. In epitheliocytes of type II, the cytoplasm contains a smaller number of mitochondria compared to type I. It should be noted that the mitochondria of cells of the second group of Prussian carp are larger, and their number is greater compared to the first group. The smooth endoplasmic reticulum in epitheliocytes of type II is more developed than that in the epitheliocytes of type I. The width of the tubules of the smooth endoplasmic reticulum is greater in the cells of Prussian carp of the second group. The length of the endocytosis zone and the length of the brush border differ insignificantly in the cells of both groups of fish (Table 3; Figs. 4i–4l).
The epithelial cells of the distal tubule are tall and very wide at the cell base. The height, area of epitheliocytes, and the thickness of the basement membrane of the distal tubules of Prussian carp of the second group are smaller than those of the first one (Table 3). Mass transfer through the wall of the distal tubule of the first group is 2.6 times lower than that of the second group. The nuclei of most cells occupy a central position; sometimes, they are shifted to the basal part. The nuclei area is smaller on the cell sections of Prussian carp of the first group compared to the second one. On the contrary, the width of nuclear pores is somewhat larger and heterochromatin is more condensed in the cells of the first group. The smooth endoplasmic reticulum with a larger width of the tubules is more developed in the cells of Prussian carp of the second group compared to the first one. It should be noted that smooth endoplasmic reticulum is located differently relative to the basement membrane of the epitheliocytes of the distal tubule. In the first group, the SER tubules are located perpendicular to the basal membrane; in the second group, they are parallel to it. In the first group, mitochondria in the cells of the distal tubules are located perpendicular to the basal membrane in its immediate vicinity; in the second group, mitochondria are more distant from the membrane and they are located in the cell randomly. The number of mitochondria in the epitheliocytes of Prussian carp of the second group is greater than that in the first. Many lysosomes and vesicles are found on sections of epithelial cells of the distal tubules. The area of vesicles on cell sections of the second group exceeds this indicator more than twofold compared to the first group (Table 3; Figs. 5a–5d).
Ultrastructure of epitheliocytes of the distal tubule of Prussian carp: (a) epitheliocyte of the distal tubule (group 1); (b) basal part of the epitheliocyte of the distal tubule (group 1); (c) basal part of the epitheliocyte of the distal tubule (group 2); (d) epitheliocyte of the distal tubule (group 2); BM, basement membrane; HC, heterochromatin; SER, smooth endoplasmic reticulum; M, mitochondria; EC, euchromatin; N, nucleus; NP, nuclear pore.
DISCUSSION
Our data testify to the peculiarities of the cell ultrastructure of the mesonephros of Prussian carp in freshwater and brackish water reservoirs. It is known that an increase in the osmotic load activates a nonspecific immune response; the increase in the size of leukocytes and the number and size of mitochondria, organelles, and inclusions associated with cytoplasmic transport are cytological markers of this process (Birrer et al., 2012; Flerova et al., 2020). Compared with Prussian carp from the freshwater reservoir, a larger area and greater number of mitochondria of lymphocytes and eosinophils, a greater number of specific granules of eosinophils, a larger area of mitochondria of neutrophils and of the cells with radially arranged vesicles, and a greater number of macrophage phagosomes in combination with a more frequent occurrence in the interstitium of mature forms of mesonephros granulocytes are characteristics of fish living at a salinity of 6‰. These peculiarities indicate the activation of cellular immunity systems under increased osmotic load. The only exception is the plasma cells of the mesonephros of Prussian carp from the Khara River. The sizes of mitochondria and lysosomes decrease without changing their number in this population.
The large size of nuclear pores in the nuclei of macrophages, neutrophils, eosinophils, and cells with radially arranged vesicles, together with an increase in the heterochromatin condensing degree in the nuclei of leukocytes of Prussian carp of the second group (compared with the first one), indicates indirectly that the synthetic activity of the nucleus increases along with the likely inactivation of a part of the chromosome. Such changes allow implementing various epigenomes by differential gene expression and, consequently, maintaining the phenotypic plasticity of the species under changing environmental conditions (Splinter et al., 2011; Lessing et al., 2013; Zenkina and Shevchuk, 2015; Eshonov, 2018).
The nephron also undergoes a number of structural changes that reflect functional adaptations to environmental conditions. In nephrons of Prussian carp sampled in a water body with a salinity of 6‰, smaller renal bodies are found; they are characterized by smaller capillary lumen and a smaller area of podocytes and by a greater thickness of the basement membrane compared to the fish inhabiting fresh water. Earlier, it was shown that adaptation to a hyperosmotic environment leads to a decrease in the diameter of the Bowman–Shumlyansky filtration capsules, accompanied by a decrease in the glomerular filtration rate in Etroplusma culatus and Alburnu starichi (Virabhadrachari, 1961; Oğuz, 2015). The decrease in mass transfer, demonstrating a decrease in the transport of substances, in combination with the ultrastructural changes described, indicates a decrease in both the formation of primary urine and the glomerular filtration rate of the renal corpuscle in the Prussian carp population living in brackish waters (6‰).
The ultrastructural features in the tubular epitheliocytes of Prussian carp living at different salinity levels are primarily associated with organelles responsible for intracellular transport. Membrane tangles, which are associated with the mechanism of secretion of divalent ions and are characteristic of epithelial cells of the proximal and distal tubules of marine fish, are not found in the cells of Prussian carp caught in brackish water of 6‰ (Natochin, 1976; Flerova, 2012). Nevertheless, the reorientation of the smooth endoplasmic reticulum with respect to the basement membrane is accompanied by an increase in the tubule width in all types of epitheliocytes of Prussian carp from the Khara River compared to the individuals from the fresh water. These structural changes indicate the increased work of pumps that provide active transport of ions under conditions of increased osmotic load and that are located mainly in the basal part of the cells (Natochin, 1976).
The number of mitochondria in the cells of the proximal and distal tubules of Trachurus mediterraneus (Staindachner) and Diplodus annularis (L.), living in the Black Sea, exceeds more than twofold the number of tubules in the epitheliocytes of freshwater fish of the Volga–Caspian basin (Flerova, 2012). This feature is associated with implementing antigradient processes occurring in fish epitheliocytes depending on the salinity of the water (Natochin, 1976; Elger and Hentschel, 1981; Flerova, 2012). In the groups of Prussian carp studied, the number and size of mitochondria in tubular epitheliocytes tend to increase according to the water salinity. Nevertheless, the number and size of mitochondria in the cells of the tubules of Prussian carp from the Khara River are lower compared to the values obtained for mitochondria of epitheliocytes of the tubules of marine fish (Flerova, 2012).
According to the results obtained, the nuclei of epitheliocytes of all types of tubules in the mesonephros of the fish from the Khara River have a greater number of nucleoli and nuclear pores compared to similar structures in the cells of individuals from the freshwater pond. In addition, a smaller area of heterochromatin is found in the nuclei of the cells of the proximal tubules of the fish from the Khara River. These features may appear as cytological markers of an increase in the environmental (water) salinity, since they indicate an increase in the synthesis of various proteins of nephron epitheliocytes; this, in turn, leads to a greater variability of possible metabolic pathways of ionocytes under increasing osmotic load (Bryan, 2000; Shatskikh and Gvozdev, 2013).
In Alburnus tarichi, the number and area of secretory granules in the cells of the proximal tubules of type I mesonephros increase significantly in accordance with the water salinity. In addition, these indicators have been compared for the fish inhabiting the salty endorheic Van Lake and the freshwater Karasu River (Oğuz, 2015). A similar change has been registered in the epitheliocytes of type I of proximal tubules in the mesonephros of Prussian carp from the Khara River and Finogenov Pond. It should be noted that the greater number of vesicles in the basal part of the distal tubules is also a cytological marker of nephrons in marine fish (Flerova, 2012).
The mass transfer of substances through the wall of the tubule decreases due to the thickening of the basement membrane and to a decrease in the length of epitheliocytes in the proximal tubules of Prussian carp from the Khara River. On the contrary, in the distal tubules, the efficiency of mass transfer of substances through the wall increases due to a decrease in the thickness of the basement membrane and the width of epitheliocytes with an increase in the osmotic load. It is known that the brush border of the proximal tubules regulates the rate of active fluid transport (Natochin, 1976). A decrease in the length of the brush border in the proximal tubules of type I in nephron of Prussian carp of the Khara River, compared with the fish from Finogenov Pond, indicates a decrease in the volume of the glomerular filtrate coming from the renal corpuscle. Taken together, these structural changes may be associated with a change in the rate of ion reabsorption in the proximal tubules and in ion secretion in the distal tubules, as well as with a change in the volume of urine excreted by the kidneys, which, in turn, allows maintaining the water–salt homeostasis of the entire organism when the water salinity changes (Natochin, 1976).
The sampling sites are close to each other. The species considered is migratory; therefore, we argue that it is highly likely that a population with a single gene pool lives in the reservoirs studied (Tupikova et al., 1989; Kolpakov and Milovankin, 2010). Therefore, we assume a high adaptive capacity of the cellular structures of the mesonephros. Comparing our own results and the literature data on the study of the ultrastructure of the nephron of marine and freshwater fish, we conclude that there are common ultrastructural differences, which are the changes in the quantitative characteristics of cells, of subcellular structures of leukocytes, and of the nephron. The degree of differences depends on the water salinity in the reservoir.
CONCLUSIONS
Under different osmotic conditions of the environment, the ultrastructure of the mesonephros of Carassius gibelio is characterized by a number of peculiarities. Most likely, a slight increase in the water salinity up to 6‰ primarily causes the multidirectional changes in the quantitative characteristics of leukocyte mitochondria and in all types of epithelial cells, as well as specialized types of inclusions in eosinophils, macrophages, proximal tubules of type I, and distal tubules of the nephron. There are changes in the nuclear structures of some types of interstitium cells and epitheliocytes. In the nephron tubules, epithelial cells of smaller sizes are found; in the epithelial cells of the tubules, a more developed smooth endoplasmic reticulum and a shorter brush border of the cells of the proximal tubule are observed. As the water salinity increases, a smaller area of renal corpuscles, glomerular capillaries, and podocytes is noted. A greater thickness of the basement membrane and a lower mass transfer is found for the renal corpuscles and proximal tubules; for the distal tubules, this pattern is the opposite (a smaller thickness of the basement membrane and a greater mass transfer).
Therefore, during the transition of a stenohaline freshwater species to brackish water, the physiological mechanisms of ionic and osmotic regulation are activated, which provide a high adaptive ability of the cellular structures of the mesonephros. The degree of cytological rearrangements depends on the absolute water salinity in the reservoir.
REFERENCES
Birrer, S.C., Reusch, T.B., and Roth, O., Salinity change impairs pipefish immune defence, Fish Shellfish Immunol., 2012, no. 33 (6), pp. 1238–1248.
Bryan, R., Cullen nuclear RNA export pathways, Mol. Cell. Biol., 2000, pp. 4181–4187.
Burkova, T.N., Characteristics of phytoplankton of the highly mineralized Khara River, Izv. PGPU im. V.G. Belinskogo, 2011, no. 25, pp. 493–496.
Elger, M. and Hentschel, H., The glomerulus of a stenohaline fresh-water teleost, Carassius auratus gibelio, adapted to saline water. a scanning and transmission electron-microscopic study, Cell Tissue Res., 1981, no. 220 (1), pp. 73–85.
Eshonov, Kh.K.O., Structural and functional organization of nuclear pores, Materialy III Nauch.-prakt. konferentsii. Novoe v biologii i meditsine (Proc. III Sci. Pract. Conf. “New in Biology and Medicine”), 2018, pp. 85–92.
Flerova, E.A., Kletochnaya organizatsiya pochek kostistykh ryb (na primere otryadov Cypriniformes i Perciformes) (Cellular Organization of the Kidneys of Teleost Fishes (by the Example of the Orders Cypriniformes and Perciformes)), Yaroslavl: Yarosl. Gos. S-kh. Akad., 2012.
Flerova, E.A., Sendek, D.S., and Yurchenko, V.V., Specific features of the ultrastructure of mesonephros of smolts of the Atlantic salmon Salmo salar L. (Baltic Sea population) and brown trout Salmo trutta L., Inland Water Biol., 2020, vol. 13, no. 3, pp. 445–454.
Folmar, L.C. and Dickhoff, W.W., The parr–smalt transformation (smoltification) and seawater adaptation in salmonids: a review of selected literature, Aquaculture, 1980, vol. 21, no. 1, pp. 1–37.
Gusakov, V.A., Benthic meiofauna of highly mineralized rivers of the Eltonskii Natural Park (Russia), Zapov. Nauka, 2019, no. 4 (1), pp. 37–63.
Kolpakov, N.V. and Milovankin, P.G., Distribution and seasonal changes in fish abundance in the estuary of the Razdol’naya River (Peter the Great Bay), Sea of Japan, J. Ichthyol., 2010, vol. 50, no. 6, pp. 445–459.
Komoroske, L.M., Jeffries, K.M., Connon, R.E., Dexter, J., Hasenbein, M., Verhille, C., and Fangue, N.A., Sublethal salinity stress contributes to habitat limitation in an endangered estuarine fish, Evol. Appl., 2016, vol. 9, no. 8, pp. 963–981.
Lessing, D., Anguera, M.C., and Lee, J.T., X chromosome inactivation and epigenetic responses to cellular reprogramming, Annu. Rev. Genom. Hum. Genet., 2013, no. 14, pp. 85–110.
Maksimovich, A.A., Serkov, V.M., Zagal’skaya, E.O., and Kudra, A.A., Ultrastructure and function of proximal tubular cells of nephrons of pacific salmons adapted to environments with different salinity, J. Evol. Biochem. Physiol., 2000, vol. 36, no. 3, pp. 33–345.
Mazzarella, A.B., Voje, K.L., Hansson, T.H., Taugbøl., A., and Fischer, B., Strong and parallel salinity-induced phenotypic plasticity in one generation of three spine stickleback, J. Evol. Biol., 2015, vol. 28, no. 3, pp. 667–677.
Mezhzherin, S.V. and Lisetskii, I.L., Genetic structure of carp populations (Cypriniformes, Cyprinidae, Carassius L. 1758) inhabiting water bodies of the Middle Dnepropetrovsk basin, Tsitol. Genet., 2004, no. 5, pp. 35–44.
Natochin, Yu.V., Ion-reguliruyushchaya funktsiya pochki (Ion-Regulating Function of the Kidney), Leningrad: Nauka, 1976.
Oğuz, A.R., A histological study of the kidney structure of van fish (Alburnus tarichi) acclimated to highly alkaline water and freshwater, Mar. Freshwater Behav. Physiol., 2015, vol. 48, no. 2, pp. 135–144.
Sezaki, K., Kobayasi, H., and Nakamura, M., Size erythrocytes in the diploid and triploid specimens of Carassius auratus langsdorfi, Jpn. J. Ichthyol., 1977, vol. 24, no. 2, pp. 135–140.
Shatskikh, A.S. and Gvozdev, V.A., Heterochromatin formation and transcription in relation to trans-inactivation of genes and their spatial organization in the nucleus, Biochemistry (Moscow), 2013, vol. 78, no. 6, pp. 603–612.
Splinter, E., Wit, E., Nora, E.P., Klous, P., Werken, H.J.G., Zhu, Y., Kaaij, L.J.T., Ijcken, W., Gribnau, J., Heard, E., and Laat, W., The inactive X chromosome adopts a unique three-dimensional conformation that is dependent on Xist RNA, Genes Dev., 2011, vol. 25, no. 13, pp. 1371–1383.
Sunde, J., Tamario, C., Tibblin, P., Larsson, P., and Forsman, A., Variation in salinity tolerance between and within anadromous subpopulations of pike (Esox lucius), Sci. Rep., 2018, vol. 8, no. 1, pp. 1–11.
Timakova, T.K., Flerova, E.A., and Zabotkina, E.A., Metody svetovoi i elektronnoi mikroskopii v biologii i veterinarii: uchebno-metodicheskoe posobie (Methods of Light and Electron Microscopy in Biology and Veterinary Medicine: Teaching Aid.), Yaroslavl: Yarosl. Gos. S-kh. Akad., 2014.
Tupikova, N.V., Sidorova, G.A., and Konovalova, E.A., Zakonomernosti formirovaniya populyatsionnoi struktury karpovykh ryb Volgo-Kaspiiskogo raiona (Patterns of the Formation of the Population Structure of Cyprinids in the Volga–Caspian Region), 1989, рр. 21–27.
Verhille, C.E., Dabruzzi, T.F., Cocherell, D.E., Mahardja, B., Feyrer, F., Foin, T.C., Baerwald, M.R., and Fangue, N.A., Inter-population differences in salinity tolerance and osmoregulation of juvenile wild and hatchery-born Sacramento splittail, Conserv. Physiol., 2016, vol. 4, no. 1, pp. 1–12.
Virabhadrachari, V., Structural changes in the gills, intestine, and kidney of Etroplus maculatus (Teleostei) adapted to different salinities, J. Cell Sci., 1961, vol. 3, no. 59, pp. 361–369.
Yang, S., Tsai, J., Kang, C., Yang, W., Kung, H., and Lee, T., The ultrastructural characterization of mitochondria-rich cells as a response to variations in salinity in two types of teleostean pseudobranch: milkfish (Chanos chanos) and Mozambique tilapia (Oreochromis mossambicus), J. Morphol., 2017, vol. 278, no. 3, pp. 1–13.
Zenkina, V.G. and Shevchuk, D.V., Changes in facultative heterochromatin in women in the age aspect, Fundam. Issled., 2015, no. 1, pp. 1831–1835.
Zinchenko, T.D., Golovatyuk, L.V., and Abrosimova, E.V., Species diversity of benthic communities of saline rivers in extreme natural conditions of the arid cis-Elton region (review), Ross. Zh. Prikl. Ekol., 2017, no. 1 (9), pp. 14–21.
ACKNOWLEDGMENTS
The authors are grateful to N.V. Lobus (Laboratory of Ocean Chemistry, Shirshov Institute of Oceanology, Russian Academy of Sciences, Moscow, Russia) for invaluable assistance in sampling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by D. Martynova
Rights and permissions
About this article
Cite this article
Flerova, E.A., Evdokimov, E.G. Peculiarities of the Mesonephros Cell Ultrastructure of the Prussian Carp Carassius gibelio (Cypriniformes, Cyprinidae) under Various Salinity Conditions. Biol Bull Russ Acad Sci 49, 1024–1036 (2022). https://doi.org/10.1134/S1062359022080064
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022080064