Abstract
The pace-of-life syndrome (POLS) hypothesis is a recent but very influential concept in life-history theory. Due to the extreme progress in research in POLS over the last decade, a review of the origin and development of this concept is topical. The roots of the POLS hypothesis go back to the r/K selection theory of MacArthur and Wilson, authors of the first idea of predictable correlations among life-history traits. Following r/K selection theory, the idea of a fast–slow life-history continuum appeared in ecology, suggesting that life-history traits covaried and formed axes from fast to slow life histories. Species physiology was soon incorporated into the fast–slow continuum theory. Thus, animal species were supposed to vary from fast species with an early development and maturation, a high rate of metabolism, a high mortality, and a short lifespan to slow species with a late development, a low metabolic rate, and a long lifespan. The theory was well supported by empirical studies in various animal species. In parallel, the concept of personality emerged in behavioral studies. The concept suggested consistent and predictable between-individual variations in behavioral phenotypes formed by syndromes of various correlated behavioral traits. More recently, the concepts of personality and fast–slow life-history continuum formed the joint and more complex POLS idea of a multivariate adaptive integration of behavior, life history, and physiology among individuals within and between species. The POLS concept suggests that various traits form a continuum from aggressive, bold, active explorers with fast life histories to shy, nonaggressive individuals with low exploration outfits and a slow life. The predictions were tested in numerous studies, and empirical data have extended the basic idea of pace-of-life: the relationships appeared to be more complex and multidimensional. The POLS hypothesis presently includes covariations among life-history, behavior, immunity, hormones, and metabolic rates, with these relationships being modulated by the environment, development, population density, and social conditions. The POLS ideas, being of great applied and theoretical significance, and long-term empirical studies in the wild populations are in high demand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
For millennia, people who interact with animals have known that animals have personality. However, until recently, this everyday knowledge existed separately from analytical research. The presence of stable differences between individuals in their behavior was not a secret to researchers of animal behavior. However, until the end of the 20th century, quantitative methods for analysis of this variability had not been developed. As a result, scientists had to deal with verbal descriptions, or, at best, differences in single parameters were considered. In statistical analysis and modeling, variability within age and sex groups was generally regarded as the result of random deviations from the average. For physical, physiological, and morphological parameters, researchers focused on the mean values within cohorts, whereas differences between individuals were regarded as an error. More than 30 years ago, such a perception of wildlife was described by Bennett as “the tyranny of the golden mean“ (Bennett, 1987). In the 21st century, due to the development of methods and the synthesis of several fields of knowledge, approaches to studying biological objects have changed. As a result, researchers began to analyze differences between individuals quantitatively, purposefully, and systematically, with an emphasis on their multidimensional and complex nature.
The concept of the pace-of-life syndrome (POLS) (Wikelski et al., 2003; Réale et al., 2010; Dammhahn et al., 2018) is one of the main concepts in ecology explaining the patterns of intraspecific variability in animals. It links together the physiology, behavior, and life history traits of individuals regarding genetic, ontogenetic, population, and evolutionary processes. This is a relatively young concept: its history is traced since the publication of the fundamental study by Réale et al. (2010), the number of citations of which approaches 1000. To date, tens of thousands of articles, including dozens of large reviews, have already been devoted to studying the pace-of-life syndrome. The accumulated amount of information, as well as the conceptual contradictions (for example, see the reviews Montiglio et al., 2018; Royaute et al., 2018) necessitates systematization of the studies in this area and tracing the formation of the concept from its origins to the present day, thereby outlining the prospects for the most relevant future research. An important aspect that determines the need for a review of studies devoted to the pace-of-life syndrome is the lack of information about this concept in the Russian-language literature. There is no corresponding terminology in Russian; for a brief glossary of basic terms, see the Appendix. Until now, there have been practically no scientific studies in this area in the Russian Federation, and this theory wasn’t included in the educational programs of universities.
The purpose of this study is to trace and comprehend the main stages in the development of the concept of the pace-of-life syndrome, to systematize the variety of modern studies in this area, and to determine the prospects for research.
FIRST IDEAS ABOUT RELATIONSHIPS BETWEEN LIFE-HISTORY CHARACTERISTICS: r/K SELECTION THEORY
By the middle of the 20th century, ideas about the correlations between various species-specific characteristics (syndromes of traits) began to appear in ecology, which primarily determined the dynamics of populations under the influence of various external conditions. The first ideas about the life history of a species as a nonrandom, stable set of traits formed under the influence of natural selection appeared (Cole, 1954; Lack, 1954). The development of these ideas and, simultaneously, the development of an adequate mathematical apparatus for simulating population processes served as prerequisites for the emergence of r/K selection theory (MacArthur and Wilson, 1967; Pianka, 1970).
This theory, which had a great influence on the development of ecology and evolutionary biology, can be considered a predecessor of the POLS concept. MacArthur and Wilson, in their outstanding monograph on island biogeography (MacArthur and Wilson, 1967), noted for the first time that, at the beginning of the colonization of islands by new species, when the abundance of species and competition are low and resources are abundant, species with a fast growth, early reproduction, high fertility, and short lifespan have an advantage. However, as the population density, competition, and saturation of the environment increase, selection begins to favor species with other traits—a slow development and late reproduction, a small number of large mature high-quality offspring, and a long lifespan.
The action of natural selection in the first phase was designated by the authors of the “r-selection” theory: it is density-independent, because the mortality of individuals does not depend on the population density. The second phase is characterized by the action of density-dependent K-selection (MacArthur and Wilson, 1967; Reznick et al., 2002). The authors assumed that, under the influence of various forms of natural selection in nature, the coexistence of two categories of living organisms is maintained, which replace each other under different conditions.
Pianka (1970, 1981) made several important contributions to r/K selection theory: first, he related different types of selection to the evolution of life histories, thereby designating the choice of r- or K-strategy as a stable trait of a species ( Pianka, 1981). In addition, he formulated ideas about the intermediate forms between r- and K-strategies and postulated the existence of an r/K continuum (Pianka, 1970). Finally, he assumed a correlation between the position of species on the r/K axis and their physical traits, primarily the body size.
Thus, r/K selection theory is one of the first predictive models for the evolution of life histories. It entailed an avalanche of comparative empirical studies and accumulation of huge amounts of data on the correlations between life history parameters in nature.
The accumulation of facts and information about real living organisms revealed the drawbacks of r/K selection theory: it turned out to be too simplified. The original version of the concept did not take into account many ecological and population parameters that play an important role in choosing the life strategy. It can be said that the too simple, dichotomous division of selection forms into density-dependent and density-independent ones, as well as the corresponding dichotomous classification of living organisms, almost challenged theoretical ecologists, who began to build more realistic models and describe new patterns in the evolution of life histories.
In particular, as early as in the 1970s, it became clear that, in modeling the processes of natural selection, factors such as the pressure of predators, the structure of resource distribution, the nature of abundance dynamics, and the differences in mortality of different age cohorts are inevitably taken into account (Gadgil and Bossert, 1970; Stearns, 1977; Reznick et al., 2002). By the 1980s, the ideas about this theory had changed dramatically, and in its original form it was practically no longer directly tested and used to interpret results (Reznick et al., 2002).
In the second half of the 20th century, simultaneously with r/K selection theory, other areas of the life-history theory, a discipline that studies the diversity of life strategies and their evolution, developed rapidly. In particular, ideas about tradeoffs and negative correlations between life functions (life-history tradeoffs) have emerged (Reznick, 1985; Stearns, 1992; Roff, 2002). These notions are based on the simple idea that the resources at the disposal of an individual are not unlimited, and, therefore, an increase in the contribution to one life process must inevitably lead to a decrease in the contribution to other processes and functions (Williams, 1966; Stearns, 1989). Indeed, if such tradeoffs did not exist, all fitness-enhancing life functions would be maintained by selection and enhanced ad infinitum (Stearns, 1989).
The key point in the tradeoff theory is the tradeoffs associated with reproduction. According to the classical theory of life histories, there are several types of reproductive tradeoffs: between growth and reproduction of an individual, between reproduction and survival, and between contribution to reproduction at present and contribution to reproduction in the future (Williams, 1966; Reznick, 1985; Stearns, 1989, 1992). In fact, for an individual, all of them can be reduced to the balance between the contribution to reproduction and the somatic contribution at a given time and, accordingly, the ability to produce offspring in the future. In other words, given a limited amount of resources that an individual can invest in the production of offspring, the reproductive trade-offs determine the portions in which this amount will be distributed throughout the entire life history and form an axis from a short period of reproductive activity to a long one. For example, this entire amount can be spent on one suicidal, catastrophic reproductive effort, as in salmonids (Crespi and Teo, 2002) or in male marsupial mice of the genus Antechinus, which all die after rut (Braithwaite and Lee, 1979), or this amount can be distributed over a large number of portions and spent over a long time interval.
Another axis along which the balance of the reproductive contribution of a living organism changes corresponds to a compromise between the quality and quantity of offspring (Stearns, 1989; Dani and Kodandaramaiah, 2017). At one end of the axis, there are species that produce thousands of small offspring, the majority of which die shortly after emergence; in this case, the parental contribution to each descendant is negligible. On the other end of the axis, there are living organisms that produce only a few offspring throughout their lives but invest a lot of resources in each of them and, as a rule, surround them with long and costly parental care.
The ideas about tradeoffs have much in common with r/K selection theory (for example, the idea of a compromise between the quality and quantity of offspring is easily integrated into it). However, these areas explore reality at different angles: r/K selection theory considers life histories in a complex way, whereas the analysis of tradeoffs decomposes the variability of life histories into separate components, which greatly simplifies their quantitative analysis and opens up prospects for studying diverse correlations.
THE FIRST STAGE OF SYNTHESIS: THE FAST–SLOW LIFE HISTORY CONTINUUM
With the accumulation of empirical data on the life histories of various plant and animal species (including growth patterns, reproduction, survival, and mortality), review studies by authors who conducted comparative analyses for large groups of species began to appear. The authors of these studies attempted to explain the diversity of life histories by ordering them along certain axes and associating these axes with the physical traits of the organisms studied.
The first step in this direction was the attempt to relate the r/K continuum of life histories to body size (Pianka, 1970; Stearns, 1983). This model predicted that relatively small species should exhibit a fast growth, intensive reproduction, and short lifespan (r‑species). Conversely, large organisms should be characterized by a low reproduction rate, slow growth and development, and long lifespan (K-species) (Dobson and Oli, 2007). The model was based on the simple idea that a large living organism needs more time to grow and reproduce (Harvey and Zammuto, 1985); thus, long-term growth and development form the life history of a species.
However, in earlier studies it was already established that, even if a correction for differences in body size between species is introduced, the axis from “fast” to “slow” life histories is retained (Stearns, 1983). For example, in mammals and birds, after the introduction of such a correction, correlations between fecundity, age of first reproduction, and lifespan were retained (Gaillard et al., 1989). These correlations are determined by the environmental conditions (e.g., by predation pressure (Promislow and Harvey, 1990; Oli, 2004)) and the phylogenetic signal and are found in different groups of living organisms, even in plants (Franco and Silvertown, 1996).
Thus, on the basis of r/K selection theory, the concept of a fast–slow continuum was formed. The main idea of this concept is that different life-history traits of species correlate with each other and form syndromes and that these correlations allow species to be located along the continuum formed, even if differences in body size are taken into account. The fast–slow continuum concept postulates restrictions on the existence of all possible combinations of life histories. At one end of the continuum, there are “fast” species with a short lifespan and a large number of small offspring produced during their lives; this number can be achieved both through early maturation and through a large brood size, clutch, etc. (Bielby et al., 2007). On the other end of this continuum, there are slow species with the opposite properties. These patterns were traced especially thoroughly in mammals, hundreds of species of which were included in the comparative analysis (Promislow and Harvey, 1990; Oli, 2004; Dobson and Oli, 2007), and in birds (Gaillard et al., 1989; Sæther and Bakke, 2000).
The next qualitative step towards understanding the principles of evolution of life histories is the interest in the proximal mechanisms of the correlations described. Here, ideas about tradeoffs between the life-history traits (Dobson and Oli, 2007) were used, which make it possible to predict reasonably, for example, a shorter lifespan for a species with a higher fecundity, all other things being equal. The first ideas that, due to the pleiotropic effect of genes, the traits that give an organism competitive advantages at the peak of its reproductive activity can be associated with subsequent early aging and death arose as early as the 1950s (Williams, 1957). In addition, comparative studies in which the physiological parameters were taken into account began to appear. First of all, attempts to relate the axis of this continuum with the metabolic rate were made (Promislow and Harvey, 1990). At the very beginning of the 21st century, Wikelski and Ricklefs with their coauthors in a series of studies (mainly on different species of birds) analyzed the correlation between the metabolic rate and the pace of life. In particular, the authors of these studies suggested a direct correlation between the metabolic rate and the pace of life: in the “slow” species, both the resting metabolic rate and the associated heart rate are lower than in the “fast” ones (Ricklefs and Wikelski, 2002; Wikelski et al., 2003). In particular, a high metabolic rate (e.g., induced by external factors such as a cold climate) may lead to a decrease in the lifespan (Wikelski and Ricklefs, 2001). Contrarywise, a short lifespan of a species makes it possible to increase energy consumption, biomass production, and the metabolic rate without compromising fitness (Ricklefs and Wikelski, 2002; Wikelski et al., 2003). Thus, the variables characterizing the living conditions of the species, population processes, demographic indicators, features of the life history, and physiology (metabolism, endocrinology, and immunology) are closely interrelated. For example, tropical bird species are characterized by slow development, long lifespan, and low metabolic rates compared to closely related species from higher latitudes (Wikelski et al., 2003).
THE EMERGENCE OF THE PERSONALITY CONCEPT
It is time to turn to a completely different direction in science. Even ancient philosophers described different temperaments in humans. However, until the end of the 20th century, methodological approaches to the quantitative study of personality traits in animals had not been developed. Moreover, the vast majority of studies on personality (i.e., stable traits of character and behavior) were performed on humans by psychologists. There was no commonly accepted term for these traits (Gosling, 2001). In the English literature, the word “personality” was literally avoided in relation to animals, it was associated with nonscientific anthropomorphisms. There was no way to translate the subjective perceptions of scientists observing the behavior of animals (for which the differences in character between individuals were obvious) into testable scientific hypotheses and analytical models. Early studies usually focused on separate behavioral characteristics of animals, and almost all studies were performed in captivity and in laboratories (Wilson et al., 1994).
The intraspecific variability in the behavior of animals was also investigated in Russia starting with the studies by Pavlov (1954) on the types of the nervous system in dogs. For example, studies of individual heterogeneity in rodent populations were carried out (Shilov, 1977; Moshkin and Shilova, 2008); individual differences in fish behavior were also studied (see the review by Budaev et al., 2015). Finally, on the basis of a huge number of case studies and due to the development of quantitative methods, by the end of the 20th century, a corresponding methodological apparatus had formed and sufficient empirical data had accumulated to form a new concept of personality in zoological studies.
Personalities are stable behavioral phenotypes in animals that are formed by several traits and vary within sex and age cohorts (Gosling, 2001; Sih et al., 2004; Vonk et al., 2017).
Stable intrapopulation differences in personalities are known for different species of fishes, amphibians, reptiles, birds, and mammals (Sih et al., 2004). Moreover, they are described for representatives of several types of invertebrates (Kralj-Fišer and Schuett, 2014): spiders, crabs, insects, squids, octopuses, and even coelenterates (Hensley et al., 2012; Niemelä et al., 2012).
In other words, the existence of stable intraspecific differences in behavioral profiles was found to be a ubiquitous phenomenon in the animal kingdom. Moreover, it became possible to describe the variability in the behavior of completely different animals in very similar categories. For example, bold and shy octopuses (Octopus rubescens) (Mather and Anderson, 1993) and chimpanzees (Pan troglodytes) (Massen et al., 2013) can be distinguished. The existence of inflexible behavioral phenotypes explains the manifestation of nonadaptive forms of behavior in different animals (Biro and Stamps, 2008): for example, an individual shows aggression towards competitors, effectively hunts, but remains aggressive towards its own offspring and partner.
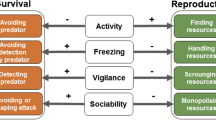
On the basis of these data, universal approaches to describing behavioral phenotypes in animals using five main traits have been formed (Budaev, 1999; Sih et al., 2004; Réale et al., 2007, 2010): (1) shyness–boldness, the response to a familiar potentially dangerous situation; (2) slow and thorough exploration–fast and superficial exploration, the response to a new situation; (3) the general level of activity in a familiar situation; (4) aggressiveness; and (5) sociability, the tendency to nonaggressive contacts with conspecifics.
It was found that the behavioral traits (especially the first four) are correlated with each other in many species. For example, back in 1976, Huntingford (1976) described a correlation between boldness towards a predator and aggressiveness towards conspecific competitors in the three-spined stickleback (Gasterosteus aculeatus): individuals that approached a predator more boldly and threatened it were more aggressive during the intrusion of conspecifics. Similar positive correlations between aggressiveness, boldness, and exploration rate in stress were also found in laboratory rodents (Koolhaas et al., 1999). As a result, the authors concluded that there is a continuum between proactive (bold, aggressive, quick explorers) and reactive individuals. Thus, the pattern of correlations between traits may be similar in different phylogenetic groups: aggressive individuals are more bold, active, and quick explorers, whereas nonaggressive individuals are shy, slow, and inactive in spiders (Riechert and Hedrick, 1993), sticklebacks, and rodents.
On the basis of such observations, they began to talk about behavioral syndromes (Sih et al., 2004)—stable associations between functionally different forms of behavior (e.g., aggression–exploratory activity) and/or correlations between manifestations of one form of behavior in different contexts (e.g., aggression towards competitors, predators, and one’s own offspring).
Finally, three components of intrapopulation variability in behavior were distinguished (Dingemanse and Wolf, 2010): (1) differences in individual behavioral traits (e.g., one individual is bolder than another); (2) differences in behavioral syndromes, i.e., in the complexes of behavioral traits in different individuals; and (3) differences between individuals in the level of behavioral plasticity (“behavioral reaction norms” (Dingemanse et al., 2010)). In other words, some individuals are able to adjust their behavior to the current context better than others (i.e., some individuals exhibit a high behavioral plasticity, and in others it is low), as in the case of laboratory rodents, which differed in their ability to change their aggressiveness (Koolhaas et al., 1999). The degree of behavioral plasticity may change in the course of ontogeny (Budaev and Zworykin, 2002). Low behavioral plasticity (the so-called behavioral carryover (Sih et al., 2004)) is one of the causes of nonadaptive behavior, which is often observed in nature.
THE SECOND STAGE OF SYNTHESIS: ANALYSIS OF THE PHYSICAL NATURE OF PERSONALITY
Having realized the reality and ubiquity of the existence of personalities in animals, researchers began to search for the physical foundations and causes for the differences in the behavioral profiles of animals. As early as the 20th century, intensive studies of the genetic basis of personalities were started (Wilson et al., 1994; Koolhaas et al., 1999; Vonk et al., 2017), and soon the hereditary nature of the variability of behavioral phenotypes was revealed in different groups of animals (Bouchard and Loehlin, 2001; Sih et al., 2004; Dochtermann et al., 2015). Selection of animals with appropriate behavioral qualities were used as a method for analyzing the genetic basis of behavior. For example, in great tits (Parus major, see the review by Groothius and Carere, 2005), two lines of birds were bred with the help of artificial selection, one of which could be called bold and fast, and the other of which was shy and slow. Similar lines were bred in mice (Mus musculus) (Koolhaas et al., 1999). Similarly, in trout (Oncorhynchus mykiss), as a result of selection for low and high post-stress levels of cortisol, bold and aggressive, on the one hand, and shy and nonaggressive, on the other hand, lines were obtained (Øverli et al., 2007). In Russia, a long-term experiment on the selection of foxes (Vulpes vulpes) for human-friendly attitude is widely known, which also showed the hereditary nature of behavioral differences between individuals and revealed correlations between behavior and other phenotypic traits of foxes (Trut et al., 2014; Belyaev et al., 1985; Trut, 1999). Today, there is no doubt that the differences in behavioral phenotypes are largely due to the differences in genotypes and can be inherited.
Given the data obtained, correlations between behavioral traits can be explained by “technical causes” such as the pleiotropic effect of genes (Sih et al., 2004; Wolf et al., 2007). In addition, correlational selection, when individuals with only certain combinations of traits receive advantages, can fix certain genotypes in a population, thereby creating expressed categories of individuals (Lande and Arnold, 1983; Sinervo and Svensson, 2002).
Simultaneously with the genetics of personalities, the physiological basis of behavioral syndromes and intraspecific behavioral differences began to be identified and analyzed in different groups of animals; however, we will only briefly touch on this key aspect of modern behavioral research. The physiological parameters studied are combined into three categories—metabolism, hormones, and immunity. It should be noted that the variability in metabolic, endocrine, and immune parameters within a species can be extremely high, varying by orders of magnitude from one individual to another and being many times greater than the variability of the mean values between species (see, for example, Burton et al., 2011). Studies of correlations between physiology and personality shed light on the nature and causes of this variability.
In different groups of animals, a correlation between the behavioral profiles of individuals and the resting metabolic rate was suggested. In this case, differences in behavioral syndromes, first of all, the most obvious and basic proactive syndrome (aggressiveness + boldness + exploration rate + level of activity correlations), are usually concerned. Namely, at the proactive end of this scale, the level of resting metabolism in different species turned out to be higher than at the reactive (“nonaggressive”) end (see reviews by Careau et al., 2008; Biro and Stamps, 2010; Houston, 2010). In other words, the proactive individuals spend and consume energy faster than the reactive ones, maintaining the intraspecific metabolic gradient (Careau et al., 2008). Then, a correlation between the place on the “proactivity–reactivity” scale and the overall level of productivity (i.e., the rate of total biomass generation through growth and reproduction) was revealed (Stamps, 2007; Biro and Stamps, 2008): more proactive individuals were also more productive. It was assumed that behavior plays a compensatory role by adjusting the animal’s lifestyle to its physiological features (Adriaenssens and Johnsson, 2009).
Correlations between the endocrinological parameters and behavioral profiles were found to be significant and multifaceted in both vertebrates and invertebrates. Neuroendocrine mechanisms can be regarded as mediators between the genotype and the behavioral phenotype. The pleiotropic effects of hormones, in addition to the pleiotropic effects of genes, explain the correlations between behavioral variables (Sih et al., 2004). For example, the testosterone level in many vertebrates is involved in the formation of the behavioral proactive syndrome (Sih et al., 2004). The pleiotropic effect of melanocortin on the behavior of vertebrates in the wild is known: darker individuals are, on average, more aggressive and active than their lighter conspecifics (Ducrest et al., 2008). It was found that the hypothalamic–pituitary–adrenal system (HPA system) plays an important role in the formation of the continuum of proactive–reactive individuals (Koolhaas et al., 1999; Øverli et al., 2007; Careau et al., 2008): the correlation between the metabolic gradient and personality is modulated by the HPA system (Careau et al., 2008). In addition, the effect of hormones at the early stages of ontogeny can determine the formation of adult behavior, reducing the behavioral plasticity (Ketterson and Nolan, 1999; Sih et al., 2004).
Finally, in a large number of studies, correlations between personality and immunity, as well as the vulnerability of an individual to various diseases in general, were found (see reviews by Cavigelli, 2005; Koolhaas, 2008; Kortet et al., 2010). These correlations are complex and ambiguous and are regulated by the external environment; however, in general, their existence is not in doubt today.
All these studies, showing the correlations between the behavioral and physiological traits of animals, gradually formed the ideas about the existence of intraspecific syndromes more complex than the behavioral syndromes, when there are continuums of individuals (possibly multidimensional) in the population, which show stable differences in a number of various traits.
In recent decades, a number of researchers have begun to search for adaptive explanations of personalities. Intuitively, it seems that selection should not maintain stable behavioral phenotypes but should favor behavioral plasticity and expand the reaction norm, which would allow individuals to adapt to changing environmental conditions (Sih et al., 2004). In addition, rigid correlations between functionally different forms of behavior are not always useful. Wolf et al. (2007) were among the first researchers to attempt to relate the evolution of personalities with the evolution of life histories. They related the tendency to risky behavior to expected future reproductive success and the tradeoff between present and future reproduction. According to their assumption, individuals that make a choice in favor of future reproduction (i.e., those who have a large residual reproductive value) are less prone to risky behavior than those who invest in reproduction at the present time and do not expect a long lifespan (Wolf et al., 2007; Dingemanse and Wolf, 2010). Thus, the first ideas appeared implying that tradeoffs in life history determine the evolution of personalities (Wolf et al., 2007; Cote et al., 2008).
THE THIRD STAGE OF SYNTHESIS: THE CONCEPT OF THE PACE-OF-LIFE SYNDROME
Thus, in the first years of the 21st century, there were the concept of a slow–fast life-history continuum in ecology and the young, though rapidly developing, concept of personality in animal behavior studies. The first of these concepts existed mostly at the interspecific level, and these concepts had little contact with each other, possibly due to their origin from different fields of knowledge.
Finally, in 2010, the key paper by Réale et al. (2010) was published, in which the authors combined small single bridges between concepts (e.g., Smith and Blumstein, 2008) into a fundamental bridge. They directly point to two circumstances: (1) despite the obvious correlations between behavior, physiology, and life-history traits, behavior is almost not considered among the variables that form the fast–slow life-history continuum; (2) although this continuum is considered in terms of interspecific and interpopulation differences, it is quite possible to apply these concepts in the studies of interindividual differences within a population. Thus, the authors for the first time related together the variability of the life-history traits, physiology, and differences in the personalities of individuals and considered these correlations not only at the interspecific and interpopulation levels, but also at the intrapopulation level, thus forming an extended concept of the pace-of-life syndrome (POLS).
Strictly speaking, the term “pace-of-life” was first used by Wikelski et al. (2003), who extended the classical concept of the fast–slow life-history continuum, having included physiological parameters into it. Réale et al. (2010) called their idea the “extended” POLS concept. However, since today the POLS concept has irreversibly integrated the behavioral component and intraspecific analysis, the word “extended” can be removed from the name of the concept.
According to the POLS concept, the metabolic rate and the life-history rate are connected by a positive two-way correlation at the intra- and interspecific levels. Metabolism serves as a fundamental determinant of the life history (Katz and Naug, 2020). One of the hypotheses relating lifespan and metabolism is the hypothesis of the synthesis of reactive oxygen species as byproducts of metabolism: the more intense the metabolism, the stronger the oxidative damage to tissues and the shorter the lifespan, i.e., the higher pace of life (Finkel and Holbrook, 2000). At the same time, metabolic and life-history rates are related to personality. The correlation with the scale of proactive–reactive individuals is the most studied: the higher are the aggressiveness, boldness, exploration rate, and activity, the higher are the pace of life and metabolic rate (Réale et al., 2010). This, for example, is illustrated well by the example of aggressive and nonaggressive dog breeds, in which both the metabolic rate and the lifespan vary accordingly (Careau et al., 2010). However, behavioral syndromes are associated with life-history parameters through mortality, which is strongly correlated with the level of risky behavior; in particular, high activity and boldness increase the risk of death from predators (see the review by Moiron et al., 2020).
The correlations that form the pace-of-life syndrome relate dozens of variables that characterize the features of the life history, behavior, and physiology of animals; the interactions are often two-way, with feedback (Sih et al., 2015). Réale et al. (2010) list at least 16 variables that change along the pace-of-life continuum. In particular, according to these authors, the following traits change among individuals, populations, and species upon the transition from a slow pace of life to a fast one:
(1) Life-history traits: lifespan (long–short), onset of maturity (late–early reproduction), and growth rate (low–high).
(2) Behavioral traits: level of parental care (high–low), degree of philopatry (philopatry–dispersal), aggressiveness (low–high), boldness (shy–bold), exploration rate (slow thorough exploration–fast superficial exploration), activity (low–high), and sociability (high–low).
(3) Physiological traits: reactivity of the hypothalamic–pituitary–adrenal system (high–low), reactivity of the sympathetic system (low–high), reactivity of the parasympathetic system (low–high), metabolic rate (low–high), sensitivity to oxidative stress (low–high), and the level of immune response (high–low).
The mechanisms of the formation and maintenance of some of these correlations were studied (see, for example, the review by Sih et al., 2015). For example, a high level of activity and aggressiveness requires high energy consumption and large volumes of metabolically active organs and, accordingly, determines a high level of basal metabolism (Careau et al., 2008; Houston, 2010; Biro and Stamps, 2010; Burton et al., 2011). However, to date, many correlations have been studied very poorly.
This is just a framework of the basic variables that greatly simplifies reality; in fact, this model can be extended not only by using universal traits but also by analyzing traits specific to certain groups of animals (e.g., the duration of hibernation in hibernating mammals). In particular, the comparison of the majority of different species of marmots and ground squirrels shows that the longer the hibernation, the slower the pace of life (Armitage, 1981; Vasilieva et al., 2009).
A number of subsequent studies consistently expanded the list of known correlations that form the pace-of-life syndrome, and the diversity of correlations revealed to date is surprising. For example, a correlation between the pace of life and the level of locomotor skills in lizards (Zootoca vivipara) was shown (Galliard et al., 2013). In the western trilling cricket (Gryllus integer), a positive correlation was found between boldness and the ability to encapsulate a foreign body (Niemelä et al., 2013). Correlations were found between the pace of life and the circadian rhythms in beetles (Matsimura et al., 2019). It was shown that the height above sea level determines the personality and the pace of life in pikas (Qu et al., 2019). It was also established that a shorter telomere length is associated with a fast pace of life as compared to a slow one (Giraudeau et al., 2019). These are just a few examples illustrating the richness of the issues covered.
MODERN DEVELOPMENT OF THE PACE-OF-LIFE CONCEPT: CRITICISM AND PROSPECTS
Réale et al. (2010) repeatedly emphasized in their landmark work that one should not try to simplify a complex reality and fold a multidimensional dynamic network of interactions into rigid correlations.
The pace-of-life syndrome in different organisms may have different architecture: different traits can be combined into blocks, and correlations between variables may have opposite signs even in several populations of the same species (Garamszegi et al., 2012). These differences can be determined by ecological factors. For example, correlations among aggressiveness, activity, and the exploration rate were present only in the populations of the three-spined stickleback that experienced active predation pressure (Dingemanse et al., 2007). Environmental factors, including seasonal ones, can have a strong effect on immunity and, accordingly, can modulate a whole range of correlations (Tieleman, 2018).
The classic situation in which, on the contrary, the expected tradeoffs between life processes are masked and predicted correlations and syndromes are not found is explained by large differences in the amount of resources between individuals, when an individual that has a lot of resources at its disposal can significantly contribute to several processes at once (Noordwijk and de Jong, 1986). Since the pace-of-life syndrome concept is based on the assumption of the existence of a tradeoff between present and future reproduction, it is meaningless to expect a pronounced pace-of-life syndrome in a population if the life-history tradeoffs are masked (Dammhahn et al., 2018). In addition, the strength and direction of correlations between variables may change as living organisms grow and develop, which is insufficiently known today (Réale et al., 2010; Kralj-Fišer and Schuett, 2014).
A separate area of research is devoted to the mechanisms of the emergence and stable coexistence in a population of several behavioral phenotypes with different paces of life. First, different phenotypes can be formed under the influence of certain forms of balancing selection (Penke et al., 2007). In particular, the resource distribution pattern and the heterogeneity of the environment play a special role in maintaining the variability of individuals along the pace-of-life continuum. Under different conditions, different phenotypes gain an advantage. For this reason, in a heterogeneous environment, balancing selection maintains the diversity of life strategies and personalities associated with them (Penke et al., 2007; Réale et al., 2010). The fluctuating density-dependent selection, the effect of which is manifested, in particular, at significant fluctuations in the population size, should be mentioned separately. Under conditions of spatiotemporal variability in the population density, individuals that are at opposite ends of the fast–slow life-history axis gain advantages at different density levels (e.g., there is evidence that the “slow” individuals are more successful at high density than the “fast” ones, and vice versa). Thus, the density-dependent selection maintains a balance between different phenotypes (Wolf and McNamara, 2012; Wright et al., 2019).
Another form of balancing selection that determines the diversity of behavioral phenotypes is the negative frequency-dependent selection (Wolf and McNamara, 2012). In this case, a certain combination of traits is favored by selection only when the frequency of this combination in the population is low, and the fitness of individuals with this combination is high as long as these individuals are rare. As frequency increases, fitness decreases, which creates equilibrium between different phenotypes. These processes are simulated well using game theory (e.g., the well-known hawk–dove model and other similar models (Maynard Smith, 1982; Hammerstein and Selten, 1994)).
In addition to genetic variability, the diversity of personalities and life strategies is determined by phenotypic plasticity (Wright et al., 2019). The ideas about bet-hedging (Starrfelt and Kokko, 2012) should be mentioned here. Bet-hedging covers a variety of phenotypic strategies, the purpose of which is to reduce the variability in fitness between individuals and maximize the geometric, rather than arithmetic, mean fitness under conditions of environmental heterogeneity (Wright et al., 2019). In other words, the bet-hedging strategy insures an individual against “complete failure” (zero reproductive success). For example, with this strategy, an individual produces offspring with different qualities (“fast” and “slow,” aggressive and nonaggressive) so that, under unpredictable changes in external conditions, at least some of the offspring could achieve high reproductive success. Studying the evolution of compound multilevel complexes of variables that constitute the pace-of-life syndrome is not an easy task, because variability in one trait may determine the variability in several other variables. Despite a large number of studies on the life-strategy evolution, the study of the pace-of-life syndrome from the standpoint of evolutionary biology is a very young direction rich in new research tasks (such as the creation of high-order models that combine the effects of frequency-dependent and density-dependent selection).
In 2018, the journal Behavioral Ecology and Sociobiology published a special set of articles devoted to the current development of the POLS concept (see the introductory article by Dammhahn et al., 2018). A significant part of these articles are fundamental reviews. In one article, the versatility and general significance of the correlations predicted by the pace-of-life syndrome concept are verified (Royaute et al., 2018): as a result of meta-analysis, it seems that this concept receives little support from empirical studies. However, this should not lead to conclusions about the inconsistency of the concept: the authors of the review point out that factors that mask correlations (primarily environmental ones) were not always taken into account when setting up the studies, model assumptions were not tested carefully, and the size of samples might be insufficient (Montiglio et al., 2018; Royaute et al., 2018).
In addition, the review of models for the origin and evolution of the pace-of-life syndrome demonstrates a lack of formal models and theoretical studies that describe the discussed correlations (Mathot and Frankenhuis, 2018). Accordingly, the not fully developed theoretical base does make it possible to test empirically all the provisions of the POLS hypothesis comprehensively.
Several articles are devoted to the sex-specific traits of the pace-of-life syndrome. Surprisingly, until recently, the obvious fact (however, see (Budaev, 1999)) that males and females differ significantly in almost all parameters involved in POLS and, as a result, have different optima in the fast– slow life-history gradient has been practically ignored in POLS studies (Hämäläinen et al., 2018). The studies included in the 2018 set are literally one of the first works that analyze the accumulated facts on the formation of differences in the pace of life in males and females in terms of metabolism, hormones, genetics, and social relationships (Hämäläinen et al., 2018; Immonen et al., 2018; Tarka et al., 2018). The available data indicate the tendency of males (especially in polygynous species) to live faster; however, these are only the first studies in this direction.
In addition, the urgent need for one simple and universal trait of the pace of life, which can measure the pace of life in different species and be used in comparative studies, is emphasized (Dammhahn et al., 2018). So far, no such standard trait has been developed.
This analysis makes it possible to identify several poorly-studied directions in POLS research (“blank spots”) that need to be filled. For example, very little is known about changes in the pace-of-life syndrome during ontogeny. Studies of species with complex life histories, including those with metamorphosis, may be especially promising here (Kralj-Fišer and Schuett, 2014). To date, quite a few studies have been devoted to these problems (and they concern the differences in personalities rather than in the pace of life in general; see, for example, Wilson and Krause (2012)). However, an analysis of such patterns can significantly improve the understanding of the evolution of life histories. Who knows how personality and the pace of life are related to the probability of neoteny? How is the reorganization of the pace of life regulated in cases when the lifestyle, the ecological niche, and the environment change dramatically among life stages, as in the case of the dragonfly?
There are data on how the pace-of-life syndrome modulates the probability of infection with parasites and other diseases (e.g., Boyer et al., 2010; Kortet et al., 2010). However, almost nothing is known about the personalities and differences in the pace of life of the parasites themselves (Kralj-Fišer and Schuett, 2014). What can be said about the pace of life of mites and fleas and the correlation of these syndromes not only with the success of feeding on the host but also with the probability of transmission of infections?
The applied significance of POLS research should be noted separately. The presence in a population of different behavioral phenotypes, for which the probabilities of being trapped and collected, as well as the probabilities of being observed during field studies may differ several times, creates methodical problems that almost no one took in account before (Réale et al., 2010). These phenotypic differences are strongly associated with the differences in life histories, physiology, and morbidity, which is an aggravating circumstance: it is possible that some part of populations is systematically skipped in field research, and this part may be very different from what is in our hands.
The same patterns are important in hunting and fishing, when part of a population is systematically withdrawn; this withdrawal is not random and, in fact, is a directed artificial selection. The same can be said about pest control with the use of poisons: as a result of using pesticides, individuals with a certain set of traits can be selected (Morales et al., 2013; Kralj-Fišer and Schuett, 2014).
The traits of personalities and the pace of life are the key characteristics of invasive species: it is known that the ability to invade is closely related to behavioral syndromes (Réale et al., 2010). The ability for synanthropic life definitely has an explanation from the standpoint of the pace-of-life syndrome. There is evidence suggestive of different sensitivity to pollution, climate change, habitat fragmentation, and other anthropogenic impacts in individuals, populations, and species with different paces of life (Réale et al., 2010; Debecker et al., 2016).
Possibly, studies of the pace-of-life continuum in different groups of animals and the role of phenotypic plasticity in its formation may contribute to understanding the mechanisms of aging. It cannot be ruled out that this knowledge could open prospects for control of these processes for humanity in the future.
Finally, studies of the pace-of-life syndrome require long-term individual-oriented observations of animals in nature and the analysis of a wide range of variables with relationships between them. Moreover, such studies should be carried out on large samples (no fewer than 100 individuals), since in such complex analytical models the effects of some factors can be masked by others (Garamszegi et al., 2012). Due to the complexity of planning and conducting such studies for many groups of animals (separate species and entire taxonomic categories), there are very few data in this regard, which restricts comparative analysis.
Thus, despite the thousands of articles published over the past decade, it can be said that the prospects for the development of the pace-of-life syndrome concept is amazing. In this review, only the main directions of work in this area are touched upon fairly superficially, and many topics were not considered at all. Today, this concept is a truly promising basis for a huge variety of studies, including completely innovative ones. The use of modern methods of research in genetics and physiology, as well as analysis of complex, multidimensional, big data opens up unlimited prospects for learning new things in ecology, evolutionary biology, and other fields of knowledge.
REFERENCES
Adriaenssens, B. and Johnsson, J.I., Personality and life-history productivity: consistent or variable associations?, Trends Ecol. Evol., 2009, vol. 24, pp. 179–180.
Armitage, K.B., Sociality as a life-history tactic of ground squirrels, Oecologia, 1981, vol. 48, pp. 36–49.
Belyaev, D.K., Plyusnina, I.Z., and Trut, L.N., Domestication in the silver fox (Vulpes fulvus Desm): changes in physiological boundaries of the sensitive period of primary socialization, Appl. Anim. Behav. Sci., 1985, vol. 13, pp. 359–370.
Bennett, A.F., Interindividual variability: an underutilized resource, New Dir. Ecol. Physiol., 1987, vol. 19, pp. 147–169.
Bielby, J., Mace, G.M., Bininda-Emonds, O.R.P., Cardillo, M., Gittleman, J.L., et al., The fast-slow continuum in mammalian life history: an empirical reevaluation, Am. Nat., 2007, vol. 169, pp. 748–757.
Biro, P.A. and Stamps, J.A., Are animal personality traits linked to life-history productivity?, Trends Ecol. Evol., 2008, vol. 23, pp. 361–368.
Biro, P.A. and Stamps, J.A., Do consistent individual differences in metabolic rate promote consistent individual differences in behavior?, Trends Ecol. Evol., 2010, vol. 25, pp. 653–659.
Bouchard, T.J. and Loehlin, J.C., Genes, evolution, and personality, Behav. Genet., 2001, vol. 31, no. 3, pp. 243–273.
Boyer, N., Réale, D., Marmet, J., Pisanu, B., and Chapuis, J.L., Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus, J. Anim. Ecol., 2010, vol. 79, no. 3, pp. 538–547.
Braithwaite, R.W. and Lee, A.K., A mammalian example of semelparity, Am. Nat., 1979, vol. 113, no. 1, pp. 151–155.
Budaev, S.V., Sex differences in the big five personality factors: testing an evolutionary hypothesis, Pers. Individ. Differ., 1999, vol. 26, pp. 801–813.
Budaev, S. and Zworykin, D., Individuality in fish behavior: ecology and comparative psychology, J. Ichthyol., 2002, vol. 42 (suppl.), pp. S189–S195.
Budaev, S.V., Mikheev, V.N., and Pavlov, D.S., Individual differences in behavior and mechanisms of ecological differentiation on the example of fish, Zh. Obshch. Biol., 2015, vol. 76, no. 1, pp. 26–47.
Burton, T., Killen, S.S., Armstrong, J.D., and Metcalfe, N.B., What causes intraspecific variation in resting metabolic rate and what are its ecological consequences?, Proc. R. Soc. London, Ser. B, 2011, vol. 278, pp. 3465–3473.
Careau, V., Thomas, D., Humphries, M.M., and Réale, D., Energy metabolism and animal personality, Oikos, 2008, vol. 117, pp. 641–653.
Careau, V., Réale, D., Humphries, M.M., and Thomas, D.W., The pace of life under artificial selection: personality, energy expenditure, and longevity are correlated in domestic dogs, Am. Nat., 2010, vol. 175, no. 6, pp. 753–758.
Cavigelli, S.A., Animal personality and health, Behaviour, 2005, vol. 142, nos. 9–10, pp. 1223–1244.
Cole, L.C., The population consequences of life history phenomena, Quart. Rev. Biol., 1954, vol. 29, no. 2, pp. 103–137.
Cote, J., Dreiss, A., and Clobert, J., Social personality trait and fitness, Proc. R. Soc. London, Ser. B, 2008, vol. 275, pp. 2851–2858.
Crespi, B.J. and Teo, R., Comparative phylogenetic analysis of the evolution of semelparity and life history in salmonid fishes, Evolution, 2002, vol. 56, no. 5, pp. 1008–1020.
Dammhahn, M., Dingemanse, N.J., Niemelä, P.T., and Réale, D., Pace-of-life syndromes: a framework for the adaptive integration of behaviour, physiology and life history, Behav. Ecol. Sociobiol., 2018, vol. 72, article 62. https://doi.org/10.1007/s00265-018-2473-y
Dani, K.G. and Kodandaramaiah, U., Plant and animal reproductive strategies: lessons from offspring size and number tradeoffs, Front. Ecol. Evol., 2017, vol. 5, article 38. https://doi.org/10.3389/fevo.2017.00038
Debecker, S., Sanmartin-Villar, I., de Guinea-Luengo, M., Cordero-Rivera, A., and Stoks, R., Integrating the pace of life syndrome across species, sexes and individuals: covariation of life history and personality under pesticide exposure, J. Anim. Ecol., 2016, vol. 85, no. 3, pp. 726–738.
Dingemanse, N.J. and Wolf, M., Recent models for adaptive personality differences: a review, Philos. Trans. R. Soc., B, 2010, vol. 365, pp. 3947–3958.
Dingemanse, N.J., Wright, J., Kazem, A.J., Thomas, D.K., Hickling, R., and Dawnay, N., Behavioural syndromes differ predictably between 12 populations of three-spined stickleback, J. Anim. Ecol., 2007, vol. 76, pp. 1128–1138.
Dingemanse, N.J., Kazem, A.J., Réale, D., and Wright, J., Behavioural reaction norms: animal personality meets individual plasticity, Trends Ecol. Evol., 2010, vol. 25, pp. 81–89.
Dobson, F.S. and Oli, M.K., Fast and slow life histories of mammals, Ecoscience, 2007, vol. 14, pp. 292–299.
Dochtermann, N.A., Schwab, T., and Sih, A., The contribution of additive genetic variation to personality variation: heritability of personality, Proc. R. Soc. London, Ser. B, 2015, vol. 282, pp. 2014–2201.
Ducrest, A.L., Keller, L., and Roulin, A., Pleiotropy in the melanocortin system, coloration and behavioural syndromes, Trends Ecol. Evol., 2008, vol. 23, no. 9, pp. 502–510.
Finkel, T. and Holbrook, N.J., Oxidants, oxidative stress and the biology of ageing, Nature, 2000, vol. 408, no. 6809, pp. 239–247.
Franco, M. and Silvertown, J., Life history variation in plants: an exploration of the fast-slow continuum hypothesis, Philos. Trans. R. Soc., B, 1996, vol. 351, no. 1345, pp. 1341–1348.
Gadgil, M. and Bossert, W.H., Life historical consequences of natural selection, Am. Nat., 1970, vol. 104, no. 935, pp. 1–24.
Gaillard, J.M., Pontier, D., Allaine, D., Lebreton, J.D., Trouvilliez, J., and Clobert, J., An analysis of demographic tactics in birds and mammals, Oikos, 1989, vol. 56, pp. 59–76.
Galliard, J.F., Paquet, M., Cisel, M., and Montes-Poloni, L., Personality and the pace-of-life syndrome: variation and selection on exploration, metabolism and locomotor performances, Funct. Ecol., 2013, vol. 27, pp. 136–144.
Garamszegi, L.Z., Marko, G., and Herczeg, G., A meta-analysis of correlated behaviours with implications for behavioural syndromes: mean effect size, publication bias, phylogenetic effects and the role of mediator variables, Evol. Ecol., 2012, vol. 26, pp. 1213–1235.
Giraudeau, M., Angelier, F., and Sepp, T., Do telomeres influence pace-of-life-strategies in response to environmental conditions over a lifetime and between generations?, BioEssays, 2019, vol. 41, no. 3, art. ID 1800162.
Gosling, S.D., From mice to men: what can we learn about personality from animal research?, Psychol. Bull., 2001, vol. 127, pp. 45–86.
Groothuis, T.G.G. and Carere, C., Avian personalities: characterization and epigenesist, Neurosci. Biobehav. Rev., 2005, vol. 29, pp. 137–150.
Hämäläinen, A., Immonen, E., Tarka, M., and Schuett, W., Evolution of sex-specific pace-of-life syndromes: causes and consequences, Behav. Ecol. Sociobiol., 2018, vol. 72, article 50. https://doi.org/10.1007/s00265-018-2466-x
Hammerstein, P. and Selten, R., Game theory and evolutionary biology, in Handbook of Game Theory, 1994, vol. 2, pp. 929–993.
Harvey, P.H. and Zammuto, R.M., Patterns of mortality and age at first reproduction in natural populations of mammals, Nature, 1985, vol. 315, no. 6017, pp. 319–320.
Hensley, N.M., Cook, T.C., Lang, M., Petelle, M.B., and Blumstein, D.T., Personality and habitat segregation in giant sea anemones (Condylactis gigantea), J. Exp. Mar. Biol. Ecol., 2012, vol. 426, pp. 1–4.
Houston, A.I., Evolutionary models of metabolism, behaviour and personality, Philos. Trans. R. Soc., B, 2010, vol. 365, pp. 3969–3975.
Huntingford, F.A., The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus, Anim. Behav., 1976, vol. 24, no. 2, pp. 245–260.
Immonen, E., Hämäläinen, A., Schuett, W., and Tarka, M., Evolution of sex-specific pace-of-life syndromes: genetic architecture and physiological mechanisms, Behav. Ecol. Sociobiol., 2018, vol. 72, article 60. https://doi.org/10.1007/s00265-018-2462-1
Katz, K. and Naug, D., A mechanistic model of how metabolic rate can interact with resource environment to influence foraging success and lifespan, Ecol. Model., 2020, vol. 416, article ID 108899.
Ketterson, E.D. and Nolan, J.V., Adaptation, exaptation, and constraint: a hormonal perspective, Am. Nat., 1999, vol. 154, no. S1, pp. S4–S25.
Koolhaas, J.M., Coping style and immunity in animals: making sense of individual variation, Brain, Behav., Immun., 2008, vol. 22, no. 5, pp. 662–667.
Koolhaas, J.M., Korte, S.M., De Boer, S.F., Van Der Vegt, B.J., Van Reenen, C.G., et al., Coping style in animals: current status in behavior and stress-physiology, Neurosci. Biobehav. Rev., 1999, vol. 23, pp. 925–935.
Kortet, R., Hedrick, A.V., and Vainikka, A., Parasitism, predation and the evolution of animal personalities, Ecol. Lett., 2010, vol. 13, no. 12, pp. 1449–1458.
Kralj-Fišer, S. and Schuett, W., Studying personality variation in invertebrates: why bother?, Anim. Behav., 2014, vol. 91, pp. 41–52.
Lack, D., The Natural Regulation of Animal Numbers, Oxford: Clarendon Press, 1954.
Lande, R. and Arnold, S.J., The measurement of selection on correlated characters, Evolution, 1983, vol. 37, pp. 1210–1226.
MacArthur, R.H. and Wilson, E.O., The Theory of Island Biogeography, Monogr. Popul. Biol., Princeton, New Jersey: Princeton Univ. Press, 1967, vol. 1.
Massen, J.J., Antonides, A., Arnold, A.M.K., Bionda, T., and Koski, S.E., A behavioral view on chimpanzee personality: exploration tendency, persistence, boldness, and tool-orientation measured with group experiments, Am. J. Primatol., 2013, vol. 75, no. 9, pp. 947–958.
Mather, J.A. and Anderson, R.C., Personalities of octopuses (Octopus rubescens), J. Comp. Psychol., 1993, vol. 107, no. 3, pp. 336–340.
Mathot, K.J. and Frankenhuis, W.E., Models of pace-of-life syndromes (POLS): a systematic review, Behav. Ecol. Sociobiol., 2018, vol. 72, Article 41. https://doi.org/10.1007/s00265-018-2459-9
Matsumura, K., Ito, R., and Miyatake, T., Pace-of-life: relationships among locomotor activity, life history, and circadian rhythm in the assassin bug, Amphibolus venator, Ethology, 2019, vol. 125, no. 3, pp. 127–132.
Maynard Smith, J., Evolution and the Theory of Games, Cambridge: Cambridge University Press, 1982.
Moiron, M., Laskowski, K.L., and Niemelä, P.T., Individual differences in behaviour explain variation in survival: a meta-analysis, Ecol. Lett., 2020, vol. 23, no. 2, pp. 399–408.
Montiglio, P.O., Dammhahn, M., Messier, G.D., and Réale, D., The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow-fast continuum, Behav. Ecol. Sociobiol., 2018, vol. 72, article 116. https://doi.org/10.1007/s00265-018-2526-2
Morales, J.A., Cardoso, D.G., Della Lucia, T.M.C., and Guedes, R.N.C., Weevil x insecticide: does ‘personality’ matter?, PLoS One, 2013, vol. 8, no. 6, article ID e67283 https://doi.org/10.1371/journal.pone.0067283
Moshkin, M.P. and Shilova, S.A., Diversity of individuals as a mechanism for maintaining the stability of population structures, Usp. Sovrem. Biol., 2008, vol. 128, no. 3, pp. 307–320.
Niemelä, P.T., DiRienzo, N., and Hedrick, A.V., Predator-induced changes in the boldness of naive field crickets, gryllus integer, depends on behavioural type, Anim. Behav., 2012, vol. 84, no. 1, pp. 129–135.
Niemelä, P.T., Dingemanse, N.J., Alioravainen, N., Vainikka, A., and Kortet, R., Personality pace-of-life hypothesis: testing genetic associations among personality and life history, Behav. Ecol., 2013, vol. 24, pp. 935–941.
van Noordwijk, A.J. and de Jong, G., Acquisition and allocation of resources: their influence on variation in life history tactics, Am. Nat., 1986, vol. 128, no. 1, pp. 137–142.
Oli, M.K., The fast-slow continuum and mammalian life history patterns: an empirical evaluation, Basic Appl. Ecol., 2004, vol. 5, pp. 449–463.
Øverli, Ø., Sørensen, C., Pulman, K.G.T., Pottinger, T.G., Korzan, W., et al., Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates, Neurosci. Biobehav. Rev., 2007, vol. 31, pp. 396–412.
Pavlov, I.P., O tipakh vysshei nervnoi deyatel’nosti i eksperimental’nykh nevrozakh (On the Types of Higher Nervous Activity and Experimental Neuroses), Moscow: Medgiz, 1954.
Penke, L., Denissen, J.J., and Miller, G.F., The evolutionary genetics of personality, Eur. J. Pers., 2007, vol. 21, pp. 549–587.
Personality in Nonhuman Animals, Vonk, J., Weiss, A., and Kuczaj, S.A., Eds., Berlin, Germany: Springer, 2017.
Pianka, E.R., On r- and K-selection, Am. Nat., 1970, vol. 104, pp. 592–597.
Pianka, E.,. Evolyutsionnaya ekologiya (Evolutionary Ecology), Moscow: Mir, 1981.
Promislow, D.E.L. and Harvey, P.H., Living fast and dying young: a comparative analysis of life-history variation among mammals, J. Zool., 1990, vol. 220, pp. 417–437.
Qu, J., Réale, D., Fletcher, Q.E., and Zhang, Y., Among-population divergence in personality is linked to altitude in plateau pikas (Ochotona curzoniae), Front. Zool., 2019, vol. 16, article 26. https://doi.org/10.1186/s12983-019-0329-6
Réale, D., Reader, S.M., Sol, D., McDougall, P.T., and Dingemanse, N.J., Integrating animal temperament within ecology and evolution, Biol. Rev., 2007, vol. 82, pp. 291–318.
Réale, D., Garant, D., Humphries, M.M., Bergeron, P., Careau, V., and Montiglio, P.O., Personality and the emergence of the pace-of-life syndrome concept at the population level, Philos. Trans. R. Soc., B, 2010, vol. 365, pp. 4051–4063.
Reznick, D., Costs of reproduction: an evaluation of the empirical evidence, Oikos, 1985, vol. 44, no. 2, pp. 257–267.
Reznick, D., Bryant, M.J., and Bashey, F., r- and K selection revisited: the role of population regulation in life history evolution, Ecology, 2002, vol. 83, pp. 1509–1520.
Ricklefs, R.E. and Wikelski, M., The physiology/life-history nexus, Trends Ecol. Evol., 2002, vol. 17, pp. 462–468.
Riechert, S.E. and Hedrick, A.V., A test for correlations among fitness-linked behavioural traits in the spider agelenopsis aperta (Araneae, Agelenidae), Anim. Behav., 1993, vol. 46, no. 4, pp. 669–675.
Roff, D.A., The Evolution of Life Histories, New York: Chapman and Hall, 2002.
Royauté, R., Berdal, M.A., Garrison, C.R., and Dochtermann, N.A., Paceless life—A meta-analysis of the pace-of-life syndrome hypothesis, Behav. Ecol. Sociobiol., 2018, vol. 72, article 64. https://doi.org/10.1007/s00265-018-2472-z
Sæther, B.E. and Bakke, Ø., Avian life history variation and contribution of demographic traits to the population growth rate, Ecology, 2000, vol. 81, pp. 642–653.
Shilov, I.A., Ekologo-fiziologicheskie osnovy populyatsionnykh otnoshenii u zhivotnykh (Ecological and Physiological Bases of Population Relations in Animals), Moscow: Mosk. Gos. Univ., 1977.
Sih, A., Bell, A.M., Johnson, J.C., and Ziemba, R.E., Behavioral syndromes: an integrative overview, Quart. Rev. Biol., 2004, vol. 79, pp. 241–277.
Sih, A., Mathot, K.J., Moirón, M., Montiglio, P.-O., Wolf, M., and Dingemanse, N.J., Animal personality and state-behaviour feedbacks: a review and guide for empiricists, Trends Ecol. Evol., 2015, vol. 30, pp. 50–60.
Sinervo, B. and Svensson, E., Correlational selection and the evolution of genomic architecture, Heredity, 2002, vol. 89, pp. 329–338.
Smith, B.R. and Blumstein, D.L., Fitness consequences of personality: a metaanalysis, Behav. Ecol., 2008, vol. 19, pp. 448–455.
Stamps, J.A., Growth-mortality tradeoffs and personality traits in animals, Ecol. Lett., 2007, vol. 10, pp. 355–363.
Starrfelt, J. and Kokko, H., Bet-hedging—a triple trade off between means, variances and correlations, Biol. Rev., 2012, vol. 87, pp. 742–755.
Stearns, S.C., The evolution of life history traits: a critique of the theory and a review of the data, Annu. Rev. Ecol. Syst., 1977, vol. 8, no. 1, pp. 145–171.
Stearns, S.C., The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals, Oikos, 1983, vol. 41, no. 2, pp. 173–187.
Stearns, S.C., Trade-offs in life-history evolution, Funct. Ecol., 1989, vol. 3, pp. 259–268.
Stearns, S.C., The Evolution of Life Histories, New York: Oxford Univ. Press, 1992.
Tarka, M., Guenther, A., Niemela, P.T., Nakagawa, S., and Noble, D.W., Sex differences in life history, behavior, and physiology along a slow-fast continuum: a meta-analysis, Behav. Ecol. Sociobiol., 2018, vol. 72, article 132. https://doi.org/10.1007/s00265-018-2534-2
Tieleman, B.I., Understanding immune function as a pace of life trait requires environmental context, Behav. Ecol. Sociobiol., 2018, vol. 72, article 55. https://doi.org/10.1007/s00265-018-2464-z
Trut, L.N., Early canid domestication: the farm-fox experiment: foxes bred for tamability in a 40-year experiment exhibit remarkable transformations that suggest an interplay between behavioral genetics and development, Am. Sci., 1999, vol. 87, no. 2, pp. 160–169.
Trut, L.N., Gerbek, Yu.E., Kharlamova, A.V., Gulevich, R.G., and Kukekova, A.V., Domesticated foxes: molecular genetic mechanisms involved in behavioral selection, Vavilov. Zh. Genet. Sel., 2014, vol. 17, no. 2, pp. 226–233.
Vasil’eva, N.A., Savinetskaya, L.E., and Chabovsky, A.V., Large body size and a short period of ground activity do not prevent the yellow ground squirrel (Spermophilus fulvus) from growing rapidly, Zool. Zh., 2009, vol. 88, no. 3, pp. 339–343.
Wikelski, M. and Ricklefs, R.E., The physiology of life histories, Trends Ecol. Evol., 2001, vol. 16, pp. 479–481.
Wikelski, M., Spinney, L., Schelsky, W., Scheuerlein, A., and Gwinner, E., Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes, Proc. R. Soc. London, Ser. B, 2003, vol. 270, pp. 2383–2388.
Williams, G.C., Pleiotropy, natural selection, and the evolution of senescence, Evolution, 1957, vol. 11, pp. 398–411.
Williams, G.C., Natural selection, the cost of reproduction, and a refinement of lack’s principle, Am. Nat., 1966, vol. 100, pp. 687–690.
Wilson, A.D. and Krause, J., Personality and metamorphosis: is behavioral variation consistent across ontogenetic niche shifts?, Behav. Ecol., 2012, vol. 23, no. 6, pp. 1316–1323.
Wilson, D.S., Clark, A.B., Coleman, K., and Dearstyne, T., Shyness and boldness in humans and other animals, Trends Ecol. Evol., 1994, vol. 9, pp. 442–446.
Wolf, M. and McNamara, J.M., On the evolution of personalities via frequency-dependent selection, Am. Nat., 2012, vol. 178, pp. 679–692.
Wolf, M., van Doorn, G.S., Leimar, O., and Weissing, F.J., Life-history tradeoffs favour the evolution of animal personalitie, Nature, 2007, vol. 447, pp. 581–584.
Wright, J., Bolstad, G.H., Araya-Ajoy, Y.G., and Dingemanse, N.J., Life-history evolution under fluctuating density-dependent selection and the adaptive alignment of pace-of-life syndromes, Biol. Rev., 2019, vol. 94, pp. 230–247.
ACKNOWLEDGMENTS
The author is grateful to A.V. Chabovskii for discussion of a future article and comments on the text.
Funding
This study was supported by the Russian Foundation for Basic Research, project no. 19-14-50232.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that she has no conflicts of interest. This article does not contain any studies involving animals or human participants performed by the author.
Additional information
Translated by M. Batrukova
APPENDIX
APPENDIX
Brief Glossary of Terms
Behavioral carryover—the use of behavior that is inadequate for a given context due to low behavioral plasticity.
Boldness–shyness scale—scale that determines the response to a familiar potentially dangerous situation.
Exploration (slow and thorough–fast and superficial)—a pattern for exploring new things; a slow but thorough study is opposed to a fast but superficial one.
Life-history tradeoffs—negative correlations between vital processes occurring under conditions of limited resources.
Life-history traits—characteristics of the life cycle.
Pace-of-life syndrome (POLS)—a concept relating the fast/slow life-history continuum, physiology, and personality at the intraspecific and interspecific levels.
Personality—a stable behavioral phenotype in animals; personalities are formed by several correlated traits and vary within sex and age cohorts.
Proactive–reactive continuum—a scale from proactive (aggressive, active, bold, and fast exploring (proactive syndrome)) individuals to reactive (nonaggressive, inactive, shy, and thoroughly exploring) individuals.
Slow–fast life-history continuum—continuum of fast/slow life history of species; a concept that relates the life-history traits (lifespan, productivity, and maturation rate) with the physiological traits of species.
Sociability—the tendency to social contacts with conspecifics.
Rights and permissions
About this article
Cite this article
Vasilieva, N.A. Pace-of-Life Syndrome (POLS): Evolution of the Concept. Biol Bull Russ Acad Sci 49, 750–762 (2022). https://doi.org/10.1134/S1062359022070238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022070238