Abstract—Features of the seasonal changes in the number of species, biomass, and the ratio of taxonomic groups of phytoplankton in flowing and regulated sections of the Ural River are described. In the unregulated section of the river, the maximum number of species was recorded in July and in the upper and lower reaches, in October. The maximum biomass of phytoplankton in the unregulated section of the river and in the lower part of the reservoir was recorded in summer, and in the upper part, in spring. The effect of a combination of a number of parameters of water bodies and environmental factors such as the typology, morphometry, flow velocity, input of substances from the catchment area and from the upper areas, and the temperature on the quantitative characteristics of communities is discussed. The leading role of the water temperature for phytoplankton development is shown in the unregulated sections of the river and in the lower part of the reservoir, which are characterized by environmental conditions that impede the abundant development of phytoplankton: high flow rates, the late onset of biological spring, and low nutrient availability. The biomass values correspond to β-mesotrophic–β-eutrophic waters in the unregulated sections of the river, to β-mesotrophic in the upper part of the reservoir, and to α-β-mesotrophic in the near-dam part. According to the saprobity index, the entire area surveyed is characterized by β-mesosaprobic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Reservoirs dramatically change the hydrological regime in rivers, which leads to transformations of the hydrobiont communities. On the one hand, many publications have been devoted to description of the features of their formation (by the example of the cascades of the reservoirs of the Volga, Angara, Dnieper, and Yenisei rivers, etc.); on the other hand, the diversity of river ecosystems subject to hydrotechnical construction is so great that each large river and different zones of its reservoir require particular study. This will make it possible to identify the general patterns and specific features of the formation of communities and the ecological state of these complex quasi-natural systems.
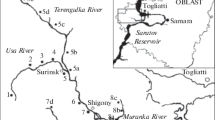
The Ural River is one of the largest rivers in Europe (2428 km in length); its ecological state is determined by a complex of anthropogenic factors, including plowing of virgin lands, destruction of the forest belt, municipal sewage, and intensive development of ore and oil fields, as well as flow regulation. The filling of the largest reservoir on the river, the Iriklinsk Reservoir, started in 1958, and the normal headwater level (NHL) was reached in 1966. It is a river-type reservoir with long-term flow regulation. The volume of the reservoir at NHL (245 m) is 3.25 km3, and the water area is 260 km2. The length is 73 km, and the maximum and average depths are 36 and 12.5 m. The catchment area of the Ural River to the hydroelectric power station (HPS) is 36 640 km2. The geology, topography, soils, vegetation, and climate have determined the specific hydrological regime of the entire river basin, which is characterized by a low water level, low runoff modulus, insignificant role of groundwater feeding, and large interannual and seasonal variations of the runoff (Solovykh et al., 2003; Sivokhip, 2014). Obviously, significant variations in the runoff cause changes in the state of hydrobiont communities in both the unregulated and regulated sections of the river during the growing season. Quantitative and qualitative characteristics of phytoplankton as one of the most crucial components of aquatic ecosystems are successfully used as indicators of changes in the biological regime of different water bodies and watercourses (Okhapkin, 1997; Datsenko, 2007; Korneva, 2015; Datsenko et al., 2017; Edelstein et al., 2017).
The aim of this work is to study the seasonal features of the phytoplankton structure in unregulated and regulated sections of the Ural River.
MATERIALS AND METHODS
Phytoplankton (PP) samples were taken in May, July, and October 2016 from the surface water layer in the channel and littoral parts of the Ural River upstream from the transient region (52°16′ N, 58°55′ E and 52°16′ N, 58°54′ E) in the upper (Chapaevsk, the second along the longitudinal profile) (52°04′ N, 58°49′ E) and lower (near dam) (51°40′ N, 58°37′ E) channel reaches of the Iriklinsk Reservoir. The depth, transparency, temperature, and flow velocity were measured simultaneously. PP samples were fixed using the Utermohl solution with formaldehyde; the cameral treatment was made according to the common method (Metodicheskie…, 1984). Phytoplankton was estimated according to the average number of species in a sample, the biomass, and the proportion of taxonomic groups of organisms. The communities were analyzed by the Shannon index computed using the biomass. The saprobity index was estimated using the Pantle–Buck method modified by Sládeček (1973). The indicator significance of the species was determined according to the Wegl list (Wegl, 1983). The analysis was made using nonparametric statistical methods including assessment of the significance of differences according to the Kruskal–Wallis test (p < 0.05) and determination of the Spearman correlation coefficient (p < 0.05).
RESULTS AND DISCUSSION
The Ural River upstream from the transient region of the reservoir. The maximum water transparency was observed at the minimum flow velocity in autumn, which varied within 0.07–0.8 m/s (on average, 0.3 m/s) in different zones, whereas it varied within 0.7–1.5 (1.0) in May and 0.03–2.6 (1.0) m/s in July. The maximum water temperature was recorded in July; the minimum temperature was in October (Table 1).
The maximum number of PP species in a sample was recorded in July; the minimum number was in October (p = 0.048). A significant variation in the specific number of species was determined by the representativeness of Chlorophyta (p = 0.048). In addition, the number of Euglenophyta species decreased during the study period (p = 0.032) (Table 2).
The maximum biomass of PP was recorded in July, which significantly exceeded the data obtained in May (p = 0.024) due to Bacillariophyta (p = 0.018) (Table 3). In October, the biomass of Chlorophyta and Cryptophyta decreased significantly; it was lower than in July (p = 0.007 and 0.021, respectively). According to the PP biomass, the trophic status of water was β‑mesotrophic in May and October and β-eutrophic in July (Zhukinskii, 1976, cited according to Kitaev, 2007).
The biomass was mainly formed by Bacillariophyta, and their proportion increased during the study period. As a result, the biomass values differed significantly between May and October (p = 0.005); on the contrary, the proportions of Chlorophyta and Cryptophyta decreased by October (p = 0.042 and 0.013, respectively). Maximum and minimum Shannon indexes were recorded in May and July, respectively (p = 0.005). The saprobity index characterized the trophic status of water as β-mesosaprobic; in July its value was at the boundary of β- and α-mesosaprobic waters; and the differences between the values in May and July were significant (p = 0.008) (Table 3). Changes in the indexes are related to the composition and the total proportion of dominant species. Thus, in spring, the species Ulnaria ulna (Nitzsch) Compere, an indicator of β-mesosaprobic conditions was dominant in the river; its average proportion was 21.3% of the total biomass. In summer, the species Stephanodiscus hantzschii Grunow and Cyclotella meneghiniana Kützing, indicators of α-mesosaprobic waters prevailed in summer; the first species constituted 23.5% of the biomass, and the second species, 55.0%. In autumn, Stephanodiscus hantzschii (23.4%) was recorded among dominant species and Surirella brebissonii var. kuetzingii Krammer & Lange-Bertalot (syn. Surirella ovata Kützing) (37.1%), an indicator of β‑mesosaprobic waters appeared.
Chapaevsk reach of the reservoir. The maximum water temperature and minimum transparency were recorded in July (Table 1). The minimum number of PP species in a sample was recorded in July, the maximum number was in October; the biomass decreased from May to October (Tables 2, 3). During the study period, the biomass of Cryptophyta increased. As a result it was higher in October than in May (p = 0.032) and the biomass of Cyanophyta was higher in July (p = 0.016). The trophic status of the reach was β-mesotrophic in all seasons. The biomass was mainly formed by Bacillariophyta; their minimum proportion was recorded in July with an increasing proportion of Cyanophyta, as a result it significantly exceeded the values recorded in May (p = 0.016) (Table 3).
There were no significant differences between the values of the Shannon index in different seasons though the minimum value was recorded in July and the maximum one was in October (Table 3). This is due to the fact that the proportion of two dominant species, Aulacoseira granulata (Ehrenberg) Simonsen and Anabaena scheremetieviae Elenkin, was 65.8% of the total biomass, and in October when three species, Aulacoseira granulata, Cryptomonas curvata Ehrenberg, and Cryptomonas sp. were dominant, their total proportion was 48%. In spring, Cyclotella meneghiniana and Ulnaria ulna dominated in the reach; their proportion was 55.9%. During the entire study period, the trophic conditions were β-mesosaprobic according to the saprobity index (Table 3).
Near-dam reach of the reservoir. As in the upper reaches of the reservoir, the minimum transparency was recorded at the maximum water temperature in July (Table 1). During the study period, the number of PP species in the samples increased, though the differences were insignificant (Table 2). The minimum biomass of PP was found in May; it significantly increased in July (p = 0.045) and decreased in October but was higher than in May (Table 3). The total biomass increased in July due to Dinophyta; their biomass and proportion in the total biomass exceeded the values recorded in October (p = 0.046 and 0.045, respectively). The biomass of Bacillariophyta increased during the study period, and in October it significantly exceeded the values recorded in May (p = 0.009). According to the biomass, the trophic status of water in the reach was α-mesotrophic in May and October and β-mesotrophic in July.
The minimum values of the Shannon index were recorded in July, and during that time, the index of saprobity corresponded to oligosaprobic conditions, whereas in spring and autumn it corresponded to β‑mesosaprobic conditions (Table 3). This is due to the composition and total proportion of dominant species. Ceratium hirundinella (O.F.M.) Bergh and Peridinium cinctum (O.F.M.) Ehrenberg, indicators of oligo- and β-mesosaprobic waters, prevailed in July, and their total proportion in the total biomass was 83.1%. Cryptomonas sp., and Cryptomonas curvata, and Rhodomonas lens Pascher (51.5%), also indicators of β-mesosaprobic conditions, prevailed in May, and the β-mesosaprobic species Aulacoseira granulata and Peridinium latum Paulsen (50.3%) prevailed in October.
In general, the data have demonstrated that seasonal changes in PP differed between the unregulated and regulated sections; differences were also found within the regulated section. The maximum specific number of PP species in the river was recorded in July, and it was observed in reaches of the reservoir in October; the seasonal differences in these sections were insignificant. The maximum biomass of PP was recorded in the river and in the near-dam reach in summer and in the upper reach in spring. Apparently, the spatial and temporal distribution of PP depends on the combination of some factors. An explanation of their role is given below.
In July, the water discharge in the river decreases, which is indicated by the literature (Pavleichik and Sivokhin, 2013) and information about the total water inflow into the reservoir in 2016 (it was on average 90.6 m3/s in May, 17 m3/s in July, and 9.8 m3/s in October).Footnote 1 In addition, in July the river reached its maximum water temperature (see Table 1), which is responsible for the increase in the number of species in the sample and the biomass of the phytoplankton (r = 0.63, 0.52). Besides, the highest concentrations of nutrients are recorded in the river in summer (Shashulovskaya et al., 2017). All these factors determined the increase in the specific number of species and biomass of PP to the maximum values in the river in summer (Tables 2, 3).
The upper reaches in the reservoir are the first to receive river waters characterized by high concentrations of organic nutrient substances (Pavleichik and Sivokhip, 2013; Shashulovskaya et al., 2017) which are accumulated in the upper reaches of the reservoir together with PP of the river. However, significant changes in the number of species and biomass of PP in different seasons were not found (Tables 2, 3), which may be explained by the volume of water inflow into the reservoir (r = 0.69) and the location of the reaches. In May a high biomass of PP, which exceeded its values in the riverine section (Table 3), was due to the high flow rate of the river, penetration of its waters into the surveyed section, and accumulation of transported substances there. In July, the flow rate of the river decreased as did the distance its waters reached. As a result, the substances from the catchment area could be accumulated to a greater extent in the uppermost Urtazymskii reach where, unfortunately, the primary material was not sampled. The limited input of substances (or its absence) during that period, apparently, promoted a slight decrease in the biomass and the number of PP species in the Chapaevsk reach (Tables 2, 3). In October, the number of species in samples and biomass of PP decreased even more, which was due to the maximum reduction in the river water inflow into the reservoir, which is characterized by the minimum concentration of nutrients during that period (Shashulovskaya et al., 2017) and a decrease in the water temperature and the end of the growing season. In general, the reach is characterized by the minimum coefficient of variation of the PP biomass, which indicates more stable conditions for the existence of communities formed by a different combination of some factors, among which the main one is the accumulation of substances transported by the river from the catchment area and simultaneously its second place in the cascade of channel reaches in the reservoir.
The highest PP biomass in the near-dam reach was recorded in summer, which is associated with the maximum water warming (r = 0.94). But some factors prevent a significant increase in the algal biomass in the lower reaches as a whole. The main factors are a slow water exchange in the reservoir (every two years (Solovykh et al., 2003)) promoting sedimentation of the greater part of substances in the upper and lower reaches, which is indirectly evidenced by a decrease in the water mineralization along the longitudinal profile of the reservoir (Pavleichik and Sivokhip, 2013) and a decrease in the concentration of organic and biogenic substances (Shashulovskaya et al., 2017); morphometry of the reach, which is characterized by maximum depths (Solovykh et al., 2003), small width, distinctness, and the area of the littoral zone, which, along with higher water transparency, characterize the section as morphometrically oligotrophic. In addition, the shift in the timing of the onset of biological spring due to later ice clearance and slow warming of the water masses at maximum depths was also observed in the lake-like part of the Rybinsk Reservoir, which may also play a certain role (Rybinskoe…, 1972).
The differences in the seasonal changes of PP in the study sections are also evidenced by the representativeness of taxonomic groups of organisms. In the river, Bacillariophyta constituted the main proportion of the specific number of species during the entire study period (31.9–59.4%), but in July, Chlorophyta were dominant (55.3% vs. 28.7% in May and 30.4% in October). In the Chapaevsk reach, the proportion of Bacillariophyta and Chlorophyta in May was 40.6 and 37.5%, respectively; in July and October the proportion of diatoms decreased (to 22.4 and 15.7%) against the background of an increase in the proportion of cyanobacteria (from 1.6 in May to 17.3 in July and 12.1% in October), and in July, the proportion of euglenids increased (from 5.5 to 10.4%). In addition, the proportion of cryptophytes in this section is higher (on average, 2.3 times) compared to the river during the entire study period. Compared to the river, the proportion of Chlorophyta species in the near-dam reach was lower (two times), while the proportion of cyanobacteria (15.8 times) and Cryptophyta (4.4 times) was higher during the entire study period. In May and October, the proportion of Bacillariophyta was lower (1.4 and 1.7 times, respectively), and compared to the upper reach, the proportion of Chlorophyta was lower (1.9 times) and the proportion of Cryptophyta (2.3 times) and Dinophyta (2.4 times) was higher. In general, it should be noted that Dinophyta appeared in the composition of PP in the regulated sections in May and October, and in July their proportion was higher by 3.6 times compared to the river.
The proportion of taxonomic groups in the total biomass was different. In the river, it was mainly formed by Bacillariophyta, and in May and October, Chlorophyta was the second largest group (Table 3). In the Chapaevsk reach, diatoms dominated in May, Cyanophyta prevailed in July, and Cryptophyta, in October. In the near-dam reach, Cryptophyta prevailed in May; Dinophyta, in July; and Bacillariophyta, in October, while Dinophyta and Cryptophyta were subdominant. Therefore, the features of PP in the study areas were determined by the combination of some of their characteristics and factors: typology, morphometry, flow velocity, trophic conditions dependent on the input of substances from the catchment area and from the upstream sections, and water temperature.
In general, according to PP parameters, the unregulated section in the Ural River corresponded to the status of highly eutrophic waters, which was more pronounced in summer at the maximum water temperature when the biomass corresponded to β-eutrophic waters (Zhukinskii, 1976, cited according to Kitaev, 2007). The community was predominantly formed by Bacillariophyta and Chlorophyta, which is typical for the most highly eutrophic watercourses as demonstrated by an example of the Middle Volga basin (Okhapkin, 1997). However, the flow prevented changes in the parameters of the community development that are observed under high biogenic and organic load in water bodies, which is evidenced by relatively high values of the Shannon index, while values of the saprobity index characterizing β-mesosaprobic conditions though the indicator species of α‑mesosaprobic waters were detected among dominants in July and October. The PP structure in the Chapaevsk reach corresponded to that in the regulated watercourses the communities in which are characterized by an increase in the biomass and proportion of cyanobacteria (Okhapkin, 1997). However, the spatial position of the reach, the second in the cascade of channel reaches in the reservoir, led to the accumulation of most of the nutrients and organic substances carried by a highly eutrophic river in the upper Urtazymskii reach, which did not result in the increase in the biomass that may expected under regulated conditions. The low biomass and poor species composition of PP in the near-dam reach, which may be explained by the effect of slow water exchange and accumulation of substances from the catchment area in the area of upstream reaches, also determined the minimum values of the saprobity index. The maximum biomasses were recorded in summer when the water temperature increased.
It is important to note that cryptophytes and dinophytes reached the highest abundance in the reservoir, especially in the near-dam reach; an increase in their abundance has been observed in many regions in recent years (Korneva et al., 1999; Trifonova and Afanasieva, 2004; Moiseenko et al., 2009; Nikulina, 2016). On the one hand, the literature shows that most of the species of Dinophyta are indicators of oligotrophic waters, which is obviously associated with the decrease in the saprobity index in the near-dam reach in July to the values typical for oligosaprobic waters.
On the other hand, there is information about the occurrence of some species in meso- and polysaprobic waters (Dogadina, 1974; Trifonova, 1990; Gorbulin, 2011), as well as of cryptophytes the abundance of which increases during eutrophication of waters (Okhapkin, 1997). Further studies on the patterns of development and distribution of Dinophyta and Cryptophyta should determine their bioindicator importance and are due to their high nutritive value determined by the high content of polyunsaturated fatty acids (Ahlgren et al., 1990; Gulati and Mott, 1997; Weers and Gulati, 1997).
CONCLUSIONS
The patterns of seasonal changes in the specific number of species, biomass, and the ratio of PP taxonomic groups in the unregulated and regulated sections in the Ural River are determined by the combination of some characteristics of water bodies and environmental factors: flow velocity, input of substances from the catchment area and upstream sections, and water temperature. Changes in the quantitative parameters of PP that developed under conditions preventing its high abundance such as in the river due to high current velocities and in the near-dam reach due to the late onset of the biological spring, as well as sedimentation and incorporation of most substances into the cycle in the upper reaches of the reservoir, were associated with the water temperature, the leading role of which is shown for communities in watercourses of the Volga basin (Okhapkin, 1997). In the Chapaevsk reach, which is the second in the cascade of channel reaches, the optimum combination of some environmental factors created the conditions for stable quantitative representativeness of PP during the study period. The biomass values make it possible to estimate the trophic status of the river as β-mesotrophic-α-eutrophic in its unregulated sections, β‑mesotrophic in the upper part of the reservoir, and α-β-mesotrophic in the near-dam section. According to the saprobity index, the entire surveyed area is characterized by β-mesosaprobic conditions.
Notes
see http://ueiv.ru/.
REFERENCES
Ahlgren, G., Lundstedt, L., Brett, M., and Forsberg, C., Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankters, J. Plankton Res., 1990, vol. 12, no. 4, pp. 809–818.
Datsenko, Yu.S., Evtrofirovanie vodokhranilishch. Gidrologo-gidrokhimicheskie aspekty (Eutrophication of Reservoirs: Hydrological and Hydrochemical Aspects), Moscow: GEOS, 2007.
Datsenko, Yu.S., Puklakov, V.V., and Eidelshtein, K.K., The analysis of the abiotic factors influence on phytoplankton development in low-flow stratified reservoir, Tr. Karel. Nauchn. Tsentra Ross. Akad. Nauk, 2017, no. 10, pp. 73–85. https://doi.org/10.17076/lim611
Dogadina, T.V., Pyrophyte algae of wastewaters, Gidrobiol. Zh., 1974, vol. 10, no. 1, pp. 73–74.
Edelshtein, K.K., Puklakov, V.V., and Datsenko, Yu.S., Experimental-theoretical basis of diagnosis and prediction of bloom in reservoirs-sources of municipal water supply, Voda Magazine, 2017, no. 4, pp. 34–40.
Gorbulin, O.S., Ecological and biological characteristics of Dinophyta flora of continental water bodies of Ukraine, Vestn. Kharkiv Nats. Univ. im. V.N. Karazina, Ser. Biol., 2011, vol. 14, pp. 43–58.
Gulati, R.D. and De Mott, W., The role of food quality for zooplankton: remarks on the state-of-the art, perspectives and priorities, Freshwater Biol., 1997, vol. 38, no. 3, pp. 353–368.
Kitaev, S.P., Osnovy limnologii dlia gidrobiologov i ikhtiologov (Basics of Limnology for Hydrobiologists and Ichthyologists), Petrozavodsk: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 2007.
Korneva, L.G., Fitoplankton vodokhranilishch basseina Volgi (Phytoplankton of Reservoirs of the Volga River Basin), Kostroma: Kostrom. Pechatn. Dom, 2015.
Korneva, L.G., Mineeva, N.M., Elizarova, V.A., Pyrina, I.L., Sigareva, L.E., Genkal, S.I., Mitropolskaia, I.V., Litvinov, A.S., and Sharapova, N.A., Ekologiya fitoplanktona Rybinskogo vodohranilishcha (Ecology of Phytoplankton of the Rybinsk Reservoir), Tolyatti: Inst. Ekol. Volzh. Bass. Ross. Akad. Nauk, 1999.
Metodicheskie rekomendatsii po sboru i obrabotke materialov pri gidrobiologicheskikh issledovaniiakh na presnykh vodoemakh. Fitoplankton i ego produktsiia (Guidelines for the Collection and Processing of Materials in Hydrobiological Studies on Freshwater Bodies. Zooplankton and Its Products), Leningrad: Gos. Nauchno-Issled. Inst. Ozern. Rechn. rybnogo khoziaistva, 1984.
Moiseenko, T.I., Gashkina, N.A., Sharov, A.N., Vandysh, O.I., and Kudryavtseva, L.P., Anthropogenic transformations of the Arctic ecosystem of Lake Imandra: tendencies for recovery after long period of pollution, Water Resour., 2009, vol. 36, no. 3, pp. 296–309.
Nikulina, V.V., Long-term changes in phytoplankton in a water body non subject to anthropogenic impact (Lake Krivoe, Northern Karelia), Tr. Zool. Inst. Ross. Akad. Nauk, 2016, vol. 320, no. 3, pp. 336–347.
Okhapkin, A.G., Phytoplankton structure and succession during regulation of river flow, Extended Abstract of Doctoral Sci. (Biol.) Dissertation, St. Petersburg, 1997.
Pavleichik, V.M. and Sivokhip, Zh.P., The formation of surface water quality in the basin of the upper reaches of the Ural River under the conditions of technogenic transformation of the natural environment, Water Resour., 2013, vol. 40, no. 5, pp. 499–509.
Rybinskoe vodokhranilishche i ego zhizn’ (The Rybinsk Reservoir and Its Life), Kuzin, B.S., Ed., Leningrad, Nauka, 1972.
Shashulovskaya, E.A., Mosiyash, S.A., Filimonova, I.G., Grishina, L.V., and Kuzina, E.G., Formation of the hydrochemical regime of the upper reaches of the Ural River under conditions of technogenic flow regulation, Povolzh. Ekol. Zh., 2017, no. 4, pp. 417–425. https://doi.org/10.18500/1684-7318-2017-4-417-425
Sivokhip, Zh.P., Analysis of the ecological and hydrological specifics of the transboundary river basin of Ural River in connection with the regulation of runoff, Vestn. Voronezh. Gos. Univ., Ser. Geogr. Geoekol., 2014, no. 3, pp. 87–94.
Sládeček, V., System of water quality from the biological point of view, Adv. Limnol., 1973, vol. 7, pp. 1–218.
Solovykh, G.N., Raimova, E.K., Osadchaya, N.D., Fabarisova, L.G., and Nikitina, L.P., Gidrobiologicheskaia kharakteristika Iriklinskogo vodokhranilishcha (Hydrobiological Characteristics of the Iriklinsk Reservoir), Ekaterinburg: Ural. Otd. Ross. Akad. Nauk, 2003.
Trifonova, I.S., Ekologiia i suktsessiia ozernogo fitoplanktona (Ecology and Succession of Lake Phytoplankton), Leningrad: Nauka, 1990.
Trifonova, I.S. and Afanas’eva, A.L., The structure and productivity of phytoplankton of the Vuoksa Lake–River system, in Sostoianie biotsenozov ozerno-rechnoi sistemy Vuoksy (The State of Biocenoses of the Vuoksa Lake–River System), St. Petersburg: Nauchno-Issled. Inst. Khim. St. Peterb. Gos. Univ., 2004, pp. 43–58.
Weers, P.M.M. and Gulati, R.D., Effect of the addition of polyunsaturated fatty acids to the diet on the growth and fecundity of Daphnia galeata, Freshwater Biol., 1997, vol. 38, no. 3, pp. 721–729.
Wegl, R., Index für die Limnosaprobität, Wasser Abwasser, 1983, vol. 26, pp. 1–175.
Zhukinskiy, V.N., Oksiyuk, O.P., Tseeb, Ya.Ya., and Georgievskiy, V.B., The project of a unified system for the characterization of continental water bodies and flows, and its application for the analysis of water quality, Gidrobiol. Zh., 1976, vol. 12, no. 6, pp. 103–111.
ACKNOWLEDGMENTS
I am grateful to the staff of the Russian Federal Research Institute of Fisheries and Oceanography, Saratov Branch, for assistance in sampling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflict of interest. This article does not contain any studies involving animals or human participants performed by the author.
Additional information
Translated by N. Ruban
Rights and permissions
About this article
Cite this article
Dzhayani, E.A. Phytoplankton of Flowing and Flow-Regulated Stretches of the Ural River in Different Seasons. Biol Bull Russ Acad Sci 48, 1747–1753 (2021). https://doi.org/10.1134/S1062359021100071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359021100071