Abstract
In parthenogenetic hybrid Darevskia armeniaca species, the development of early oogenesis and early meiosis were analyzed. It has been shown for the first time that the number of synаptonemal complexes (SCs) of bivalents during the stages of early pachуtena–diplotena of the meiotic prophase 1 is constantly equal to the haploid number, 19. The SC karyotype is presented. The results of comparative molecular–cytogenetic (C/CMA3/DAPI) analysis of the mitotic chromosomes (2n = 38: 34A + 2m + Zw-sex chromosomes) of parthenogenetic females and of the meiotic Zw-sex chromosomes (n = 19 bivalents) of a male D. armeniaca specimen with sex inversion have been presented. Finally, the results obtained demonstrate the absence of premeiotic endoreplication of chromosomes, standard early stages of meiosis, and the formation of the haploid number of SC-bivalents (n = 19) of homeological chromosomes in D. armeniaca.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The discovery of a significant number of fish, amphibians, and reptiles that reproduce through gyno-, hybrido-, and parthenogenesis has raised for researchers a number of serious questions, in particular, the question of the cytological features of oogenesis in unisexual species. In early works, it was shown that, in such forms during oogenesis, meiosis occurs and the mechanisms of restoration of the somatic number of chromosomes in unisexual vertebrates and invertebrates are often the same. The classifications of the mechanisms of diploidization differ from one another in the cytological picture of oogenesis disorders or in their genetic effect (pre-, intra-, and postmeiotic types of meiosis). The genetic consequences of these types of meiosis are different; therefore, the results of meiosis and the fate of individuals in general will depend on the mechanisms of restoration of the somatic number of chromosomes in unisexual individuals. These consequences become even more important when we consider that, evolutionarily, unisexual reproduction is associated with hybridization and polyploidy.
Preservation and maintenance of a high degree of heterozygosity of hybrids is possible with the premeiotic type of diploidization, namely, with the suppression of cytokinesis in the last premeiotic mitosis. Moreover, this mechanism allows avoiding many of the difficulties associated with the synapsis of hybrid homeologs and the divergence of pseudo-bivalents during meiosis of hybrid triploid forms. Apparently, it is precisely these circumstances that explain the spread of this mechanism in unisexual vertebrates and the discovery of this type of meiosis in North American parthenogenetic hybrid diploid (2n) and triploid (3n) lizards of the genus Aspidoscelis of the family Teiidae (Cuerllar, 1971; Lutes et al., 2010). In hybrid diploid parthenogenetic females of this genus, a tetraploid (4n) cell enters meiosis, and during prophase 1 of meiosis at the pachytene–diplotene stages in the oocyte, the pairing of sister chromosomes occurs with the formation of a diploid number of pseudo-bivalents (Lutеs et al., 2010). However, in meiosis, in the hybrid gynogenetic fish Carassius gibelio, there is no such mechanism (Cherfas, 1969).
Caucasian parthenogenetic lizards of the genus Darevskia of the family Lacertidae also have a hybrid origin (Moritz et al., 1992b) and, in contrast to North American parthenogenetic species, are always characterized by a diploid typical for the family (2n) with the number of acrocentric (A) chromosomes equal to 38 with heteromorphic sex Zw chromosomes (2n = 38A, Zw).
In previous studies, the stages of early pachytene–diplotene–diakinesis were studied in the nuclei of oocytes of hybrid parthenogenetic females of this genus, and figures formed by homoeologous chromosomes with terminal chiasmas in the proximal and distal regions of meiotic chromosomes were found (Cuellar, 1971; Kupriyanova, 1992, 1994, 2010). In addition, in the analysis of synaptonemal complexes (SCs), the number of bivalents approached 19, i.e., like the haploid number (n = 19). These and other indirect facts allowed us to assume the absence of a mechanism of endoreduplication of chromosomes in premeiotic mitosis and the possibility of recombination of genetic material, and, consequently, the increasing of genetic diversity due to recombination (Kupriyanova, 1992, 1994, 1997, 2010).
It is obvious that the cytogenetic mechanisms of meiosis are key in the evolution of parthenogenesis in hybrid species. For a more detailed description and clarification of the mechanisms of early meiosis of hybrid parthenogenetic species of the genus Darevskia, we examined oocytes of the species Darevskia armeniaca (Méhely, 1909) and analyzed the structure of the elements of the meiotic nucleus and the morphology of SC chromosomes, on the basis of which the SC karyotypes of the hybrid nucleus chromosomes were made. Such SC karyotypes were compared with previously obtained mitotic chromosomes of D. armeniaca, as well as with the characteristics of prophase 1 of meiosis, namely with chromosomes at the stage of diakinesis in rare diploid males of D. armeniaca with abnormal fertility (Darevskii and Kupriyanova, 1982; Kupriyanova, 2010).
MATERIALS AND METHODS
Female D. armeniaca specimens were collected in various years in the regions of the Armenian cities of Tsaghkadzor (40°32′7′′ N, 044°42′30′′ W) and Dilijan (40°44′2′′ N, 044°49′4′′ W). The characteristics of the capture sites and the modern ranges of parthenogenetic species of D. armeniaca and the parent species D. valentini and D. mixta have been described earlier (Petrosyan et al., 2019a, 2019b, 2020).
Meiotic chromosomes. For microscopic analysis, SC preparations of the total smear of the nuclei of oocytes were prepared from a cell suspension obtained from the embryonic cavity of a female ovary by the method of Dresser and Moses (Dresser and Moses, 1980). Spreading of oocytes was performed on a drop of 0.5% NaCl solution. The preparations were fixed with 4% paraformaldehyde, and for SC visualization they were stained with a 50% silver nitrate solution, then examined and photographed under a Leica light microscope (Germany). The lengths of the SC bivalents of oocytes were measured using the Leica Application Suite software.
Mitotic chromosomes. We used the method of obtaining mitotic chromosomes with the preliminary injection of a 0.1% solution of phytohemagglutinin (PHA P PanEco (Russia), 0.03 mL of solution per 10 g of mass) and colchicine (Merck (Germany); 0.1 mL per 10 g of mass). The preparations were stained with Giemsa dye according to the standard method and the method of sequential C-banding with the fluorochrome dyes DAPI specific to the AT-base pairs. The preparations were examined and photographed under a Leica light microscope. The chromosome lines were measured using the Leica Application Suite software.
RESULTS
In the karyotype of the D. armeniaca females studied, there are 38 acrocentric (A) chromosomes, including two microchromosomes (m) and sex Zw (2n = 38A: 34A + 2m + Zw; n = 19). Comparative fluorochrome differential (C/CMA3/DAPI) staining of metaphase chromosomes of D. armeniaca showed that most of the chromosomes of the set have small blocks of AT pairs in the pericentromeric region and GC pairs in the telomeric region of C-heterochromatin (Figs. 1, 2). At the same time, molecular cytogenetic analysis identified differences in the structure of some chromosomes with heteromorphism of telomeric regions of chromosome C-heterochromatin. For example, one first chromosome of the first pair of the karyotype has large bright blocks containing GC pairs in these regions, while in the other chromosome of the first pair these blocks are much less pronounced. A bright C-block, including GC-pairs, is also present in the sex w-microchromosome, although in size and morphology it practically does not differ from the other two microchromosomes of the karyotype (Fig. 1). In addition, one macrochromosome of D. armeniaca differs in morphology from other chromosomes of the set, since it has short arms and small blocks and enriched AT and GC pairs in the pericentromeric and telomeric regions, respectively. In size, this unpaired chromosome belongs to the middle group of chromosomes, to the fifth or sixth pair, and is considered as a sex Z-chromosome (Figs. 1, 2). It is important to note that, in various species of this family, the sex Z-chromosome of the fifth-sixth pair often has short arms, which is explained by its instability. Early meiosis (prophase 1 of meiosis) of the parthenogenetic species D. armeniaca (2n = 38A) was studied on preparations of a smear of oocyte nuclei 0.8–1.1 mm in size.
Metaphase plates of blood cells of Darevskia armeniaca (female) after C-banding and a specific GC-fluorochrome (CMA3) staining (Kupriyanova, 2010, as amended with changes) 2n = 38A: 34A + 2m + Zw (sex chromosomes). The arrows indicate heteromorphic auto- (first pair) and sex chromosomes Z and w (fifth pair).
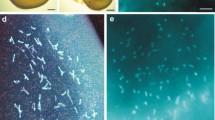
Analysis of SCs at the early pachytene and diplotene stages showed that they were completely synaptized and formed 19 SC bivalents. This meiotic prophase analysis was performed on five nuclei of oocytes (Figs. 3a–3d). However, the flatness of SC-bivalents (complex) does not occur similarly, as was shown during spermatogenesis (Safronova and Kupriyanova, 2016), since the oocyte nuclei are immersed in a liquid medium with a large amount of fat. This process prevents good spreading on the glass, which leads to waviness and, in some cases, to twisting of the ends. In Fig. 3 the numbering of SC-bivalents is presented in decreasing order of their linear sizes of the measurements taken. SC-bivalent no. 1, formed by the chromosomes of the set, the first in size and heteromorphic in structure (the first SC), differed in morphology from the other SC bivalents. It should be especially emphasized that on all the studied preparations of spreading oocyte nuclei, the number of SC bivalents is always equal to the haploid number of chromosomes of the species, i.e., 19.
DISCUSSION
In a number of previous complex works, it was shown (Kupriyanova, 1989, 1992, 2010) that the hybrid parthenogenetic species D. armeniaca (D. mixta × D. valentini), like other unisexual and bisexual species of the genus Darevskia, family Lаcertidae, is characterized by a 38 one-arm morphology and acrocentric (A) chromosomes and a stable karyotype structure similar in size (2n = 38A, NF (fundamental number) = 38). In addition, this species, like two other hybrid parthenospecies (D. dahli, D. unisexualis) of the genus Darevskia, has similar numbers and morphology of sex Zw chromosomes. As a result of hybridization, the female D. armeniaca could have received the Z chromosome from the paternal D. valentini and the w‑chromosome from the maternal D. mixta parental species (Kupriyanova, 1992; Moritz et al., 1992a; Murphy et al., 2000). The karyotypes of the Lаcertidae are characterized by the localization of structural heterochromatin (C-staining) in the pericentromeric and telomeric regions of chromosomes and rarely in intercalary regions (Olmo et al., 1986; Kupriyanova, 1994). This uniformity of chromosomes constrains the possibility of using the karyotype traits when analyzing the origin of these hybrid species, the meiotic process, and their evolution in general. It is for this reason that the identification of chromosomes of species of the genus Darevskia on the whole is still a difficult task. However, these results also indicated the cyto- and genetic similarity of the parental species of the genus Darevskia (Kupriyanova, 2010, 2014).
Nevertheless, molecular cytogenetic analysis of one of the parental species for D. rostombekowi of this genus, D. raddei, demonstrated the presence of a dicentric chromosome in the karyotype (Spangenberg et al., 2019). The results of this work identified some differences in the molecular-cytogenetic structure of telomeric regions of the chromosomes of D. armeniaca. To date, it is known that the amplification of some genes and the formation of tandemly located high-copy repeats, for example, in the pericentromeric regions of chromosomes, are a common genomic disorder found in hybrids. The pericentromeric and telomeric regions are often of key importance for the spatial orientation of chromosomes in the nucleus and are very important for the coincidence of the sites of communication of the hybrid chromosomes with the nuclear envelope, as well as for the conjugation of chromosomes during meiosis. Large chromosomal reorganization and systemic mutations play an essential role in the process of saltation speciation and affect the early stages of an individual’s development (Stegny, 2019).
Analysis of cells in the early stage of prophase 1 of meiosis indicates the fact that, in the early oogenesis of the parthenogenetic hybrid species D. armeniaca, there are no polyploid oocytes nor, correspondingly, a mechanism of chromosome endoreduplication in the last mitotic divisions. All the data obtained indicate that, in early oogenesis, diploid nuclei of oocytes females of D. armeniaca (2n = 38) enter early meiosis (in prophase 1 of meiosis) and, at the early pachytene–diplotene stage, 19 SC bivalents are formed. Pictures of early meiosis once again demonstrate the genetic similarity of the parental species of hybrid unisexual D. armeniaca.
Comparative differential fluorochrome (C/CMA3/DAPI) staining of the chromosomes of D. armeniaca also allowed us to consider once again the sex Zw chromosomes of species of the genus Darevskia. It is known that the sex Z-chromosome of a number of Lаcertidae species is assigned in size to the fifth or sixth chromosome and often has short arms. For example, according to molecular-cytogenetic mapping of chromosomes, the Z-sex chromosome of Lacerta agilis (2n = 38, Zw) is related in length to the fifth pair of the karyotype and has partial homology with the sixth and ninth chicken chromosomes (Skikulnath et al., 2014). Presumably, the fifth to sixth longest sex chromosomes Z1 and W were found in the cryptic group of Zootoca vivipara (Kupriyanova and Rudi, 1990; Odierna et al., 1998; Kupriyanova and Boehme, 2012). The unpaired fifth to sixth chromosome by length in D. armeniaca is interpreted as the sex Z chromosome. Note that the sex Z chromosome of the species Takydromus, Gallotia, and Eremias is assigned to the average size group of the karyotype, which is 12–13 in length (Olmo et al., 1986; Lisachov et al., 2019). The facts listed clearly indicate the difficulties in solving the problems of identifying the sex chromosomes of lacertid lizards and the need for further detailed comparative analysis of the sex chromosomes of different groups of families and lizards as a whole.
It should be recalled that previous cytogenetic and molecular-cytogenetic studies have convincingly demonstrated the essential role of sex Zw chromosomes in the phylogenetic constraints of the rise of parthenogenesis in the genus Darevskia (Kupriyanova, 1989, 1992, 1997, 1999, 2010). These facts also indicated that the “balance” hypothesis of the hybrid transition to the unisexual mode of reproduction proposed earlier (Moritz et al., 1989, 1992a) did not take into account all the factors of the forming of the unisexual lizards (Kupriyanova, 1997, 1999).
Due to the importance of the sex Zw chromosomes in the successful hybridization of lizards of the genus Darevskia and in the transition of the emerging hybrids to the parthenogenetic type of meiosis, we again analyzed the previously published data on the karyotype of a rare male individual of the parthenogenetic hybrid species D. armeniaca (Darevskii and Kupriyanova, 1982). In contrast to the numerous triploid hybrids that appear in sympatric populations between parthenogenetic and bisexual species, a rare male was captured in a “pure” population of D. armeniaca (district of Stepanavan, Armenia). Chromosomal studies have shown complete coincidence of the karyotype of the male with that of the females of this and other populations of the species: diploid number of chromosomes 2n = 38, among which there were sex Zw chromosomes (Fig. 4). As a result, it was concluded that the interaction of sex Zw chromosomes in the hybrid genome of D. armeniaca is disrupted and that there was sex reversion in the Zw individual. At the same time, cytological abnormalities were established during meiosis of an individual: in the segregation of homeologs in meiotic division I and in the formation of aneuploid spermatocytes of order II, spermatids and spermatozoa, and in disturbances of the processes of spermatogenesis. During meiosis, 19 bivalents were formed at the stage of diakinesis of prophase 1 of meiosis. Among them, the fifth to sixth largest bivalent, presumably formed by sex chromosomes, was identified, and the features of its morphology were noted. The large element of the “bivalent” is probably represented by a large Z-chromosome and a small element, probably, by the w-microchromosome (Fig. 5). Dense conjugation of these chromosomes is not observed. One can note the association of the ends of the w microchromosome with the intercalary region of the Z chromosome and the significant size of the w microchromosome, possibly due to its decondensation.
It should be recalled that a certain spectrum of cytogenetic, genomic, and functional disturbances was also established in hybrid parthenogenetic females of the genus Darevskia. In this regard, an important conclusion was made that, despite the cyto- and genetic similarity of the parental species, the instability of their hybrid genomes is observed in hybrid parthenogenetic species. As a result, “hybrid” instability and recombination exchange in prophase 1 of meiosis can serve as mechanisms of genetic variability of parthenogenetic species of the genus (Kupriyanova, 1989, 1992, 1997, 1999, 2010, 2014). For example, rare alleles for protein loci found in D. armeniaca (MacCullach et al., 1995) and the variability and mutations of a certain type at the microsatellite DNA loci of these parthenogenetic species (Ryskov, 2008; Vergun et al., 2014; Girnyk et al., 2018) can be explained the cytogenetic mechanisms indicated (Kupriyanova, 2010, 2014).
CONCLUSIONS
Data received on early oogenesis in females of the hybrid parthenogenetic species D. armeniaca (2n = 38) indicate the typical course of the early stages of meiosis and the passage of early prophase 1 of meiosis. At the pachytene–diplotene stage, 19 SC elements are formed, and later there are 19 bivalents of homeologous chromosomes. This indicates that an oocyte with a diploid chromosome number enters meiosis. Thus, the data obtained confirm the scarce information that the mechanisms of meiosis in parthenogenetic lizards of the genera Aspidoscelis and Darevskia are different. In lizards of the genus Darevskia during oogenesis in the last premeiotic mitoses there is no endoreduplication of chromosomes or, therefore, doubling of the number of chromosomes to 4n. During early meiosis in a diploid oocyte at the pachytene–diplotene stage, there develops a haploid number of SC bivalents (n = 19). The data obtained once again confirm the cyto- and genetic similarity of the parental species of the hybrid parthenospecies D. armeniaca, formation of SC-bivalents in meiosis of homeologous chromosomes, and the possibility of recombination exchanges. At the same time, they indicate heteromorphism in the cytogenetic structure of some autochromosomes and sex mitotic chromosomes, the special morphology of their SC bivalents, and, in an sex-reversed male, a specific morphology and structure of a sex bivalent with a “decondensed” w chromosome and the type of association of sex chromosomes. Different types of sex-determining mechanisms are noted in lizards: Aspidoscelis females are characterized by homomorphic XX-sex chromosomes, while in Darevskia there are heteromorphic Zw-sex chromosomes.
Cytological characteristics and features of meiosis at later stages of oogenesis D. armeniaca and other parthenogenetic species of the genus Darevskia not fully investigated. The mechanisms of restoration of the diploid number of chromosomes in Caucasian parthenogenetic species, as well as the mechanisms of maintaining their heterozygosity and the system of sex Zw chromosomes, are the goals of further studies by the authors.
REFERENCES
Cherfas, N.B., The main results of cytogenetic analysis of unisexual and bisexual forms of goldfish, in Genetika, selektsiya i gibridizatsiya ryb (Fish Genetics, Breeding, and Hybridization), Moscow: Nauka, 1969, pp. 85–97.
Cuellar, O., Reproduction and the mechanism of meiotic restitution in the parthenogenetic lizard Cnemidophorus uniparens, J. Morphol., 1971, vol. 133, no. 2, pp. 139–165.
Darevskii, I.S. and Kupriyanova, L.A., Rare males in parthenogenetic lizard Lacerta armeniaca Méhely, Vertebr. Hung., 1982, vol. 21, pp. 69–75.
Dresser, M. and Moses, M., Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus gricus). IV. Light and electron microscopy of synapsis and nucleolar development by silver staining, Chromosoma, 1980, vol. 76, pp. 1–22.
Girnyk, A., Vergun, A., Semyenova, S.K., Guliaev, A., Arakelyan, M., Danielyan, F., Martirosyan, I., Murphy, R., and Ryskov, A.P., Multiple interspecific hybridization and microsatellite mutations provide clonal diversity in the parthenogenetic rock lizard Darevskia armeniaca, BMC Genomics, 2018, vol. 19, no. 979. https://doi.org/10.1186/s12864=018-5359-5
Kupriyanova, L., Cytogenetic evidence on genome interaction in hybrid Lacerta. Evolution and ecology of unisexual vertebrates, Bull. N.Y. State Mus. (Albany), 1989, vol. 466, pp. 236–239.
Kupriyanova, L.A. and Rudi, E.R., Comparative karyological analysis of populations of viviparous lizard (Lacerta vivipara, Lacertidae, Sauria), Zool. Zh., 1990, vol. 69, no. 6, pp. 93–101.
Kupriyanova, L., Diversity in parthenogenetic lacertid lizards: cytogenetic studies, in Proc. 6th Ordinary General Meeting of the Societas Europaea Herpetologica, Budapest: SHE, 1992, pp. 273–279.
Kupriyanova, L., Structure, localization and stability of chromosome in karyotype evolution in lizards of the Lacertidae family, Russ. J. Herpetol., 1994, vol. 1, pp. 1–12.
Kupriyanova, L.A., Some cytogenetic patterns of reticular speciation of unisexual species of lizards (Reptilia, Lacertidae) and other groups of vertebrates, Tsitologiya, 1997, vol. 39, no. 12, pp. 1089–1108.
Kupriyanova, L.A., Genetic diversity of hybrid in unisexual species and forms of the genus Lacerta (Reptilia, Lacertidae): its possible cytogenetic mechanisms and cytogenetics of meiosis of natural polyploid forms, Tsitologiya, 1999, vol. 41, no. 12, pp. 1038–1047.
Kupriyanova, L., Cytogenetic and genetic trends in the evolution of unisexual lizards, Cytogenet. Genet. Res, 2010, vol. 127, pp. 273–279.
Kupriyanova, L.A., The concept of hybridogenic speciation of vertebrates: comprehensive studies of unisexual reptile species, Tr. Zool. Inst. Ross. Akad. Nauk, 2014, vol. 318, no. 4, pp. 382–390.
Kupriyanova, L.A. and Bohme, W., Viviparous lizard (Zootoca vivipara (Lichtenstein, 1823), Lacertidae) from northeastern and central regions of Europe: intraspecific karyotypic diversity, Zool. Zh., 2012, vol. 91, no. 11, pp. 1428–1432.
Lisachov, A.P., Galkina, S.A., Saifitdinova, A.F., Romanenko, S.A., Andreyushkova, D.A., Trifonov, V.A., and Borodin, P.M., Identification of sex chromosomes in Eremias velox (Lacertidae, Reptilia) using lampbrush chromosome analysis, Comp. Cytogenet., 2019, vol. 13, no. 2, pp. 121–132. https://doi.org/10.3897/CompCytogen.v13i2.34116
Lutes, A.A., Neaves, W.B., Baumann, D.P., Wiegrabe, W., and Baumann, P., Sister chromosome pairing maintains heterozygosity in parthenogenetic lizards, Nature, 2010, vol. 11, no. 464 (7286), pp. 283–286.
MacCulloch, R.D., Murphy, R.W., Kupriyanova, L.A., Darevsky, I.S., and Danielyan, F.D., Clonal variation in the parthenogenetic rock lizard Lacerta armeniaca, Genome, 1995, vol. 38, pp. 1057–1060.
Moritz, C., Brown, W.M., Densmore, L.D., Wright, J., Vyas, D., Donnellan, S., Adams, M., and Baverstock, P., Genetic diversity and the dynamics of hybrid parthenogenesis in Cnemidophorus (Teiidae) and Heteronotia (Gekkonidae), Bull. N.Y. State Mus. (Albany), 1989, vol. 466, pp. 87–112.
Moritz, C., Wright, J.W., and Brown, C.M., Mitochondrial DNA analysis and the origin and relative age of parthenogenetic Cnemidophorus: phylogenetic constraints on hybrid origins, Evolution, 1992a, vol. 46, pp. 184–192.
Moritz, C., Uzzell, T., Spolsky, C., Hotz, H., Darevsky, I., Kupriyanova, L., and Danielyan, F., The material ancestry and approximate age of parthenogenetic species of Caucasian rock lizards (Lacerta: Lacertidae), Genetics, 1992b, vol. 87, pp. 53–62.
Murphy, R., Darevsky, I., Kupriyanova, L., MacCulloch, R., and Fu, J., A fine line between sex and unisexuality: the phylogenetic constraints on parthenogenesis in lacertid lizards, Zool. J. Linn. Soc., 2000, vol. 130, pp. 527–549.
Odierna, G., Aprea, G., and Capriglione, T., Progressive differentiation of the w sex chromosome between oviparous and viviparous populations of Zootoca vivipara (Reptilia, Lacertidae), Ital. J. Zool., 1998, vol. 65, pp. 295–302.
Olmo, E., Odierna, G., and Cobror, O., C-band variability and phylogeny of Lacertidae, Genetics, 1986, vol. 71, pp. 63–74.
Petrosyan, V.G., Osipov, F.A., Bobrov, V.V., Dergunova, N.N., Danielyan, F.D., and Arakelyan, M.S., New records of Darevskia armeniaca (Mehely, 1909) and Darevskia valentini (Boettger, 1892) (Squamata, Sauria, Lacertidae) from Armenia and updated geographic distribution maps, Check List, 2019a, vol. 15, pp. 21–31.
Petrosyan, V., Osipov, F., Bobrov, V., Dergunova, N., Nazarenko, E., Omelchenko, A., Danielyan, F., and Arakelyan, M., Analysis of geographical distribution of the parthenogenetic rock lizard Darevskia armeniaca and its parental species (D. mixta, D. valentini) based on ecological modeling, Salamandra, 2019b, vol. 55, no. 3, pp. 173–190.
Petrosyan, V., Osipov, F., Bobrov, V., Dergunova, N., Kropachev, I.I., Danielyan, F., and Arakelyan, M., New records and geographic distribution of the sympatric zones of unisexual and bisexual rock lizards of the genus Darevskia in Armenia and adjacent territories, Biodiversity Data J., 2020, no. 8. e56030. https://doi.org/10.3897/BDJ.8.e56030
Ryskov, A.P., Genetically unstable microsatellite containing loci and genome diversity in clonally reproduced unisexual vertebrates, in International Review of Cell and Molecular Biology., Jeon, K.W., Ed., New York: Academic, 2008, vol. 270, pp. 319–349.
Safronova, L.D. and Kupriyanova, L.A., Metaphase and meiotic chromosomes, synaptonemal complexes (SC) of the lizard Zootoca vivipara, Russ. J. Genet., 2016, vol. 52, no. 11, pp. 1186–1191.
Skikulnath, K., Matsubara, K., Uno, Y., Olsson, M., and Matsuda, Y., Identification of the linkage group of the Z sex chromosomes of the sand lizard (Lacerta agilis, Lacerttidae) and elucidation of karyotype evolution in lacertid lizards, Chromosoma, 2014, vol. 123, pp. 563–575.
Spangenberg, V., Arakelyan, M., Galoyan, E., Pankin, M., Petrosyan, R., Stepanyan, I., Grishaeva, T., Danielyan, F., and Kolomiets, O., Extraordinary centromeres: differences in the meiotic chromosomes of two rock lizards of species Darevskia portschenskii and Darevskia raddei, 2019.https://doi.org/10.7717/peerj.6360
Stegnii, V.N., Genetika sal’tatsionnogo vidoobrazovaniya i sistemnye mutatsii (Genetics of Saltation Speciation and Systemic Mutations), Tomsk: Tomsk. Gos. Univ., 2019.
Vergun, A.A., Martirosyan, I.A., Semyenova, S.K., Omelchenko, A.V., Petrosyan, V.G., Lazebny, O.E., Tokarskaya, O.N., Korchagin, V.I., and Ryskov, A.P., Clonal diversity and clone formation in the parthenogenetic Caucasian rock lizard Darevskia dahlia, PLoS One, 2014. e91674. https://doi.org/10.1371/journal.pone.0091674
ACKNOWLEDGMENTS
The authors are grateful to A.P. Ryskov for valuable comments, attention to this work, and assistance in preparing the manuscript.
Funding
This work was supported by the Zoological Institute, Russian Academy of Sciences (project no. AAAA-A19-119020590095-9) and the Russian Foundation for Basic Research (project nos. 17-00-00427 and 17-00-00430).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kupriyanova, L.A., Safronova, L.D., Sycheva, V.B. et al. Oogenesis (Prophase 1 of Meiosis) and Mitotic Chromosomes of Parthenogenetic Species Darevskia armeniaca (Family Lacertidae). Biol Bull Russ Acad Sci 48, 274–280 (2021). https://doi.org/10.1134/S1062359021030080
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359021030080