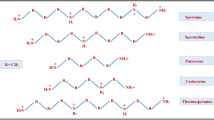
Abstract
It is revealed that dipeptides glycylglycine (GlyGly) and glycylaspartic acid (GlyAsp), as well as the amino acid glycine (Gly), appreciably stimulated the growth and development of tobacco (Nicotiana tabacum L.) regenerants and seedlings if they were present in a medium at 10–7 М. All three compounds GlyGly, GlyAsp, and Gly influenced the cell differentiation and morphogenic processes in the calli. The compounds modulated expression of the KNOX and GRF family genes. The profiles of induction or repression of gene expression by one and the same peptide were found to differ in tobacco regenerants and seedlings. It is concluded that GlyGly, GlyAsp, and Gly may be considered as regulators of plant growth and development, the mode of action of which is signaling and mainly epigenetic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Peptides are involved in an extensive diverse regulatory network, which controls the growth and development of plants and animals (Khavinson and Malinin, 2005; Vanyushin et al., 2017). Most of the plant peptides examined are small signaling molecules or antimicrobial peptides that originated from nonfunctioning precursor proteins (Goyal and Mattoo, 2014). Recently, data have appeared on peptides formed from functional proteins and directly translated on small open reading frames (Hanada, 2013). Secretable peptides (containing from 2 to 100 amino acid residues) play important roles as regulators of intercellular interactions, physiological activities, and responses to various influences and environmental signals (Czyzewicz et al., 2013; Motomitsu et al., 2015; Tavormina et al., 2015).
Evidence concerning the effects of exogenous peptides, which are capable of plant penetration, is meager. Exogenous short peptides may selectively modulate expression of genes and synthesis of proteins including those participating in DNA replication and reparation and responsible for animal cell differentiation (Khavinson and Malinin, 2005). The action of such peptides is gene-specific. Its character is regulatory signaling, and its nature is presumably mainly epigenetic (Vanyushin et al., 2017). For example, exogenous peptides (epitalon) lengthen the animal lifespan (Khavinson et al., 2014); tetrapeptide bronchogen regulates differentiation, proliferation, and apoptosis in human cultured cells of the bronchial epithelium; and vilon exerts the same action on human and animal thymus cells and lymphocytes of the peripherial blood (Khavinson et al., 2015). These peptides are tissue-specific and induce expression of the genes governing DNA reparation and replication (Khavinson et al., 2014, 2015). It is supposed that peptide-dependent regulation of vital activity is common in eucaryots and originated at early phases of evolution (Vanyushin et al., 2017). An important issue of the problem regards the simplest short peptides, which might also be of abiogenic origin at early stages of the formation of life; the effects of these compounds on various organisms and biological systems are of interest.
We found earlier that the exogenous short peptides epitalon (AlaGluAspGly), bronchogen (AlaGluAspLeu), and vilon (LysGlu) influenced the growth, development, and differentiation of the tobacco (Nicotiana tabacum) callus (Fedoreyeva et al., 2017).
The purpose of the present work was comparative study of the effects of low concentrations of the dipeptides glycylglycine (GlyGly) and glycylaspartic acid (GlyAsp), as well as the amino acid glycine (Gly), on the growth, development, and gene expression in tobacco seedlings and calli.
MATERIALS AND METHODS
Seeds of tobacco (Nicotiana tabacum L., Samsun serotype) were sterilized for 15 min in 1.5% sodium hypochlorite containing 0.01% Triton X-100. They were washed with distilled water three times and were allowed to germinate in flasks with an agarized hormone-free Murashige and Skoog (MS) nutrient medium (Sigma, United States). The emerging cotyledons were detached with a scalpel and were placed on an agarized MS medium containing 10–7 M GlyGly, GlyAsp, or Gly (four cotyledons per Petri plate). The control MS medium did not contain the peptides or amino acid. The medium also contained phytohormones: 2 mg/L 6-benzylaminopurine, 0.2 mg/L α‑naphtylacetic acid, and 0.2 mg/L indole-3-butyric acid. One experiment included 4–5 Petri plates with explants, which were thermostated at 25°C for 14 days in the dark. Afterwards, the explants were maintained under light 5000 lx (16 h daily) at 20–22°C (day) and 16–18°C (night). The experiments of regenerant derivation from the explants were carried out with four replications.
On day 21 after explantation, the rate of callus formation was estimated together with the morphology of the regenerants emerging from the explants, namely, the color, texture, size, and number of regenerants and leaves appearing. At the end of the experiment (after 28 days), the normally developed regenerated plants possessing shoots and roots were registered. The regeneration effectiveness was calculated as the number of regenerants per explant.
To obtain tobacco seedlings, the seeds were treated as mentioned above followed by a transfer to an agarized MS medium with 10–7 M GlyGly, GlyAsp, or Gly or without these additions in the control. After 28 days, the seedling height and fresh mass, the length of the main root and shoot, and the leaf area were evaluated. The last parameter was measured under an Olympus BX51 microscope (Japan) furnished with the Cell program. Statistical treatment of all data was performed with the help of Microsoft Excel program.
RNA was isolated from tobacco regenerants and seedlings by the standard method using the corresponding RNK-Ekstran (Synthol, Russia) reagent kits. The RNA concentration in the isolated samples was determined spectrophotometrically. Preparations of cDNA were obtained with the standard method using a Synthol reagent kit intended for reverse transcription.
Data on the primary structure of the KNOX1 and GRF genes of N. tabacum and N. sylvestris were taken from the NCBI database. The primers of these genes were selected with the help of the NCBI Primer-BLAST online service and were synthesized by Synthol Tables 1, 2).
Real-time polymerase chain reaction analysis (PCR-RT) was carried out in a CFX 96 Real-Time System thermocycler (Bio-Rad, United States). Samples were prepared by the standard method with the reagent kit for PCR-RT in the presence of SYBR Green (Synthol). PCR-RT reaction was performed under uniform conditions for all samples: 5 min at 95°C (polymerase activation) and later 45 cycles for 30 s at 94°C, 30 s at 5°C, and 30 s at 72°C. The reaction was undertaken in 2–3 parallels in three replications. The GaPDh (LOC107828122) gene encoding the protein glyceraldehyde-3-phosphate dehydrogenase was taken as the reference counterpart. The relative rate of gene expression was calculated using the calibration curve drawn with the PCR products prepared with the primers of the GaPDh gene.
The PCR-RT efficiency (E, %) was calculated by the formula E = (10–1/s – 1) – 100, where s is the slope angle of the dependency chart of decimal logarithms of values of the threshold cycle (Ct) on the cDNA concentration. This efficiency was 95–96% with the primers of the genes tested.
RESULTS AND DISCUSSION
It was found that GlyGly, GlyAsp, or Gly, when present at 10–7 M in a medium of tobacco, stimulated callus formation and mass accumulation of calli and regenerants and also influenced leaf formation. The regenerants derived from the callus tissue of the cotyledons had a mass 35–90% higher in the peptide-treated counterparts than in the untreated control (Fig. 1, Table 3). The leaf emergence started on days 11–12 in the tobacco regenerants subjected to peptides in contrast to days 14–15 in the control regenerants. Therefore, GlyGly, GlyAsp, and Gly affected the cell differentiation and were involved in form-building processes in the plants. The most active callus formation occurred on the medium containing the GlyGly peptide. In the control, areas of friable and firm morphogenic calli, together with rare large morphogenic zones containing multiple small regenerants, arose after eight days (Fig. 1). The presence of the peptides increased the total number of regenerants per explant. The regeneration efficiency depended on particular peptides and the amino acid applied (Table 3). On the Gly- and GlyGly-containing media, large regenerants appeared bearing bigger leaves than in the control (Fig. 1, Table 3). Thus, the lamina area of most regenerants was 1.3 times higher and that of large regenerants was almost two times higher than in the control. In the presence of GlyAsp, large leaves did not emerge, and the mean leaf area of the regenerants was even 30% smaller in comparison with the control. Therefore, the neutral Gly and GlyGly, but not the acidic GlyAsp dipeptide, stimulated formation of large leaves in tobacco regenerants.
The growth and development of tobacco seedlings were also influenced by peptides and glycine. GlyAsp and GlyGly were the most stimulative towards development. The root system appeared more developed in their presence. Here, the length of the main root increased approximately 40% against the control (Fig. 2), and new lateral roots arose. In this case, the total height of the seedling increased only 15%. With allowance for the lower height of the seedling, the lamina area increased 1.5 times in the presence of peptides. The tobacco seedling biomass was approximately 40% higher in the peptide-treated counterparts (Fig. 3, Table 4). Apparently, the tested compounds are quite biologically active at such a low concentration (10–7 M) because they fulfill a signaling regulatory function in the cell.
Glycine also stimulated plant development but more weakly than peptides did (Table 4): the lamina area was 1.3 times larger than in the untreated control, and the seedling height and the main root length increased by 10–20 and 10–30%, respectively (Fig. 2b, Table 4). However, Gly, as well as dipeptides, promoted the emergence of lateral roots and, consequently, also stimulated development of the tobacco root system.
Therefore, the stimulation of tobacco plant development by the tested dipeptides and amino acid glycine was accompanied by the appearance of leaves and additional roots, formation of a more developed leaf blade, and enhancement of the seedling total biomass as a result (Fig. 3, Table 4).
The fact of the physiological activity of the examined peptides (induction of morphogenesis, stimulation of callus and leaf growth, and augmentation in biomass of both callus-originated regenerants and seedlings of tobacco) prompted us to study the expression of some genes of the KNOX and GRF families, which encode transcription factors and are responsible for cell differentiation and leaf emergence.
Growth-regulating factors of plants are specific factors of transcription. They play leading roles in the stem growth; formation of leaves, flowers, and seeds; root development; and coordination of growth processes under unfavorable conditions (Omidbakhsfar et al., 2015). For the first time, the GRF gene was identified in rice (OsGRF1); it regulates the gibberellin- induced elongation of the stem cells (Van der Knaap et al., 2000).
The GRF family genes were recently discovered and characterized in tobacco (Zhang et al., 2018). The proteins encoded by them differ in the molecular masses (24.4–65.4 kDa) and physicochemical properties. Their functions are not defined thus far. It is supposed that the genes of this family are involved in plant responses to biotic and abiotic stressors (Zhang et al., 2018).
Almost all the GRF family genes display a high level of expression in all types of actively growing tissues, for example, in germinating seeds, calli, and flower buds. The transcription level of any GRF genes is relatively higher in the younger than in elder leaves. This means that these genes mainly operate in the early phases of leaf growth and development (Kim and Kende, 2004). However, GRF3 manifests, for example, higher expression in leaves and stems, while GRF4 does this in roots. Therefore, these genes may be related to regulation of growth and development of these organs.
In Arabidopsis thaliana, the GRF1, GRF2, and GRF3 genes function as regulators of cell proliferation during leaf development (Kim and Lee, 2006). The high GRF4 expression level correlates with the increase in a total biomass and seed size leading to the increase in these parameters (Van Daele et al., 2012). In A. thaliana, GRF4 participates not only in leaf cell proliferation but also in embryonic development of the cotyledons and meristem (Kim and Lee, 2006).
The proteins encoded by four GRF genes of growth-regulating factors were identified in tobacco (Table 1). This fact has determined the choice of the genes whose expression in response to peptides was important to study. All proteins coded by the examined genes bound to DNA (Table 1). Those were DNA-apurinic/apyrimidinic ligase (GRF1), DNA-topoisomerase 3α (GRF2), 3',5'-exonuclease (GRF3), and endonuclease 8 (GRF4).
Figure 4 depicts the data on expression of the GRF genes in tobacco regenerants and seedlings grown in the presence or absence of short peptides. The level of expression of this family of genes was markedly lower in the seedlings than in the regenerants derived from the callus tissue. As mentioned above, all known GRF genes are more active and demonstrate a higher expression level in the young developing plants, especially in their leaves. This may explain why the leaves of the 4-week-old seedlings were better developed than the leaves of 4-week-old regenerants from the callus.
Peptides and glycine suppressed the expression of the GRF2 and GRF1 genes in regenerants from calli but boosted the expression of the GRF3 and GRF4 genes (Fig. 4). Thus, GlyGly and especially GlyAsp enhanced the GRF4 expression almost fourfold in comparison with the control.
Peptides influenced the expression of the GRF gene family in the tobacco seedlings in a different manner than in the regenerantes. In the seedlings, peptides and glycine considerably enhanced expression of the GRF3 and GRF4 genes but were almost indifferent towards the expression of GRF2. The expression of the GRF1 gene was also unchanged by GlyGly or Gly but it almost doubled in the presence of GlyAsp. The GRF4 gene expression is known to be maximal in the roots (Kim and Kende, 2004; Kim and Lee, 2006). The 4-week-old regenerants only start the formation of roots, whereas seedlings of equal age already possess well-formed roots. Apparently, this may explain the fact that the GRF4 gene activity is higher in regenerants than in seedlings and can be additionally stimulated by dipeptides.
Therefore, GlyGly, GlyAsp, and Gly affect the expression of the genes encoding the proteins of growth-regulating factors. It is not excluded that the peptides can bind to the proteins encoded by the GRF family genes and so participate directly in growth regulation. Gly and possibly GlyGly are known to bind preferentially to cystein residues similarly to the animal situation where glycine binds to the cysteine loop located at the end of the glycine receptor (Lynch et al., 2017), and the negatively charged peptide GlyAsp binds in preference to the positively charged residues of lysine or arginine. In our experiments, the effects of neutral Gly and GlyGly on tobacco development differed from those of negatively charged GlyAsp. Here, neutral Gly and GlyGly increased to the greatest extent the biomass of the callus-derived regenerants, whereas GlyAsp mainly stimulated seedling growth.
It is revealed that the proteins coded by the GRF genes from barley Hordeum vulgare can act as repressors through binding to the intervening sequence of the Knotted3 gene; the AtGRF4, AtGRF5, and AtGRF6 proteins from A. thaliana repress the promoter activity of the KNAT2 (Knotted2) gene (Kuijt et al., 2014). These data demonstrate that the transcription factors of the GRF Knotted (KNOX) families interact with each other and, due to binding to a certain sequence of the KNOX family genes, control their activity.
The proteins of the KNOX class (KNOTTED1-like homedomain) are critical regulators of the stem cell homeostasis in plant seedlings. The KNOX genes encode transcription factors that are involved in cessation of cell differentiation in the seedling apical zone; they are identified in all monocot and dicot plants (Lynch, 2004). Ectopic expression of the KNOX genes causes dramatic changes in the leaf and flower morphology in various plants (Srinivasan et al., 2011).
The KNOX gene family is divided into the KNOX1 and KNOX2 classes. The Arabidopsis genome contains four genes of the KNOX1 class: SHOOT MERISTEMLESS (STM), BREVIPEDICELLUS (BP or KNAT1), KNAT2, and KNAT6. These genes operate at different stages of various developmental processes in the plants. Thus, the STM gene is expressed in early embryogenesis in all meristematic zones (Zhang and Rongming, 2014), and the gene KNAT6 is expressed at the stage of embryoniс development and exhibits the maximal expression level over the entire boundary of the meristem (Long et al., 1996). The expression of BP is the most pronounced in the meristem during the postembryoniс period (Belles-Boix et al., 2006). The KNAT2 expression rises in the period of embryogenesis mainly in the central part of the meristem zone (Byrne et al., 2002). An increase in the STM expression entails earlier formation of leaves in callus cultures. Modulation of the KNOX1 activity makes leaf shapes of flowering plants more variable. The KNOX1 and KNOX2 genes differ functionally in that the expression of the first one is confined to less differentiated tissues, while the second one is expressed in both differentiating tissues and already formed organs (flowers and inflorescences) (Dockx et al., 1995). Evidence of a reciprocal suppression of the KNOX1 and KNOX2 expression has been reported (Hay and Tsiantis, 2010). In Arabidopsis, the KNAT3 gene encodes β‑glucuronidase and belongs to the KNOX2 class (Serikawa, et al., 1997). The KNAT3 gene is active only in the tissues of the completed root (Truernit and Haseloff, 2007).
The LЕT6 and LЕT12 genes were characterized for the first time in the tomato Solanum lycopersicum (Janssen et al., 1998). LЕT6 is attributed to the KNOX1 class, and LЕT12, to KNOX2. The LЕT6 gene is supposed to bear a resemblance to the STM gene from Arabidopsis, and its expression is related to the formation of lateral organs. Expression of LET12 occurs in all tissues of the developing plant.
GlyGly, GlyAsp, and Gly affected expression of the KNOX family gene in tobacco regenerants and seedlings (Fig. 5). The KNOX family genes, which are responsible for leaf differentiation and belong to the KNOX1 family, manifested a higher expression in the regenerants than in the seedlings. These data establish the processes related to leaf formation and proceeding in the arising regenerates.
The KNAT2 and LET6 expression levels changed only slightly under the influence of peptides in the regenerants from tobacco callus tissue. Furthermore, the presence of dipeptides and glycine in the medium of seedling cultivation even decreased the expression of these genes. In the seedlings, GlyGly and GlyAsp inhibited 2–3 times the KNAT2 gene expression; all the preparations tested suppressed the expression of LET6. It is known that a high expression level of the KNOX1 genes leads to earlier leaf differentiation in A. thaliana (Srinivasan et al., 2011).
In the presence of peptides or glycine, leaf formation started 4–6 days earlier than in the untreated control in the regenerants from tobacco callus tissue (data not shown). It can be suggested that dipeptides initiate earlier expression of these genes, which decreased after four weeks of seedling development. The contact of calli with Gly or especially GlyGly considerably diminished the KNAT1 gene expression in the regenerants. By contrast, GlyAsp strongly increased this gene expression in the regenerants. In the seedlings, the effects of the tested substances on KNAT1 expression were differently directed: a slight (approximately 10%) increase caused by GlyGly or Gly and almost twofold decrease caused by GlyAsp were observed.
The expression level of the KNAT3 gene was more than two times higher in the seedlings than in the regenerants. These data are consistent with the report that the KNAT3 gene, which belongs to the KNOX2 class, is more actively expressed in the developing roots (Serikawa et al., 1997). In the regenerants, the KNAT3 expression level appreciably depended on the presence of peptides or glycine in the medium; these compounds might elevate the expression 3–5 times or more. In turn, such an increase in the KNAT3 expression might apparently promote earlier development of the root system in the regenerants. In fact, the calli treated with Gly, GlyGly, or GlyAsp formed roots earlier than the untreated ones.
GlyAsp boosted KNAT3 gene expression in both the callus-originated regenerants and seedlings and diminished the KNAT6 expression in the seedlings. Dipeptides and glycine scarcely affected the LET12 gene expression in the seedlings but increased it 1.5–2 times in the regenerants.
Therefore, the KNOX family genes actively participate in the development of the regenerants from callus tissue and growth of the seedlings of tobacco as well as in leaf and root formation. GlyGly, GlyAsp, and Gly influenced the process of leaf formation in the regenerants from tobacco callus tissue (Fig. 1) that was accompanied by modulation of the expression levels of the KNOX1 family genes (Fig. 5). GlyGly, GlyAsp, and Gly even inhibited the expression of these genes in the tobacco seedlings, which bore leaves of larger area than leaves of the regenerants. Nonetheless, the expression level of the gene KNAT3, belonging to the KNOX2 class, was enhanced 3–5 times in the presence of dipeptides (especially GlyAsp) or glycine. Presumably, the expression of this gene was initiated at later stages of seedling development; the gene activity might be related to formation of lateral roots of the seedlings that we actually observed (Fig. 1, Table 4).
The molecular machinery, by which GlyGly, GlyAsp, and Gly regulate gene expression, is still uncertain. For example, it seems possible that the tested compounds are capable of binding to signaling proteins and affecting gene expression this way. GlyGly, GlyAsp, and Gly, like other short bioactive peptides (Khavinson et al., 2011), might directly link to the promoter regions of genes and epigenetically control gene expression through blocking promoter methylation. In general, the regulation of gene expression by short peptides may be of epigenetic nature. It is not also excluded that peptides may interact with N- and C-terminal histone sequences in chromatin through a blockade of their enzymatic modification. Finally, peptides may also interact with small interfering RNA (siRNA) and thus prevent RNA binding to genes.
The reported results mean that dipeptides and glycine are able to affect the growth and development of tobacco regenerants and seedlings appreciably so that the character of their effects is different in these plant objects. Regenerants and seedlings also differ in the efficiency of expression regulation of the GRF and KNOX family genes by the chemical agents tested. Therefore, similarly to different short exogenous peptides (Fedoreyeva et al., 2017), the dipeptides glycylglycine and glycylaspartic acid, as well as the amino acid glycine, possess a pronounced physiological activity and may be attributed to efficient regulators of plant growth and development.
REFERENCES
Belles-Boix, E., Hamant, O., Witiak, S.M., Morin, H., Traas, J., and Pautot, V., KNAT6: an Arabidopsis homeobox gene involved in meristem activity and organ separation, Plant Cell, 2006, vol. 18, pp. 1900–1907.
Byrne, M.E., Simorowski, J., and Martienssen, R.A., Asymmetric leaves reveals knox gene redundancy in Arabidopsis,Development, 2002, vol. 129, pp. 1957–1965.
Czyzewicz, N., Yue, K., Beeckman, T., and De Smet, I., Message in a bottle: small signalling peptide outputs during growth and development, J. Exp. Bot., 2013, vol. 64, pp. 5281–5296.
Van Daele, N., Gonzalez, I., Vercauteren, L., de Smet, D., Inze, I., Roldan-Ruiz, M., and Vuylsteke, A., A comparative study of seed yield parameters in Arabidopsis thaliana mutants and transgenics, Plant Biotechnol. J., 2012, vol. 10, pp. 488–500.
Dockx, J., Quaedvlieg, N., Keultjes, G., Kock, P., Weisbeek, P., and Smeekens, S., The homeobox gene ATK1 of Arabidopsis thaliana is expressed in the shoot apex of the seedling and in flowers and inflorescence stems of mature plants, Plant. Mol. Biol., 1995, vol. 28, pp. 723–737.
Fedoreyeva, L.I., Dilovarova, T.A., Ashapkin, V.V., Martirosyan, Yu.Ts., Khavinson, V.Kh., Kharchenko, P.N., and Vanyushin, B.F., Short exogenous peptides regulate expression of CLE, KNOX1, and GRF family genes in Nicotiana tabacum,Biochemistry (Moscow), 2017, vol. 82, pp. 521–528.
Goyal, R.K. and Mattoo, A.K., Multitasking antimicrobial peptides in plant development and host defense against biotic/abiotic stress, Plant Sci., 2014, vol. 228, pp. 135–149.
Hanada, K., Higuchi-Takeuchi, M., Okamoto, M., Yoshizumi, T., Shimizu, M., Nakaminami, K., Nishi, R., Ohashi, C., Iida, K., Tanaka, M., Horii, Y., Kawashima, M., Matsui, K., Toyoda, T., Shinozaki, K., Seki, M., and Matsui, M., Small open reading frames associated with morphogenesis are hidden in plant genomes, Proc. Natl. Acad. Sci. U. S. A., 2013, vol. 110, pp. 2395–2400.
Hay, A. and Tsiantis, M., Knox genes: versatile regulators of plant development and diversity, Development, 2010, vol. 137, pp. 3153–3165.
Janssen, B.J., Williams, A., Chen, J.J., Mathern, J., Hake, S., and Sinha, N., Isolation and characterization of two knotted-like homeobox genes from tomato, Plant. Mol. Biol., 1998, vol. 36, pp. 417–425.
Khavinson, V.Kh. and Malinin, V.V., Gerontological Aspects of Genome Peptide Regulation, Basel, Switzerland: Karger, 2005, p. 104.
Khavinson, V.Kh., Fedoreeva, L.I., and Vanyushin, B.F., Short peptides modulate the effect of endonucleases of wheat seedling, Doklady Biochem. Biophys., 2011, vol. 437, pp. 64–67.
Khavinson, V.Kh., Tendler, S.M., Vanyushin, B.F., Kasyanenko, N.A., Kvetnoy, I.M., Linkova, N.S., Ashapkin, V.V., Polyakova, V.O., Basharina, V.S., and Bernadotte, A., Peptide regulation of gene expression and protein synthesis in bronchial epithelium, Lung, 2014, vol. 192, pp. 781–791.
Khavinson, V.Kh., Tendler, S.M., Kasyanenko, N.A., Tarnovskaya, S.I., Linkova, N.S., Ashapkin, V.V., Yakutseni, P.P., and Vanyushin, B.F., Tetrapeptide KEDW interacts with DNA and regulates gene expression, Am. J. Biomed. Sci., 2015, vol. 7, pp. 156–169.
Kim, J.H. and Kende, H., A transcriptional coactivator, AtGRF1, is involved in regulating leaf growth and morphology in Arabidopsis, Proc. Natl. Acad. Sci. U. S. A., 2004, vol. 101, pp. 13374–13379.
Kim, J. and Lee, B., Growth-regulating factor4 of Arabidopsis thaliana is required for development of leaves, cotyledons, and shoot apical meristem, J. Plant Biol., 2006, vol. 49, pp. 463–468.
Van der Knaap, E., Kim, J.H., and Kende, H., A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth, Plant Physiol., 2000, vol. 122, pp. 695–704.
Kuijt, S.J.H., Greco, R., Agalou, A., Shao, J., Hoen, C.C., Overnäs, E., Osnato, M., Curiale, S., Meynard, D., van Gulik, R., de Faria Maraschin, S., Atallah, M., de Kam, R.J., Lamers, G.E., Guiderdoni, E., Rossini, L., Meijer, A.H., and Ouwerkerk, P.B., Interaction between the growth-regulating factor and knotted1-like homebox families of transcription factors, Plant Physiol., 2014, vol. 164, pp. 1952–1966.
Long, J.A., Moan, E.I., Medford, J.I., and Barton, M.K., A member of the KNOTTED class of homeodomain proteins encoded by the SHOOTMERISTEMLESS gene of Arabidopsis,Nature, 1996, vol. 379, pp. 66–69.
Lynch, J.W., Molecular structure and function of the glycine receptor chloride channel, Physiol. Rev., 2004, vol. 84, pp. 1051–1095.
Lynch, J.W., Zhang, Y., Talwar, S., and Estrada-Mondeagon, A., Glycine receptor drug discovery, Adv. Pharmacol., 2017, vol. 79, pp. 225–253.
Motomitsu, A., Sawa, S., and Ishida, T., Plant peptide hormone signaling, Ess. Biochem., 2015, vol. 58, pp. 115–131.
Omidbakhsfar, M.A., Proost, S., Fujikura, U., and Mueller-Roeber, B., Growth-regulating factors (GRFs): a small transcription factor family with important functions in plant biology, Mol. Plant, 2015, vol. 8, pp. 998–1010.
Serikawa, K.A., Martinez-Laborda, A., Kim, H.S., and Zambryski, P.C., Localization of expression of KNAT3, a class 2 knotted1-like gene, Plant J., 1997, vol. 11, pp. 853–861.
Srinivasan, C., Liu, Z., and Scorza, R., Ectopic expression of class 1KNOX genes induce adventitious shoot regeneration and alter growth and development of tobacco (Nicotiana tabacum L.) and European plum (Prunus domestica L.), Plant Cell Rep., 2011, vol. 30, pp. 655–664.
Tavormina, P., De Coninck, B., Nikonorova, N., De Smet, I., and Cammue, B., The plant peptidome: an expanding repertoire of structural features and biological functions, Plant Cell, 2015, vol. 27, pp. 2095–2118.
Truernit, E. and Haseloff, J., A role for knat class ii genes in root development, Plant Signal Behav., 2007, vol. 2, pp. 10–12.
Vanyushin, B.F., Ashapkin, V.V., and Aleksandrushkina, N.I., Regulatory peptides in plants, Biochemistry (Moscow), 2017, vol. 82, pp. 89–94.
Zhang, W. and Rongming, Yu., Molecular mechanism of stem cells in Arabidopsis thaliana,Pharmacogn. Rev., 2014, vol. 8, pp. 105–112.
Zhang, J., Li, Z., Jin, J., Xie, X., Zhang, H., Chen, Q., Luo, Z., and Yang, J., Genome-wide identification and analysis of the growth-regulating factor family in tobacco (Nicotiana tabacum), Gene, 2018, vol. 639, pp. 117–127.
ACKNOWLEDGMENTS
The authors are grateful to Ya.I. Alekseev and A.V. Kuzubov for synthesis of the primers.
The equipment of the Center for Collective Use, All-Russia Research Institute of Agricultural Biotechnology, was employed.
Funding
This work was performed according to State Task no. AAAA-A17-117091460012-8 and was supported in part by the Russian Science Foundation (project no. 14-50-00029) and the Russian Foundation for Basic Research (project no. 18-016-00150).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Aver’yanov
Rights and permissions
About this article
Cite this article
Fedoreyeva, L.I., Kononenko, N.V., Baranova, E.N. et al. Dipeptides and Glycine Modulate Development of Seedlings and Regenerants of Tobacco Nicotiana tabacum L.. Biol Bull Russ Acad Sci 47, 364–373 (2020). https://doi.org/10.1134/S1062359020030036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359020030036