Abstract—
Examples of the use of different types of chromosome aberrations as diagnostic indicators to solve the practical problems of radioecology were considered. The classifications of the chromosome aberrations used to estimate the clastogenic effect of factors of radiation and chemical nature according to the results of cytogenetic studies with uniform staining of the chromosomes were analyzed. Some terminological inconsistency and ambiguity when designating various types and categories of chromosome aberrations, reflecting the clastogenic effect, was detected. It was demonstrated that this inconsistency can complicate the use of such cytogenetic indices in radioecological practice. According to the results of the Allium test using a digital imaging system, original microimages demonstrating the configurations of aberrant chromosomes used in classifications were obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cytogenetic indicators of biodiagnostics are traditionally used in radiobiological and radioecological studies to solve scientific and applied problems: to study molecular mechanisms of the effect of ionizing radiation and the effects of adaptation and evolutionary processes in conditions of acute and chronic irradiation and to estimate the levels of radionuclide and complex contamination of technogenic territories [1]. The ability of ionizing radiation and a number of chemical mutagens to have a clastogenic effect on chromosomes (causing DNA molecule breaks) can be detected by standard methods of cytogenetic study in the form of metaphase or ana-telophase analysis. The analysis of structural mutations of the chromosomes using undifferentiated staining of genetic material without stopping the division at the stage of metaphase is the most simple and universal procedure [2]. The anaphase method is widely used in radioecology, especially using the plant test organisms and bioindicators (members of phytocenoses characterized by large chromosomes). At the same time, integral indicators such as the total frequency of mitosis (meiosis) pathologies and/or the frequency of chromosomal aberrations are used as test functions. Taking into account the peculiarities of the radiation effect mechanism, it is informative to allocate from the total frequency of aberrations that part of the spectrum of violations that reflects the clastogenic effect. At the stage of anaphase, the fragments and bridges that represent the effects on the genetic material realized in the chromosome breaks are easily detected. Depending on the phase of the break occurrence, the chromatid or chromosome fragments and bridges are allocated [3] that relate to the chromatid or chromosome type of aberrations, respectively. The same structural abnormalities of the chromosomes are mentioned as single fragments and bridges according to another trait (morphology of anaphase configuration) as opposed to paired configurations [4].
Since differences in the spectra of the chromosome aberrations (reflecting the clastogenic effect on the chromosomes) were detected in studies on the cytogenetic effects of factors of radiation and chemical nature [5], the use of the ratio of the chromosomal aberrations of different types as an indicator of the nature of the mutagen is natural in radioecological studies. Thus, the predominance of the chromosomal type of aberrations in the total spectrum of structural mutations of the chromosomes reflects the priority role of the factor of the radiative nature in the genotoxic effect of the combined effects of mutagens. However, there are certain contradictions and terminological discrepancies when designating the aberrations in different classifications; this was noted in the studies on the peculiarities of chemical and radiation mutagenesis. “There is some terminological inaccuracy in the literature when designating the structural mutations of the chromosomes. All types of changes are frequently designated by the term chromosome rearrangements. In this case, it is more correct to use the term rearrangements of the chromosomes, while the expressions chromosome rearrangements and chromatid rearrangements will be independent” (cited by [6]). To some extent, this is associated with differences in the spectra of the chromosome abnormalities detected with different forms of cytogenetic study. The spectrum of abnormalities of the chromosome structure, detected by metaphase analysis and underlying major classifications, is significantly wider and differs from the types of aberrations available for accounting in cytogenetic study without stopping mitosis at the stage of metaphase [7]. The absence of a unified generally accepted approach to designation of the types of the chromosomal aberrations, detected at the stage of anaphase, and a certain subjectivity in the choice of classifications significantly complicates the comparison of the results of researchers and generalization of the data obtained.
The aim of this work was to compare the classifications of the chromosome aberrations detected in cytogenetic studies with undifferentiated chromosome staining and reflecting the clastogenic effects, as well as to analyze the use of the spectrum of chromosome abnormalities as bioindication and test indicators in the practice of radioecological studies.
A METHOD FOR OBTAINING DIGITAL IMAGES OF ABERRANT CHROMOSOMES
The procedure for obtaining micrographs documenting the configurations of aberrant chromosomes that correspond to classifications was performed based on the results of a series of Allium tests without stopping mitosis at the stage of metaphase. The bulbs and seeds of the onion Allium cepa L. (Stuttgart Riesen cultivar) served as a phytotester. For cytogenetic analysis, the root section with a apical meristem (about 1.5 cm in size) was placed in a Clarke fixator for a period of 2 to 14 days. If long-term storage was required, biomaterial was converted into 70% (v/v) ethanol with preliminary washing from the fixator with an alcohol solution of the same concentration. The preparation for microscopy was conducted according to a standard method of preparing the preparations from the root meristem [8], washing the material from alcohol in distilled water in small containers. The preparations were stained with 2% acetoorcein (Sigma-Aldrich, United States) for 30 min, after which the roots were washed for 15 min from the dye in 45% acetic acid. To prepare standard squeezed preparations, the apical region at least 2 mm in length was taken from the stained root. The analysis of the cells was conducted using a light field microscopy in transmitted light at a magnification of 10 × 40 using a blue light filter. Digital microimages of the chromosome aberrations were obtained using a visualization system based on a Mikmed-6 microscope with a trinocular attachment (OAO LOMO, Russia), opto-mechanical adapter (NPK Zenit, Russia), digital camera Canon EOS 1100 D (Canon Inc., Japan), and software supplied in a package with the visualization complex EOS Utility, version 2.10.00 (Canon Inc.) [9]. The conversion of digital images was conducted in the computer block using the Microsoft Office Picture Manager photo editor.
CHROMOSOME ABERRATIONS AS DIAGNOSTIC INDICATORS OF CLASTOGENIC EFFECT OF RADIONUCLIDE AND COMPLEX CONTAMINATIONS
In radioecological studies, the total frequency of chromosomal abnormalities in somatic and generative plant cells was a dose-dependent indicator of the level of radiation effect both for the acute period and for long-term periods after the Chernobyl radiation accident [10, 11]. The frequency of chromosome aberrations was used during cytogenetic biomonitoring of technogenic territories with radionuclide and chemical contamination [12, 13] as an indicator of the level of complex contamination.
However, the attempts at differentiated use of cytogenetic indicators are increasingly being made in recent years. Those parts of the spectrum that are associated with ideas about different mechanisms for realization of a clastogenic effect are allocated from the total frequency of the chromosome aberrations for diagnostic purposes, thus revealing the leading role of either chemical mutagens or the factors of radiation nature in genotoxicity. At the same time, the classification of chromosome aberrations can rely on the morphology of the anaphase chromosome configuration (single or double bridges and fragments) or the cell cycle phase at which there was a chromosome break with its subsequent realization in the aberration in the cycle “break–fusion–structural mutation” with the allocation of subgroups of chromatid or chromosome types of aberrations as the basis.
For example, the spectrum of cytogenetic abnormalities with the allocation of subgroups of chromosome and chromatid types of aberrations in the Alliumschoenoprasum onion plants, growing on technologically contaminated soil for a year, was used in the work [14] as an indicator of the nature of a mutagen, a priority for genotoxicity of several test sites with different contents of HNRN, heavy metals, and arsenicum. The predominance of chromatid-type abnormalities (single chromatid fragments and bridges) in the spectrum of cytogenetic effects allowed the suggestion of the leading role of chemical mutagenesis associated with high concentrations of metals in the soil. At the same time, an increase in the frequency of paired bridges and fragments related to the chromosome type of chromosome damages was considered as a sign of the radiation effect of ionizing radiations from HNRN.
The results of estimation of the groundwater genotoxicity from the industrial site of the center for radioactive waste management in conditions of combined contamination were similarly analyzed. A slight contribution of the chromosome type of aberrations (double bridges and fragments) was detected according to the results of biotesting with the Allium cepa; this allowed the authors to allocate the leading role of the chemical contamination factor (substances of the 3rd hazard class) in the genotoxicity of the samples against the background of contamination with 137Cs and 90Sr [15].
The authors recommended a part of the spectrum of chromosomal aberrations in the form of double bridges as a biological indicator diagnosing the level of the effect of radiation on natural plant populations under conditions of radioactive contamination of the Semipalatinsk Test Site. When studying cytogenetic indices of the dominant Koeleria gracilis species, detailed analysis of the spectrum revealed varying intensity of the growth in the frequency for different types of aberrations. The growth in the number of single bridges and fragments and double fragments did not depend on the value of Sr90-specific activity in biomaterial. On the contrary, the change in the frequency of double bridges showed a linear dose dependence up to the value of 10 MBq/kg [16].
The researchers in [17] used a slightly different way of using the indicator of the frequency of chromosome aberrations, analyzing the nature of the chromosome aberration distribution in the cells of hydrobiont indicators under experimental conditions of mutagenesis. The effect of only ionizing radiation or its leading role under conditions of the combined effect with chemical mutagens was characterized by the distribution of the chromosomal aberrations in the cells according to Poisson’s laws, while with chemical mutagenesis or its leading role in the combined effect, it is characterized by a distribution close to geometrical. In identifying differences in the type of cell-by-cell distribution of the chromosome aberrations depending on the role of chemical mutagens or ionizing radiation in the abnormalities, the authors analyzed natural populations of hydrobionts to estimate the efficiency of real operating factors of contamination and to take into account their contribution in the chromosome damage under complex contamination. The regularities detected in the model experiment were confirmed, and it was concluded that the type of distribution of a chromosomal aberration can be used to indicate the cytogenetic effect of ionizing radiation and chemical mutagens with a combined effect.
In general, it is clear that it is informative to use both the analysis of the type of cell-by-cell distribution of the chromosomal aberrations and the ratio of the chromosome aberrations of different types in the spectrum when trying to identify the nature of the mutagen with the leading effect under conditions of complex contamination with mutagens of chemical and radiative nature. According to the authors, these peculiarities can indicate the leading role of the factors of either radiation or chemical nature in the genotoxicity of natural environments [14–17].
ANAPHASE CONFIGURATIONS OF ABERRANT CHROMOSOMES USED FOR CLASSIFICATION
Comparing the contribution of chemical mutagens or factors of radiation nature in the clastogenic potential, the researchers prefer to use classifications based on the morphology of metaphase/anaphase chromosomes or rely on ideas about the stage of the break occurrence and different molecular genetic events leading to its realization in structural mutations of the chromosomes. As is known, depending on the cell cycle phase of the appearance of a DNA strand break, the chromosomal type of aberrations (occurring in presynthetic stage G1) and those of the chromatid type (the formation of which is possible both during and after replication in the phase S and G2, respectively) are allocated. In the phase G1, a chromosome is represented by a single-stranded structure; therefore, emerging at this stage, the chromosomal abnormalities after DNA synthesis phase S are doubled and are morphologically represented by double structures in metaphase and anaphase. The chromatid type of aberrations occur after replication and affect a change in one of the chromatids, which is morphologically manifested by single aberrations as the final form of violations of only one of two sister chromatids at the stage of metaphase and anaphase [4, 5, 18–20].
Table 1 summarizes data on the types of aberrant chromosomes used for the classification according to the results of anaphase study and subdivided on the basis of the following:
—stage (S, synthetic; G1 and G2, pre- and postsynthetic, respectively) of the cell cycle of the appearance of the chromosome break;
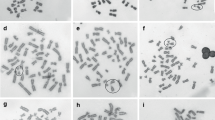
—morphology of the anaphase chromosome configuration in mitosis. Micrographs demonstrating the configurations of aberrant chromosomes used in classifications and obtained according to the results of the Allium test with undifferentiated staining are presented in Fig. 1.
Digital microimages (magnification 10 × 40) of mitosis pathologies in the Allium cepa onion root apical meristem cells associated with a clastogenic effect on the chromosomes during biotesting: (1) soils with maximal activity of 137Cs 7.1 ± 5.9; Ra226, 44.3 ± 15.2; Th232, 27.6 ± 16.0; K40, 420 ± 151 Bq/kg and the content of the amount of polycyclic aromatic hydrocarbons 91–297, including 3,4-benzpyrene 1–15 µg/kg (a, c, d, e, i); (2) γ-radiation from the soils contaminated with 137Cs with the equivalent dose rate 0.3–1.53 µSv/h (b, f, h, j, k, m); (3) mobile phone radiation with the intensity 1.5–2.5 µW/cm2 (g, l, n, o); (a, b), single bridge in anaphase; (c) pair of single bridges in anaphase of a single cell; (d) double parallel bridge in anaphase; (e) configuration of single and double crossed bridge with a pair of long fragments in anaphase of a single cell; (f, g, h) double crossed bridge in anaphase; (i, j) double bridge in the form of two linked chromatids like links in the chain; (k) double bridge as a large dicentric anaphase ring; (l, m) multiple fragmentation of the chromosomes into aberrant metaphase with an implicit double nature of fragments; (n, o) multiple fragmentation of the chromosomes in anaphase with implicit single nature of fragments; (p) microscale (division value 10 µm).
The description of anaphase bridges according to the classification [18] with their division according to morphology into the chromosome (usually double) and chromatid (usually single) bridges is quite often cited. The chromosome double bridge is considered as a result of reunification of two broken chromosomes, while the chromatid single bridge (Table 1, no. 1; Figs. 1a, 1b) is formed during the sister compound of the chromatids of an isolocus break of a single chromosome and is frequently designated as a dicentric chromatid or equal arm anaphase bridge. The second possible mechanism of the formation of a single (unequal arm) bridge of the chromatis type consists in asymmetrical interchromosomal exchange (chromatid translocation) as a result of the fusion of proximal ends after the breakdown of chromatids of two different chromosomes (Table 1, no. 2).
The possibility of a “single bridge” configuration in anaphase as the chromosome type of aberration when there is a break in an unreplicated chromosome of the cell cycle phase G1 with subsequent doubling and subsequent fusion of the proximal fragments [19] (Table 1, no. 3) is not recognized by all researchers [4].
All researchers attribute the double anaphase bridges (Table 1, nos. 4, 5) to the chromosome type of aberrations formed on the basis of asymmetric interchromosomal exchange [7] after the breaks in two uncleaved chromosomes with subsequent replication. In the metaphase configuration, these are dicentric chromosomes (radiation effect markers); in anaphase, the chromatids can go to different poles forming a crossing double bridge or a configuration of parallel bridges designated as a “double bridge” [4, 19], without specifying a specific form (Figs. 1c–1h). Only a cross bridge is more frequently mentioned in classifications as a possible anaphase fate of a dicentric [20, 21].
When analyzing double bridges, it is also necessary to note the anaphase fate of ring centric chromosome of metaphases (radiation effect markers). It is known that ring-shaped chromosome anaphase bridges are formed in the cycle “break–fusion (repair errors)–structural violation” and are preceded by the existence of ring chromosomes [7, 22]. Separating in anaphase, centric rings can straighten by the type of a Möbius strip forming a large dicentric loop (Table 1, no. 7; Fig. 1k) or intertwine with two smaller rings and interlock by the type of chain links, which resembles the shape of an “eight” [20] (Table 1, no. 6; Figs. 1i, 1j). Asymmetrical intrachromosomal exchange based on interarm deletion at the cell cycle stage G1 with subsequent doubling during replication is a mechanism of this chromosomal type of rearrangement.
The chromatid rearrangements based on an isolocus break (isochromatid deletion) of two chromatids and different variants of the fusion of fragments are manifested in anaphase by a single bridge or dicentric chromatid (sister fusion of proximal fragments (Table 1, no. 1)), as well as by a single or double fragment (Table 1, nos. 8, 10; Figs. 1n, 1o) when the distal ends are fused/not fused, respectively.
At the same time, another mechanism for the formation of a single fragment (Table 1, no. 9), which allows us to attribute it to the chromosomal type of aberrations (however, not recognized by all researchers) [4], was described. This is the chromosome break or terminal deletion at the stage G1 (according to the author’s terminology, “terminal shortage in the uncleaved” chromosome) with subsequent replication and fusion of distal fragments after replication [19]. Similarly, a morphologically double configuration “two unpaired fragments,” according to the mechanism of occurrence, refers to the chromatid type of aberrations, since it represents an asymmetric interchromosomal exchange (chromatid translocation) as a result of nonfusion of distal fragments after the break of chromatids of two different replicated chromosomes (Table 1, no. 10).
ANALYSIS OF THE CHROMOSOME ABERRATION CLASSIFICATION TO TAKE INTO ACCOUNT PECULIARITIES OF THE CLASTOGENIC EFFECT OF FACTORS
According to modern ideas about the mechanism of a radiation effect, directly double strand DNA breaks (and/or superposition of single strand breaks) are basic events of radiation-induced chromosome damages that are realized in aberrations in the cycle “break–crosslinking (repair errors)–structural mutation” and can be detected by the usual cytogenetic methods. Some mutagens of chemical nature can have a similar clastogenic effect on the chromosomes, imitating the effects of ionizing radiation. At the same time, a more complex sequence of molecular genetic events associated with the formation of “primary, initial” damage to a single strand (realized under certain conditions in the cell cycle phases in true damages (double strand breaks) of the chromosomes) was described [23, 24].
The approaches to classification of the chromosomal aberrations to take into account the clastogenic effects of the factors can be based on a different typological basis. Depending on the classification used and the goals of cytogenetic study, stable and unstable types of the chromosome aberrations, symmetric and asymmetric structural rearrangements, chromosome and chromatid type of aberrations, based on inter- and intrachromosomal exchanges, etc., are allocated. At the same time, the spectrum of the chromosomal aberrations can include different forms identified according to the technique of cytogenetic study and ideas about the mechanisms involved in realization of the double break in the microscopic structural rearrangement [25, 26].
In metaphase analysis, centric rings (intrachromosomal exchanges, when the interstitial fragment is closed into a ring after two breaks on different sides of the centromere and fusion of the sticky ends of the centric fragment between each other) and acentric rings (the result of intrachromosomal exchanges during the fusion of acentric regions of a single chromosome arm into the ring), dicentric chromosomes (the result of asymmetric translocations with interchromosomal exchange formed after the breaks in different chromosomesand subsequent unification of their centromeric fragments), and paired terminal or interstitial fragments formed as a result of one or two breaks, respectively, in an unreplicated chromosome are available for accounting [2, 4, 18, 19]. For diagnostic purposes, it was suggested to use, for example, the ratio of the chromosome type of aberrations based on inter- and intrachromosomal exchanges to characterize the quality (by the value of linear energy transfer) of radiation [27]. At the same time, the technique of simple uniform chromosome staining does not allow us to distinguish symmetrical intrachromosomal exchanges (para- and pericentric inversions) when analyzing the metaphase spectrum [25, 27].
With the anaphase method, about 40% of metaphase violations are underestimated. At the same time, the fate of metaphase chromosome changes in anaphase is manifested by another set of aberrations fixed using undifferentiated chromatin staining [7, 19, 20]. When using the anaphase method in radioecological studies, single bridges and single fragments (m′–f′) are traditionally referred to the chromatid type of aberrations; and double bridges and double fragments (m′′–f′′), to the chromosome type of aberrations [14–16]. Some ambiguity concerning the distribution of anaphase aberrations by these subtypes was noted in work [6], when seven types of abnormalities were allocated during the analysis of anaphases, including chromatid, (1) single fragment (−); (2) paired fragments (= as partial chromosome rearrangements); (3) bridges with a single fragment ([−); (4) bridges with a pair of fragments ([=); (5) bridges without fragments ([ as a result of leaving one or a pair of fragments with the mass of chromosomes to the cell pole), as well as chromosomal (1) translocations with a pair of fragments (Х=) and (2) translocations without fragments (Х as a result of leaving of the fragments to the cell pole). As is seen, the chromatid double fragments (a symbol =) were partially attributed to the chromosome rearrangements, and the possibility of their underestimation when leaving to the cell poles was noted.
At the same time, the chromatid type of aberrations is possible based on an isolocus break, which occurs in the replicated chromosome in the phase S or G2. According to this trait, the single bridge and related single fragments belong to the chromatid type of aberrations. However, the single bridge is allocated in the classification [19] as an anaphase figure manifesting the chromosomal type of aberrations based on the chromosome break (end shortage) in an “uncleaved” chromosome with subsequent doubling without the fusion of broken ends. After replication, the possibility of fusion of already sister chromatids was demonstrated in a number of objects (similarly to the fusion of proximal ends after the isolocus break) with the formation of dicentric chromatid, which forms a bridge in anaphase. In this case, based on the morphology of the anaphase figure, this type should be attributed to the chromatid type of aberrations as a “single bridge.” However, it should be attributed to the chromosome type according to the stage of the appearance of the break in the phase G1. The mechanism of the formation of the anaphase single fragment as the chromosome type of aberration [19] emerged as a result of terminal deletion (end shortage in the “uncleaved” chromosome) with subsequent replication and fusion of distal fragments is also allowed (Table 1, no. 9). Although a number of authors consider that the fusion of distal chromatid fragments with the formation of a single fragment is unlikely: “…the assumption that a single fragment can be due to the fusion of sister chromatids of a paired fragment and due to their deployment in length is unlikely” (cited by [2]).
Based on isochromatid breaks, when the change occurred after replication and affects sister chromatids, chromosome aberrations are possible as paired terminal or interstitial fragments morphologically identical to the chromosome type of aberrations (double), but emerging in the replicated chromosome (consequently, the chromatid type). Thus, the double fragment (the result of no fusion of distal fragments) belongs to the chromosome type of aberrations according to the morphology, although the break emerged at the stage S or G2 (Table 1, no. 11). Therefore, paired fragments can be of both isochromatid and chromosomal origin (Table 1, nos. 11, 12).
Thus, when using the method of common light field microscopy, typical chromosome configurations of metaphase plates and mitosis anaphases are morphological markers of clastogenic effects and subsequent translocations. At the same time, the chromosome type of aberrations is more often described as double, similarly affecting both chromatids of the replicated chromosome, while the chromatid type of aberrations are described as single figures, although its single or paired nature is recognized as difficult to distinguish by the fragment width when using the technique of undifferentiated nuclear chromatin staining (Figs. 1l–1o). Undifferentiated (uniform) staining of an anaphase chromosome imposes similar instrumental limitations on the process of recognition of the nature of bridges and, correspondingly, the mechanism of the formation of the preceding metaphase dicentrics [25]. The chromatid bridge or dicentric chromatid is designated as single on the schemes; however, when microscoping the preparations, “…by the thickness of the bridge, it is impossible to judge about its chromosome or chromatid character…, it is not always possible to differentiate the nature of the bridge and, therefore, it is hardly advisable to make such a separation when using the total chromosome staining method, which does not allow us to identify individual chromatids” (cited by [2]).
CONCLUSIONS
(1) For a number of aberrations, terminological contradictions and an ambiguous correlation of the anaphase morphological view with the chromatid or chromosomal type, reflecting the peculiarities of clastogenic effect of the factors of radiation or chemical nature and using as a bioindication or test indicator, were detected.
(2) Using the anaphase spectrum of chromosomal aberrations for diagnostic purposes, it is informative to present the results not only with the division of abnormalities into the types of the chromosome or chromatid nature, but also to give a more differentiated morphological characterization of the anaphase configuration both for the bridges and for the fragments.
(3) The technique of total chromosome staining with a simple variant of cytogenetic study without stopping the division at the stage of metaphase ambiguously diagnoses the single or paired nature of the fragments, complicating their assignment to the chromosomal or chromatid type.
(4) For the anaphase bridges, it is desirable to present not only a description of their double or single nature, but also to characterize the way of crossing the strands, including description of ring-shaped structures. This gives information about the mechanism of occurrence and the process of metaphase morphology of the chromosome, which is used as a marker of the radiative effect.
REFERENCFES
Esnault, M.-A., Legue, F., and Chenal, Ch., Ionizing radiation: advances in plant response, Environ. Exp. Bot., 2010, vol. 68, pp. 231–237.
Kalaev, V.N. and Karpova, S.S., Tsitogeneticheskii monitoring: metody otsenki zagryaznenii okruzhayushchei sredy i sostoyaniya geneticheskogo apparata organizma (Cytogenetic Monitoring: Methods of Assessment of Environmental Pollution and State of the Genetic Apparatus of the Body), Voronezh: Voronezh. Gos. Univ., 2004.
McClintock, B., The stability of broken ends of chromosomes in Zea mays,Genetics, 1940, vol. 26, pp. 234–282.
Pausheva, Z.P., Praktikum po tsitologii rastenii (A Practical Course in Plant Cytology), Moscow: Kolos, 1988.
Dubinin, N.P., Radiatsionnyi i khimicheskii mutagenez (Radiation and Chemical Mutagenesis), Moscow: Nauka, 2000.
Dubinin, N.P., Dubinina, L.G., and Nesterova, N.I., Irradiation dose and nuclear changes in human cells in tissue culture in different phases of the cell cycle, Radiobiologiya, 1964, vol. 4, no. 5, pp. 713–725.
Nicoloff, H. and Gecheff, K., Methods of scoring induced chromosome structural changes in barley, Mutat. Res., 1976, vol. 34, pp. 233–244.
Barykina, R.P., et al., Spravochnik po botanicheskoi mikrotekhnike. Osnovy i metody (A Handbook of Botanical Microtechnology: Fundamentals and Methods), Moscow, 2004.
Stolbova, V.V., Manakhov, D.V., and Shcheglov, A.I., The use of imaging complex based on Mikmed-6 microscope in the record of Allium test results for the estimation of soil genotoxicity, Moscow Univ. Soil Sci. Bull., 2016, vol. 71, no. 2, pp. 78–82.
Kal’chenko, V.A. and Fedotov, I.S., Genetic effects of acute and chronic ionizing irradiation on Pinus sylvestris L. inhabiting the Chernobyl meltdown area, Russ. J. Genet., 2001, vol. 37, no. 4, pp. 341–350.
Geraskin, S.A., Dikarev, V.G., Zyablitskaya, Y.Y., Oudalova, A.A., Spirin, Y.V., and Alexakhin, R.M., Genetic consequences of radioactive contamination by the Chernobyl fallout to agricultural crops, J. Environ. Radiat., 2003, vol. 66, pp. 155–169.
Geraskin, S.A., Kim, J.K., Oudalova, A.A., Vasiliyev, D.V., Dikareva, N.S., Zimin, V.L., and Dikarev, V.G., Bio-monitoring the genotoxicity of populations of Scots pine in the vicinity of a radioactive waste storage facility, Mutat. Res., 2005, vol. 583, pp. 55–66.
Geras’kin, S.A., Oudalova, A.A., Michalik, B., Dikareva, N.S., and Dikarev, V.G., Geno-toxicity assay of sediment and water samples from the Upper Silesia post-mining areas, Poland by means of Allium-test, Chemosphere, 2011, vol. 83, no. 8, pp. 1133–1146.
Belykh, E.S. and Maistrenko, T.A., Cytogenetic effects in Allium schoenoprasum plants growing in technogenically polluted soil, Radiats. Biol. Radioekol., 2015, vol. 55, no. 1, pp. 5–15.
Udalova, A.A., Pyatkova, S.V., Geras’kin, S.A., Kiselev, S.M., and Akhromeev, S.V., Evaluation of cyto- and genotoxicity of groundwater sampled at the site of the Far Eastern Center for Radioactive Waste Management, Radiats. Biol. Radioekol., 2016, vol. 56, no. 2, pp. 208–219.
Minkenova, K.S., Baigazinov, Zh.A., Lukashenko, S.N., Mamyrbaeva, A.N., and Karimbaeva, K.S., Cytogenetic changes in Koeleria gracilis Pers. growing in the areas of field trials of combat radioactive materials at the Semipalatinsk Test Site, in VII Mezhdunar. Nauchno-Prakt. Konf., Tezisy Dokladov (VII Int. Sci.-Pract. Conf., Abstracts of Papers), Pavlodar: Dom Pechati, 2016, pp. 69–70.
Polikarpov, G.G. and Tsytsugina, V.G., Patterns in the distribution of chromosome aberrations in the cells of hydrobionts under the action of ionizing radiation and environmental chemical mutagens, Radiobiologiia, 1993, vol. 33, no. 2, pp. 205–213.
Alov, I.A., Tsitofiziologiya i patologiya mitoza (Cytophysiology and Pathology of Mitosis), Moscow: Meditsina, 1972.
Li, D.E., Deistvie radiatsii na zhivye kletki (Effects of Radiation on Living Cells), Moscow: GosAtomIzdat, 1963.
Koggl, J., Biologicheskie effekty radiatsii (Biological Effects of Radiation), Dedenkov, A.N., Ed., Moscow: Energoatomizdat, 1986.
Gisselson, D., Classification of chromosome segregation errors in cancer, Chromosoma, 2008, vol. 117, no. 6, pp. 511–519.
McClintock, B., The production of homozygous deficient tissues with mutant characteristics by means of the aberrant behavior of ring-shaped chromosomes, Genetics, 1938, vol. 23, pp. 215–237.
Kovaleva, O.A., Cytogenetic aberrations in mammalian somatic cells, Tsitol. Genet., 2008, vol. 42, no. 1, pp. 58–72.
Dubinin, N.P., Potentsial’nye izmeneniya v DNK i mutatsii. Molekulyarnaya tsitogenetika (Potential Changes in DNA and Mutations. Molecular Cytogenetics), Moscow: Nauka, 1978.
Obe, G., Pfeiffer, P., Savage, J.R.K., et al., Chromosomal aberrations: formation, identification and distribution, Mutat. Res., 2002, vol. 504, pp. 17–36.
Durante, M., Bedford, J.S., Chen, D.J., et al., From DNA damage to chromosome aberrations: joining the break, Mutat. Res., 2013, vol. 756, pp. 5–13.
Cornforth, M.N. and Durante, M., Radiation quality and intra-chromosomal aberrations: size matters, Mutat. Res. Gen. Tox. En., 2018, vol. 836, pp. 28–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Stolbova, V.V., Mamikhin, S.V., Kotelnikova, A.D. et al. Detailed Classification of Chromosome Aberrations with Undifferentiated Staining to Account for Clastogenic Effects of Radionuclide and Complex Contaminations. Biol Bull Russ Acad Sci 46, 1611–1618 (2019). https://doi.org/10.1134/S1062359019120082
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359019120082