Abstract
Craniometric features (measurements, indexes) in several species of the genus Sylvaemus (S. sylvaticus, S. flavicollis, and S. uralensis from Eastern Europe and some adjacent territories, as well as S. ponticus and S. witherbyi from the Caucasus) have been studied using multivariate statistical analysis. For the first time, craniometric characters of the genus Sylvaemus are considered in relation to the feeding ecology of species. A total of 614 skulls were studied. Based on original material, discriminant keys have been developed using skull measurements. The keys appear to be suitable for the diagnostics of the studied Sylvaemus species, including sibling ones. The largest skulls are shown to be characteristic of S.flavicollis, and the smallest ones, of S. uralensis. Cluster analysis of the absolute measurements of the skull cumulative selection has revealed the greatest isolation of S. flavicollis and the similarity of coexisting but phylogenetically remote S. ponticus and S. witherbyi. Factor analysis showed that, within the genus Sylvaemus, the general sizes are less essential than the characters related to food smashing (the length of the lower jaw and rostrum, the width of the occipital part of the skull), and possibly also to the thickness of the incisors. Comparative analyses of the relative skull measurements (the indexes related to the food preferences of the animals, granivory or folivory) allow us to conclude that the food adaptations to granivory in the structure of the skull and teeth increase in the series S. uralensis–S. sylvaticus–S. witherbyi–S. flavicollis–S. ponticus. The cranial evolution in species within the genus Sylvaemus is suggested to have been directed to increased adaptations in the mechanisms in seed peeling and processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The distribution of mice of the genus Sylvaemus covers the mountain and flat landscapes of Europe, the Caucasus, North Africa, and also the Near East, Middle East, and Southwestern Asia, where these rodents are often common species (Pavlinov et al., 1995). Until recently, all species of wood and field mice were united into a single transpalearctic genus Apodemus Kaup 1829, consisting of four subgenera: the Western Palearctic wood mice Sylvaemus Ognev 1924 and Karstomys Martino 1939, as well as the Eastern Palearctic Apodemus Kaup 1829 and Alsomys Dukelski 1929 (Musser et al., 1996). The generic status of Sylvaemus including the subgenera Sylvaemus and Karstomys, was substantiated by biochemical data (Bonhomme et al., 1985; Mezhzherin and Zykov, 1991; Mezhzherin, 1997), and later it was both supported (Zagorodnyuk, 1993; Pavlinov et al., 1995; Zagorodnyuk et al., 1997; Kartavtseva, 2002; Chelomina and Suzuki, 2006; etc.) and not recognized (Vorontsov et al., 1992; Lavrenchenko and Likhnova, 1995; Gromov and Erbaeva, 1995; Frynta et al., 2001; Baskevich et al., 2004; Musser and Carleton, 2005; etc.). In the latest taxonomic report (Pavlinov and Lissovskii, 2012), the Western Palearctic wood and field mice are given the generic rank Sylvaemus Ognev 1924, and we accept this point of view in this communication. Paleontological data date the formation of the Western Palearctic species of wood mice to the Pleistocene (Gromov et al., 1963). According to molecular (Michaux et al., 2002; Chelomina and Suzuki, 2006; etc.) and genetic and biochemical data (Mezhzherin, 1997), the genus Sylvaemus appeared before the Pleistocene. Mezhzherin (1997) dates this event to the Pliocene. Obviously, subsequent events have led to intense morphogenesis in the taxon, which, as is commonly believed, is still ongoing. As a result of large-scale genetic studies (Vorontsov et al., 1989, 1992; Mezhzherin and Zagorodnyuk, 1989; Mezhzherin, 1991, 1997a; Mezhzherin and Zykov, 1991; Orlov et al., 1996; Michaux et al., 2002; Filippucci et al., 2002; etc.), the genus was substantially revised and supplemented, mainly by identifying the sibling species of Eastern Europe and the Caucasus. Currently, the majority of researchers recognize in the genus Sylvaemus nine species: the wood mouse (S. sylvaticus Linnaeus 1758), the yellow-necked mouse (S. flavicollis Melchior 1834), the alpine mouse (S. alpicola Heinrich 1952), the pygmy wood mouse (S. uralensis Pallas 1881), the steppe field mouse (S. witherbyi Thomas 1902 = S. fulvipectus Ognev 1924, S. arianus Blanford 1881, S. hermonensis Filippucci, Simson et Nevo 1989), the Caucasus field mouse (S. hyrcanicus Vorontsov, Boeskorov et Mezhzherin 1992), the Black Sea wood mouse (S. ponticus Sviridenko 1936), the eastern broad-toothed field mouse (S. (Karstomys) mystacinus Danford et Alston 1877), and the western broad-toothed field mouse (S. (K.) epimelas Nehring 1902). Until now, the position of individual taxa in the structure of the genus has been controversial. Thus, some researchers have given species status to the ciscaucasicus and vohlynensis forms (Orlov et al., 1996), with which other zoologists disagree (Zagorodnyuk, 1993; Mezhzherin, 1997a; Bogdanov, 2001, 2004; Bogdanov et al., 2009, 2012). Not all mammalogists agree (Gromov and Erbaeva, 1995; Frynta et al., 2001) with the majority of authors recognizing the species rank of S. ponticus, which is a sibling species of S. flavicollis (Mezhzherin, 1991; Musser and Carleton, 1993, 2005; Pavlinov, 2003; etc.). There is still no morphological and taxonomic interpretation of genetically discrete intraspecific groups identified as part of certain species (Bogdanov, 2001, 2004; Filippucci et al., 2002; Michaux et al., 2002; Bogdanov et al., 2009, 2012, 2014; Klempa et al., 2008); depending on the trait used, the interspecific and intraspecific relations of Sylvaemus are considered ambiguous (Mezhzherin, 1997; Chelomina and Suzuki, 2006; Bogdanov et al., 2012; etc.).
To solve controversial issues in the construction of the taxonomic system of Sylvaemus, as well as for the diagnosis of species and intraspecific forms, researchers used different signs: allozymes (Mezhzherin, 1987, 1990; Vorontsov et al., 1989, 1992; Mezhzherin and Zykov, 1991; Lavrenchenko and Likhnova 1995; Macholán et al., 2001; Bogdanov, 2004; etc.), features of the differential staining of chromosomes and FISH (Kozlovsky et al., 1990; Bulatova et al., 1991; Vorontsov et al., 1992; Boeskorov et al., 1995; Orlov et al., 1996, 1996a; Bogdanov, 2001; Bogdanov and Rozanov, 2005; Kartavtseva, 2002; Baskevich et al., 2004; Karamysheva et al., 2010; Rubtsov et al., 2011), molecular genetic markers (Chelomina, 1998, 2005; Chelomina et al., 1998, 1998a, 2007; Chelomina and Suzuki, 2006; Balakirev et al., 2007; Bogdanov et al., 2009, 2012, 2014; Amshokova et al., 2015; etc.), as well as behavioral (Stopka, 1999, cit. ex Baskevich et al., 2004), morphological, based on a comparative analysis of the features of the generative system of males (Baskevich et al., 2004a), and nonmetric signs of the skull (Orlov and Okulova, 2001).
The use of predominantly genetic research methods made it possible to clarify the boundaries of a number of species and intraspecific groups of animals. Within this context, several questions arise: what features of the structure and way of life of animals distinguish the selected groups? What mechanisms of evolution cause the revealed differences? Do they have adaptive meaning, and if so, which one? What could explain the emergence of such groups? Answers to these questions can be obtained by comparing the morphological features and ecology of groups. However, investigations of the ecological and morphological differences of species of the genus advance slowly. The ecology of the former polytypic species was studied in detail, but the latest scientific achievements such as identifying sibling species (S. ponticus, S. flavicollis, etc.), as well as intraspecific chromosomal, allozyme, molecular-genetic forms, drive a significant review of this work. Craniometric approaches with the use of multidimensional statistics methods have had limited application and have mainly concerned regional samples of individual species in particular sites or areas of ranges where species are sympatric (Mezhzherin and Mikhailenko, 1991; Boeskorov, 1992; Zagorodnyuk, 1993; Lavrenchenko and Likhnova, 1995; Rеutter et al., 1999; Frynta et al., 2001; Stakheev, 2009; etc.). The degree of coincidence of molecular-genetic variability with morphological and morphometric variations and ecological peculiarities of the identified sibling species and intraspecific genetically discrete groups has not been studied. Methods for species diagnostics for practical purposes are not always developed. At the same time, craniometric studies are certainly of interest because they expand the range of diagnostic features in identifying sibling species and intraspecific forms of Sylvaemus and open up new possibilities for studying the problems of evolution and ecological dependencies in the formation of the morphological variability of Sylvaemus.
The aim of this report is to compare, on the basis of methods of multidimensional statistics, the craniometric features of the following species of the genus Sylvaemus: S. sylvaticus, S. flavicollis, and S. uralensis from Eastern Europe, as well as S. ponticus and S. witherbyi from the Caucasus.
MATERIALS AND METHODS
We used our own original data on representatives of the genus Sylvaemus (S. flavicollis, S. ponticus, S. witherbyi, S. sylvaticus, and S. uralensis) from Southeastern and Western Ukraine, Northwestern Caucasus, the Republic of Dagestan, and Ivanovo oblast. In addition we involve craniometric data on our measurements of the skulls from the collections of the Zoological Museums of Moscow State University and the Zoological Institute, Russian Academy of Sciences (ZI RAS). Table 1 represents the material on these species. The skulls of adults and semiadult individuals of both sexes were used in this work. Individual laboratory diagnostics of specimen species affiliation were not carried out, with the exception of animals from Dagestan (Lavrenchenko and Likhnova, 1995). Genetic confirmations of the presence of a particular species in the study areas were obtained for S. uralensis from Ivanovo oblast (protein electrophoresis was carried out by S.V. Mezhzherin), for the same species and S. ponticus from Krasnodar krai (karyological analysis was provided by V.N. Orlov and M.I Baskevich; protein electrophoresis and molecular-genetic analysis, by A.E. Balakirev, A.S. Bogdanov, and B. Klempa), for S. uralensis, S. witherbyi, and S. ponticus from Dagestan (protein electrophoresis by O.N. Likhnova), and for S. sylvaticus and S. uralensis from Dniprovsko Orilskii Nature Reserve (karyological analysis were done by A.I. Kozlovskii).
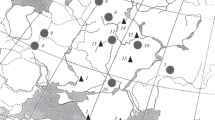
The measurements were made for 309 skulls of the representatives of the genus Sylvaemus from our original summer–autumn samplings and for 305 skulls from the materials of Moscow State University and ZI RAS; in total 614 skulls were processed (Table 1). Our collections from the Northwestern Caucasus relate to the area of the municipal district of the city of Sochi, including the vicinity of the town Krasnaya Polyana. Descriptions of the environmental conditions and fauna of small rodents were reported previously for the Dniprovsko Orilskii Nature Reserve (Okulova and Antonets, 2002, 2007; Antonets and Okulova 2014), for the Northwestern Caucasus (Okulova et al., 2005), and for Dagestan (Lavrenchenko and Likhnova, 1995). In Ivanovo oblast animals were collected by N.M. Okulova and her assistants in the Privolzhskii (near the town of Plyos) and Zavolzhskii (settlement of Krasnogor’e) districts, in the subzone of coniferous–deciduous forests (for a description of the area, see Okulova and Khelevina, 1989). The sampling localities are presented in Fig. 1.
Sampling localities (their numbers correspond to Table 1): 1, Dniprovsko Orilskii Nature Reserve (S. uralensis and S. sylvaticus); 2, Northwestern Caucasus, the region of the municipal district of the city of Sochi (S. ponticus and S. uralensis); 3, Republic of Dagestan (S. uralensis, S. ponticus, and S. witherbyi); 4, Roztochya Biosphere Reserve, Ukraine (S. flavicollis); 5, Sweden (S. flavicollis); 6, Tver oblast (S. flavicollis); 7, Bashkirskii Nature Reserve (S. flavicollis); 8, Leningrad oblast (S. flavicollis); 9, Belovezhskaya Pushcha National Park, Belarus (S. flavicollis); 10, Republic of Mordovia (S. flavicollis); 11, Moscow oblast (S. uralensis); 12, Ivanovo oblast (S. uralensis); 13, Prioksko-Terrasny Nature Biosphere Reserve, Moscow oblast (S. flavicollis and S. uralensis); 14, Yaroslavl oblast (S. uralensis); 15, Ryazan oblast (S. uralensis); 16, Tellerman Forestry, Voronezh oblast (S. flavicollis).
The animals (except for representatives of S. witherbyi) were caught mainly at the end of summer and at the beginning of autumn, usually over two or more years, which, in our opinion, eliminates the variability in the age and sex composition. Special studies are needed to assess fully the role of this factor in craniometric variability. The intraspecific geographical variability of widespread species (mainly S. uralensis and S. flavicollis) will be discussed in Report 2.
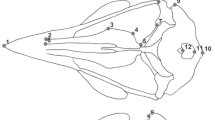
Measurement of the skulls in millimeters was performed using an MBS microscope and an ocular micrometer at magnifications of ×04, ×7.5, and ×25. The measurement scheme is shown in Fig. 2, and the results are presented in Table 2. Twenty-one signs were measured on the skull: (1) Cbl, condylobasal length; (2) Zyg, zygomatic width; (3) Hmax, height of the upper jaw in front of M1; (4) Lmd, length of the lower jaw; (5) Iob, interorbital width; (6) Lna, the length of the nasal bones; (7) Bna, the width of the nasal bones; (8) Lbull, the length of the auditory bulla; (9) Lfi, the length of the incisor foramen; (10) Bfi, the width of the incisor foramen; (11) Lm1-3, the length of the upper dentition; (12) Lm1-3, the length of the lower dentition; (13) Lm1, the alveolar length of the first upper molar; (14) Bm1, the width of the first upper molar; (15) D1, upper diastema length; (16) D2, lower diastema length; (17) M1-1, minimum distance between the first upper molars; (18) Bcra, the width of the skull in the area of the auditory bulla; (19) Hcra, the height of the skull in the area of the auditory bulla; (20) Li, the length of the incisor from the alveoli; (21) Bi, the width of the incisor in the lateral direction at the end of the tooth. For Dagestan specimens, 17 cranial signs were measured (all except Hmax, Li, Bi, and D2).
Based on these measurements, 24 indices were calculated.
Statistical data processing was carried out on a computer using Statistic software in modules: descriptive statistics, arithmetic average M, its error m, and the standard deviation of data σ2. To analyze the differences in the indices, the maximum and minimum species values were compared by Student’s criterion (t ≥ 2). We also used cluster analysis (one linkage method and calculation of Euclidean distances) and factorial and discriminant analyses. The construction of clusters was carried out according to average values for all signs or their separate group; for factor analysis, the axes were rotated using the varimax row method. In discriminant analysis, Rcan, the canonical correlation coefficient in the system was also determined. The reliability of the coefficients of the equations and the canonical correlation coefficients in the discriminant analysis were estimated using the Fisher criteria F, Wλ, χ2, F with p < 0.05. Only those calculations were considered reliable that had significant coefficients for all the terms of the equation with eigenvalues of two or more. We aimed for 100% separation of species, but in some cases it was necessary to use and consider convincing results with an average accuracy of identification of more than 95%. As a result of the calculations, the animal was attributed to the species for which the maximum value of the discriminant function was obtained.
RESULTS AND DISCUSSION
Species Variability
Table 2 shows that among the five species of Sylvaemus, the largest skulls are characteristic of S. flavicollis, and the smallest, of S. uralensis. Accordingly, the dentition (Lm1-3, Lm1-3) is usually larger in the first species and smallest in the latter. However, reliably maximum values of Zyg, Lmd, D1, M1-1, Li, and Bi were noted not in S. flavicollis, in which the skull is the longest, but in S. ponticus. Four features are maximal in S. sylvaticus (Bfi, Lm1, Bсra, and Hсra), and two features, in S. witherbyi (Lna and Bna). The minimum values of the features according to the average indicators for the species were observed in the smallest S. uralensis in 12 cases out of 21 (Zyg, Lmd, Lna, Lfi, Lm1-3, Lm1-3, Lm1, Bm1, D1, M1-1, Bcra, and Bi); in one case, in S. sylvaticus (Hmax); in three cases, in S. ponticus (Bna, Iob, and Bfi); and in one case, in S. flavicollis (Li).
Thus, according to the absolute terms, the length of the skull and the length of the upper and lower dentitions are maximal in the yellow-necked mouse (S. flavicollis), but the length of its incisors is minimal. The Black Sea field mouse (S. ponticus) is distinguished by a rather large, but not maximal skull length, with a significantly maximal width of the archus zygomaticus, lengths of mandible and upper diastema, and width of the incisor and of the interorbital space. At the same time, this species has the narrowest muzzle (the width of the nasal bones and the incisor foramen are minimal).
The steppe wood mouse (S. witherbyi) has an average length of the skull, but at the same time, elongated and enlarged nasal bones. The wood mouse (S. sylvaticus) with a smaller skull has a maximum width of the incisor foramens and skull in the area of the auditory bullas, as well as the maximum M1 length with a minimum height of the upper jaw before the start of dentition. The pygmy wood mouse (S. uralensis) was characterized by half of the minimum values of the skull parts with a minimum length of the skull; maximum values were not found at all.
The similarity of species and groups of the genus in the dimensions of the skull and its parts was studied by cluster analysis methods. We used our original own materials. To assess the degree of similarity, we used the absolute values of dimensions and the one-linkage method. Euclidean distances were used as linkage distances. The results are presented in Fig. 3. It is seen clearly that S. flavicollis differs greatly from other species. S. uralensis is distinguished from the others; S. ponticus and S. witherbyi are most similar (among others), and S. sylvaticus is somewhat separated. The alpine mouse (S. alpicola Heinrich 1952), which is close to S. flavicollis (Reutter et al., 1999) according to its dimensions, appeared to be closer to S. ponticus.
Proportions (Indices) of Signs in Different Species
Calculation of the proportions (indices) allows us to compare the dimensions of individual parts, regardless of the size of the whole. Consideration of the skull proportions of Sylvaemus species (Table 3) leads to the following conclusions. S. flavicollis differs from other species in a number of features. More than half of the indices (13 out of 24: 3/1, 4/1, 6/1, 9/1, 14/1, 14/13, 11/1, 15/1, 15/11, 17/11, 13/12, 18/1, and 19/1) in this species are distinguished by minimal values (in comparison with other species of the genus).
The skull of S. flavicollis is characterized by the following features: the lowest upper jaw and the shortest lower jaw, the shortest nasal bones and incisor foramens relative to the condylobasal length, the narrowest M1 relative to its length, the shortest upper dentition, and both diastemas short relative to Cbl (and the upper diastema, also relative to the length of the upper dentition), narrowed interdental space M1-1, and shortened Lm1 relative to Lm1-3, narrowed width Bcra/Cbl, and a minimum height of the skull in the area of the auditory bullas Hcra/Cbl. No maximum values of indices for this species were identified.
Thus, the skull of S. flavicollis is maximally elongated, narrowed, and flattened. The lengths of the mandible, the upper dentition, the upper diastema, the interdental space, the nasal bone, and the incisor foramens are relatively short. M1 is narrowed; its proportion in the length of the upper dentition is minimal.
For S. ponticus, five minimal-value indices were found (5/1, 10/9, 7/6, 21/20, and 12/4) and 12 out of 24 were maximum (2/1, 4/1, 14/1, 14/13, 15/1, 15/11, 17/11, 13/12, 14/13, 12/1, 19/1, and 20/1). In other words, in the Black Sea wood mouse (S. ponticus) the zygomatic width and height of the skull, the length of the lower jaw and upper diastema relative to Сbl and the upper dentition, and the interdental space and the length of the 1st upper molar relative to the upper dentition are maximum. The incisors are the longest and narrowest relative to Сbl and Li, respectively; the 1st upper molar is widest relative to its length; the nasal bones, the interorbital space, and the incisor foramens are narrowed.
Thus, the skull of S. ponticus is somewhat shorter than in S. flavicollis, but the highest, with the widest zygomatic part, while the interorbital space is minimal. The upper diastema and interdental space are the largest, M1 is wide and the M1 proportion in the length of the upper dentition is maximal, while the nasal bones and incisor foramens are narrowed. The skull of S. ponticus in comparison with the skulls of other species seems to be the largest.
In S. witherbyi five minimum values of indices were revealed (5/1, 5/2, 8/1, 20/1, and 14/13); i.e., it has the minimum length of the auditory bullas, the minimum interorbital space relative to Cbl and the zygomatic width, and the smallest incisors relative to the length of the skull. This species also has a minimum width of the 1st upper molar relative to its length. At the same time, four features are of maximum values: 4/1, 6/1, 10/9, and 12/4; i.e., the nasal bones and lower jaw are the longest relative to the condilobasal length of the skull and the incisor foramens are the widest relative to their length. In addition, the length index of the upper dentition relative to the length of the lower jaw is maximal. In general, with the average size of the skull of S. witherbyi, the lower jaw, nasal bones, and upper dentition are elongated relative to the length of the lower jaw, but the interorbital space is narrowed relative to the length of the skull and its zygomatic width; the zygomatic bullas and incisors are shortened.
In S. sylvaticus with a rather small skull size, minimal indices were not found, but there were revealed six maximal indices relative to the average values: 5/1, 7/1, 9/1, 18/1, 5/2, and 7/6. In general, this means that S. sylvaticus is characterized by an extended interorbital space (in two proportions) and the nasal bones, the longest auditory bullas and incisor foramens, and the maximal skull width in the area of the auditory bullas relative to the condylobasal length.
In S. uralensis, one minimal index (11/1) and one maximal index (3/1) were revealed; i.e., with the minimum size, the skull of S. uralensis is distinguished by a minimum dentition and maximally high upper jaw in front of the molars.
Previously (Lebedkina, 1949; Vorontsov, 1967; Okulova, 2000) it was shown that dental indices (the ratio of the upper dentition, diastema, and the proportions of teeth) in rodent families are quantitatively related to nutrition: some indices increase with folivory increasing, others increase with the bias to granivory. For Dniprovsko Orilskii Nature Reserve (Okulova and Antonets, 2002; Antonets and Okulova, 2014), we noted the relation of some dental indices to the feeding preferences of mice. However, being clearly pronounced in the comparison of rodents of different genera or species, these patterns are not significant within the genus Sylvaemus. It turned out that the proportion of seeds in the autumn feeding of the mice studied by us does not correspond to the average values of the indices related to nutrition in these species.
Quite possibly the strength of seed shells, which animals have to crack, also plays a significant role in the nutrition of the species studied. In this case, differences in nutrition may also appear in other indicators, such as the width of the skull (the wider the skull, the greater the area for attachment of the masticatory muscles), the strength of the bones, and the thickness of the incisors. It is possible that for this reason, the yellow-necked mouse (S. flavicollis) has a narrower and flatter skull, since they mainly feed on acorns with relatively soft shells; for cracking hazelnuts, as well as other nuts, the thickness of the incisors may be important. Unfortunately, we did not measure this parameter in most of the species. The widest in the zygomatic part skull (Zyg/Cbl), apparently due to the large volume of muscles associated with the incisors, were found in S. ponticus, the food of which often contains nuts of hornbeam and beech with very strong shells.
Factor Analysis
To understand the features that have the greatest value in the divergence of mice of the genus studied, we conducted a factor analysis based on our own data. Two groups of factors (axes) were distinguished: the 1st axis has an eigenvalue of 5.365 and explains 67.06% of the dispersion of characters, and the 2nd axis is characterized by values of 1.09 and 12.61%, respectively. Overall, the system explains 79.67% of the variance. Table 4 shows that the greatest loads bear signs associated with an increase in the longitudinal dimensions of the rostral part of the skull and lower jaw in the absence of tendencies to an increase in the overall size of the skull (factor 1, length of the lower jaw, nasal bones, incisor foramens, and upper diastema) and dental system (upper dentition and 1st upper molar); in addition, there is an increase in the occipital width of the skull (factor 1) and the width of the interorbital space (significantly weaker factor 2). Elongation of the lower jaw and diastema indicates the predominance of the gnawing type of food processing (Vorontsov, 1967) and the increase of granivory; at the same time, there is a weaker tendency toward an increase in the dimensions of the upper dentition, which may indicate an increase in folivorous mood in some species. The width of the interorbital space is most likely related to the age variation of the feature.
Thus, the skull of Western Palearctic wood mice, without a directional change in the overall size, tends to narrow the rostral part and expand the occipital part with an increase in the interorbital space and the prevailing development of signs characterizing the granivory type of diet. At the same time, in some forms there seems to be a tendency towards an increase of folivory.
Discriminant Analysis
We used our original measurements of the skulls of five Sylvaemus species on the entire complex of characters (absolute values) in the discriminant analysis. The results are presented in Tables 5 and 6.
Equations for the identification of five species in a single system were compiled using 12 signs with an eigenvalue of 26.4 and a canonical correlation coefficient of 0.98. Figure 4 shows that S. flavicollis, S. sylvaticus, and S. ponticus are distinguished in the best way, while S. uralensis interferes with other species.
We used from two to nine signs for pairwise comparison of species, usually around five (Table 5). In all pairwise comparisons, the results were located on the same canonical axis. The eigenvalues did not reach two in two cases (S. flavicollis–S. witherbyi and S. sylvaticus–S. uralensis); in the same cases, the minimal canonical correlation coefficients were obtained (0.72–0.75).
Most combinations of pairs of species exist in nature sympatrically: S. sylvaticus and S. flavicollis or S. uralensis and S. flavicollis in different biotopes of Rostov oblast (Stakheev, 2009; Stakheev et al., 2011), S. uralensis and S. flavicollis in the forests of the black earth regions and the southern non–black earth regions (Okulova et al., 2007a), S. uralensis and S. ponticus in the Northwestern Caucasus (Okulova et al., 2005, 2007), etc. A system for determining species by craniometric characteristics was developed using discriminant equations for three Dagestan field mouse species (Lavrenchenko and Likhnova, 1995). The same systems were developed for pairs of species in two other studied sites, Dniprovsko Orilskii Nature Reserve and the Northwestern Caucasus (Table 7). Therefore, these equations can be used to create local diagnostic systems and practical definition of species.
Ecological and Morphological Interpretation of the Data
In general, within the genus Sylvaemus, according to the data of factor analysis, the overall dimensions of the skull are less significant than the signs associated with food crushing: the length of the mandible and the rostrum (as a pressure lever of incisors) and the width of the occipital part of the skull (as a place of the muscles attaching, the increase in volume of which causes an increase in the pressure of the incisors). Dispersion of the features occurs mainly in accordance with the change in the food preferences. It is also possible that changes in the dispersion will be noticeable during comparison of the thickness of the incisors, which helps to increase the strength and potency of their cracking movements. Unfortunately, we do not have full data on the thickness of the incisors.
In vertebrate ecology, the separation of ecological niches according to the body size of animals has often been reported (Dzhiller, 1988). In particular, it was noted in the work (Dickman, 1988) for common shrews of the genus Sorex (order Lipothyphla). A similar phenomenon was observed by us for the representatives of the family Muridae in the Dniprovsko Orilskii Nature Reserve (Okulova and Antonets, 2002). Species of mice coinhabiting this area have a ratio of sizes that corresponds to the proportions determined by the Hutchinson rule. A similar phenomenon was noted for representatives of Sylvaemus in Rostov oblast (Stakheev, 2009).
The mice of this genus are characterized by a joint habitat of several species, and the segregation of ecological niches occurs in accordance with the Hutchinson rule by the size of individuals: for the coliving species, the overall size or body parts of the smaller one are approximately 0.7–0.8 of the length of the larger one. In addition, it is important for the structure of mammalian communities that species share biotopes, being confined to certain types of landscape, food, and abiotic environmental conditions. Sometimes, the even smaller house mouse (Mus musculus) or Eurasian harvest mouse (Micromys minutus) joins the Sylvaemus species. They have lower food requirements and subsist on small seeds, and their requirements for abiotic conditions also differ (Okulova et al., 2016). Field and house mice inhabiting the same area often compete with each other for resources, as was noted, for example, in the forests and protecting forest belts of the northern foothills of the Caucasus (Tarasov, 1989) or in the lowlands of the Northwestern Caucasus (Okulova et al., 2005, 2007). The advantage is obtained by those species the needs of which are better fulfilled by the region’s natural resources. Thus, according to Lindeman and coauthors (2005), house mice for a long time dominated man-made forests in western Kazakhstan, while plantings were young and did not produce the proper amount of seeds. But as plants matured and fruiting increased, the pygmy wood mouse prompted them to leave this biotope. In the years of abundance peaks of S. uralensis, there are almost no house mice in the Northern Ciscaucasia, and in the years of their depression, the house mouse dominates. The same was noted in the lowlands of the Northwestern Caucasus for S. ponticus and S. uralensis. It can be assumed that within the genus Sylvaemus during the process of species evolution there was a quantitative increase in differences in the mechanisms of peeling seed shells and seed processing, as well as a change in dietary patterns: an increase in the proportion of vegetative fodder and seeds of trees or lower forest levels. Thus, the group of large consumers of tree seeds, representatives of which are more closely related to the tree layer of vegetation, should be considered more ancient. It is known that the grassy layer in the forest zone evolved much later than the woody one.
REFERENCES
Amshokova, A.K., Tembotova, F.A., and Kuchinova, E.P., Genetic diversity and geographic distribution of mitochondrial DNA haplotypes of cryptic species of wood mice of the subgenus Sylvaemus in Western Caucasus, Izv. Samar. Nauchn. Tsentra Ross. Akad. Nauk, 2015, vol. 17, no. 2-4, pp. 402–411.
Antonets, N. and Okulova, N., Mammals of the Dnieper-Orelsky Nature Reserve, Saarbrücken, Germany: Lap Lambert Academic Publishing, 2014.
Balakirev, A.E., Baskevich, M.I., Gmyl, A.P., Okulova, N.M., Andreeva, T.A., et al., On the taxonomic rank of ciscaucasicus and its relationships with the pygmy wood mouse Sylvaemus uralensis inferred from the mtDNA cytochrome b gene sequence, Russ. J. Genet., 2007, vol. 43, no. 12, pp. 1386–1399.
Baskevich, M.I., Potapov, S.G., Okulova, N.M., and Balakirev, A.E., Diagnosis of wood and field mice of the genus Apodemus (Rodentia, Muridae) in the western part of the Greater Caucasus under the conditions of symbiotopy, Zool. Zh., 2004, vol. 83, no. 2, pp. 220–233.
Baskevich, M.I., Potapov, S.G., Okulova, N.M., Balakirev, A.E., Krapivko, T.P., and Sapel’nikov, S.F., Comparative analysis of spermatozoa in the six species of mice of the genus Apodemus (Muridae, Rodentia) from Eastern Europe and Transcaucasia, Zool. Zh., 2004a, vol. 83, no. 6, pp. 725–732.
Boeskorov, G.G., Genetic diagnosis of sibling species of wood mice of the subgenus Sylvaemus in the Caucasus, Extended Abstract, Cand. Sci. (Biol.) Dissertation, Moscow, 1992.
Bogdanov, A.S., Chromosomal differentiation of populations of pygmy wood mouse Sylvaemus uralensis in the eastern part of the species range, Zool. Zh., 2001, vol. 80, no. 3, pp. 331–342.
Bogdanov, A.S., Allozyme variation of the pygmy wood mouse Sylvaemus uralensis (Rodentia, Muridae) and estimation of the divergence of its chromosome forms, Russ. J. Genet., 2004, vol. 40, no. 8, pp. 897–909.
Bogdanov, A.S. and Rozanov, Yu.M., Variability in size of the nuclear genome in pygmy wood mouse Sylvaemus uralensis (Rodentia, Muridae), Russ. J. Genet., 2005, vol. 41, no. 10, pp. 1123–1129.
Bogdanov, A.S., Atopkin, D.M., and Chelomina, G.N., Analysis of genetic variation and differentiation in the pygmy wood mouse Sylvaemus uralensis (Rodentia, Muridae) aided by the RAPD-PCR method, Biol. Bull. (Moscow), 2009, vol. 36, no. 3, pp. 227–241.
Bogdanov, A.S., Stakheev, V.V., Zykov, A.E, Yakimenko, V.V., and Mal’kova, M.G., Genetic variation and differentiation of wood mice from the genus Sylvaemus inferred from sequencing of the cytochrome oxidase subunit 1 gene fragment, Russ. J. Genet., 2012, vol. 48, no. 2, pp. 186–198.
Bogdanov, A.S., Stakheev, V.V., Zykov, A.E., Okulova, N.M., Mironova, T.A., et al., Intraspecific differentiation of yellow-necked mice Sylvaemus flavicollis in the eastern part of the range: the results of the study of variability of the mitochondrial cytochrome oxidase gene fragment, in Materialy 3-i Mezhdunar. konf. “Zoologicheskie issledovaniya v regionakh Rossii i sopredel’nykh territorii” (Proc. 3rd Int. Conf. “Zoological Research in the Regions of Russia and Adjacent Countries”), Nizhny Novgorod, 2014, pp. 258–264.
Bonhomme, F., Iskandar, D., Thaler, L., and Petter, F., Electromorphs and phylogeny in muroid rodents, in Evolutionary Relationships among Rodents, New York: Plenum, 1985, pp. 671–683.
Boskorov, G.G., Kartavtseva, I.V., Zagorodnyuk, I.V., Belyanin, A.N., and Lyapunova, E.A., Nucleolus organizer regions and B-chromosomes of wood mice (Mammalia, Rodentia, Apodemus), Genetika, 1995, vol. 31, no. 2, pp. 185–192.
Bulatova, N.Sh., Nadjafova, R.S., and Kozlovsky, A.I., Cytotaxonomic analysis of species of the genera Mus, Apodemus and Rattus in Azerbaijan, Zeitschrift für Zoologische Systematik und Evolutionsforschung. B, 1991, pp. 139–153.
Chelomina, G.N., Molecular phylogeny of wood and field mice of the genus Apodemus (Muridae, Rodentia) based on the data on restriction analysis of total nuclear DNA, Russ. J. Genet., 1998, vol. 34, no. 9, pp. 1084–1089.
Chelomina, G.N., Lesnye i polevye myshi: molekulyarno-geneticheskie aspekty evolyutsii i sistematiki (Wood and Field Mice: Molecular Genetic Aspects of Evolution and Systematics), Vladivostok: Dal’nauka, 2005.
Chelomina, G.N. and Suzuki, H., Molecular evolution and phylogeography Western Palearctic wood mice of the subgenus Sylvaemus inferred from the data on the variability of nuclear (IRBP) and mitochondrial (cytochrome b) DNA genes, Zool. Zh., 2006, vol. 85, no. 2, pp. 219–234.
Chelomina, G.N., Pavlenko, M.V, Kartavtseva, I.V., et al., Genetic differentiation of Caucasian wood mice: comparison of isozymic, chromosomal, and molecular divergence, Russ. J. Genet., 1998, vol. 34, no. 2, pp. 151–162.
Chelomina, G.N., Suzuki, Kh., Suchiya, K., Morivaki, K., Lyapunova, E.A., and Vorontsov, N.N., Sequencing of the mtDNA cytochrome b gene and reconstruction of the maternal relationships of wood and field mice of the genus Apodemus (Muridae, Rodentia), Russ. J. Genet., 1998a, vol. 34, no. 5, pp. 529–539.
Chelomina, G.N., Atopkin, D.M., and Bogdanov, A.S., Phylogenetic relationships between species and intraspecific forms of forest mice from the genus Sylvaemus as determined by partial sequencing of the cytochrome b gene of mitochondrial DNA, Dokl. Biol. Sci., 2007, vol. 416, pp. 356–359.
Dickman, C.R., Body size, pray size, and community structure in insectivorous mammals, Ecology, 1988, vol. 69, pp. 569–580.
Filippucci, M.G., Macholan, M., and Michaux, J.R., Genetic variation and evolution in the genus Apodemus (Muridae: Rodentia), Biol. J. Linnean Soc., 2002, vol. 75, no. 3, pp. 395–419.
Frynta, D., Mikulova, P., Suchomelova, E., and Sadlova, J., Discriminant analysis of morphometric characteristics in four species of Apodemus (Muridae: Rodentia) from Eastern Turkey and Iran, Israel J. Zool., 2001, vol. 47, pp. 243–258.
Giller, P., Community Structure and the Niche, London: Chapman and Hall, 1984.
Gromov, I.M. and Erbaeva, M.A., Mlekopitayushchie fauny Rossii i sopredel’nykh territorii (Mammals of the Fauna of Russia and Adjacent Countries), St. Petersburg: Ross. Akad. Nauk, 1995.
Gromov, I.M., Gureev, A.A., Novikov, G.A., et al., Mlekopitayushchie fauny SSSR (Mammals of the Fauna of the USSR), Moscow, 1963, part 1.
Karamysheva, T.V., Bogdanov, A.S., Kartavtseva, I.V., et al., Comparative FISH analysis of C-positive blocks of centromeric chromosomal regions of pygmy wood mice Sylvaemus uralensis (Rodentia, Muridae), Russ. J. Genet., 2010, vol. 46, no. 6, pp. 712–724.
Kartavtseva, I.V., Kariosistematika lesnykh i polevykh myshei (Rodentia, Muridae) (Karyosystematics of Wood and Field Mice (Rodentia, Muridae)), Vladivostok: Dal’nauka, 2002.
Klempa, B., Tkachenko, E.A., Dzagurova, T.K., Yunicheva, Yu.V., Morozov, V.G., et al., Hemorrhagic fever with renal syndrome caused by 2 lineages of Dobrava hantavirus, Russia, Emerg. Infect. Dis., 2008, vol. 14, no. 4, pp. 617–625.
Kozlovskii, A.I., Nadzhafova, R.S., and Bulatova, N.Sh., Cytogenetic hiatus between sympatric forms of wood mice of Azerbaijan, Dokl. Akad. Nauk SSSR, 1990, vol. 315, no. 1, pp. 219–222.
Lavrenchenko, L.A. and Likhnova, O.P., Allozyme and morphological variability of three species of wood mice (Rodentia, Muridae, Apodemus) in Dagestan under the conditions of symbiotopy, Zool. Zh., 1995, vol. 74, no. 5, pp. 107–119.
Lebedkina, N.S., Morphofunctional analysis of the skull of rabbits and voles, Extended Abstract, Cand. Sci. (Biol.) Dissertation, Moscow, 1949.
Lindeman, G.V., Abaturov, B.D., Bykov, A.V., and Lopushkov, V.A., Dinamika naseleniya pozvonochnykh zhivotnykh Zavolzhskoi polupustyni (Population Dynamics of Vertebrates in the Zavolzhskaya Semidesert), Moscow: Nauka, 2005.
Macholán, M., Filippucci, M.G., Benda, P., Frynta, D., and Sadlova, J., Allozyme variation and systematics of the genus Apodemus (Rodentia, Muridae) in Asia Minor and Iran, J. Mammal., 2001, vol. 82, pp. 799–813.
Mezhzherin, S.V., Genetic divergence of wood mice of the subgenus Sylvaemus, Dokl. Akad. Nauk SSSR, 1987, vol. 286, no. 5, pp. 1255–1257.
Mezhzherin, S.V., Allozyme variability and genetic divergence of wood mice of the subgenus Sylvaemus (Ognev et Vorobiev), Genetika, 1990, vol. 26, no. 8, pp. 1046–1054.
Mezhzherin, S.V., Specific differences in Apodemus (Sylvaemus) ponticus (Rodentia, Muridae), Vestn. Zool. (Kiev), 1991, no. 6, pp. 34–40.
Mezhzherin, S.V., Genetic differentiation and phylogenetic relationships among Palearctic mice (Rodentia, Muridae), Russ. J. Genet., 1997, vol. 33, no. 1, pp. 65–72.
Mezhzherin, S.V., Revision of mice of the genus Apodemus (Rodentia, Muridae) of Northern Eurasia, Vestn. Zool., 1997a, vol. 31, no. 4, pp. 29–41.
Mezhzherin, S.V. and Mikhailenko, A.G., Species affiliation of Apodemus sylvaticus tscherga (Rodentia, Muridae) of Altai, Vestn. Zool. (Kiev), 1991, no. 3, pp. 35–44.
Mezhzherin, S.V. and Zagorodnyuk, I.V., A new species of mice of the genus Apodemus (Rodentia, Muridae), Vestn. Zool., 1989, vol. 23, no. 4, pp. 55–59.
Mezhzherin, S.V. and Zykov, A.E., Genetic divergence and allozyme variability of mice of the genus Apodemus s. lato (Muridae, Rodentia), Tsitol. Genet., 1991, vol. 25, no. 4, pp. 51–59.
Michaux, J.R., Chevret, P., Fillipuccci, M.J., and Macholán, M., Phylogeny of the genus Apodemus with a special emphasis on the subgenus Sylvaemus using the nuclear IRBP gene and two mitochondrial markers: cytochrome b and 12s rRNA, Mol. Phylogenet. Evol., 2002, vol. 23, pp. 123–136.
Musser, G.G. and Carleton, M.D., Family Muridae, in Mammal Species of the World: A Taxonomic and Geographic Reference, Wilson, D.E. and Reeder, D.M., Eds., 2nd ed., Smithsonian Institution, 1993, pp. 501–755.
Musser, G.G. and Carleton, M.D., Superfamily Muroidea, in Mammal Species of the World: A Taxonomic and Geographic Reference, Wilson, D.E. and Reeder, D.M., Eds., 3rd ed., Baltimore: Johns Hopkins Univ. Press, 2005, pp. 894–1531.
Musser, G.G., Brother, E.M., Carleton, M.D., and Hutterer, R., Taxonomy and distributional records of Oriental and European Apodemus, with a review of the Apodemus/Sylvaemus problem, Bonner zoologische beiträge, 1996, vol. 46, pp. 143–190.
Okulova, N.M., Morphometric differentiation of populations of the yellow-necked mouse in connection with the analysis of trophic adaptations, in Sistematika i filogeniya gryzunov i zaitseobraznykh (Systematics and Phylogeny of Rodents and Lagomorphs), Moscow, 2000, pp. 121–125.
Okulova, N.M. and Antonets, N.V., Comparative characteristics of ecology of mice of the genus Apodemus (Rodentia, Muridae) of the Dnieper–Orelsky Nature Reserve, Povolzh. Ekol. Zh., 2002, no. 2, pp. 108–128.
Okulova, N.M. and Antonets, N.V., Craniometric characterization and differentiation of mice of the genus Apodemus (Muridae, Rodentia) of the southeast of Ukraine, in Teriofauna Rossii i sopredel’nykh territorii. Materialy 8-go s"ezda Teriologicheskogo obshchestva (Proc. 8th Congr. Theriol. Soc. “Theriofauna of Russia and Adjacent Countries”), Moscow, 2007, p. 348.
Okulova, N.M. and Khelevina, S.A., Melkie lesnye mlekopitayushchie Ivanovskoi oblasti i ee okrestnostei (Small Forest Mammals of the Ivanovo Oblast and Its Vicinities), Ivanovo, 1989.
Okulova, N.M., Baskevich, M.I., Ryabova, T.E., Potapov, S.G., and Vasilenko, L.E., New data on the morphometry and diagnosis of sibling species of wood mice (Sylvaemus, Muridae, Rodentia) of Northwest Caucasus, in Mnogoletnyaya dinamika populyatsii zhivotnykh i rastenii na OOPT i sopredel’nykh territorii (Long-term Dynamics of Populations of Animals and Plants in Specially Protected Areas and Adjacent Territories), Cherepovets: Port. Aprel’, 2005, pp. 78–80.
Okulova, N.M., Ryabova, T.E., Vasilenko, L.E., and Yunicheva, Yu.V., Biotopic localization of small mammals in the mountains and foothills of the Krasnodar krai and Adygea Republic, in Mlekopitayushchie gornykh territorii. Materialy Mezhdunar. konf. (Proc. Int. Conf. “Mammals of Mountain Areas”), Moscow: Tov. Nauchn. Izd. KMK, 2007, pp. 234–239.
Okulova, N.M., Sapel’nikov, S.F., Baskevich, M.I., Vlasov, A.A., Maiorova, A.D., et al., Comparative data on the species composition, abundance, and localization of small mammals in the forest–steppe of the Central Black Soil region, Tr. Voronezh. Gos. Zapov., Voronezh: Voronezh. Pedagog. Gos. Univ., 2007a, no. 25, pp. 45–68.
Okulova, N.M., Grazhdanov, A.K., and Neronov, V.V., Struktura i dinamika soobshchestv mlekopitayushchikh Zapadnogo Kazakhstana (Structure and Dynamics of Communities of Mammals in Western Kazakhstan), Moscow: Tov. Nauchn. Izd. KMK, 2016.
Orlov, V.N. and Okulova, N.M., Application of the Hardy–Weinberg equation for analyzing geographical variation of the yellow-necked mouse Apodemus flavicollis (Muridae, Rodentia), Zool. Zh., 2001, vol. 80, no. 5, pp. 607–617.
Orlov, V.N., Bulatova, N.Sh., Nadjafova, R.S., and Kozlovsky, A.I., Evolutionary classification of European wood mice of the subgenus Sylvaemus based on allozyme and chromosome data, Bonner zoologische beiträge, 1996, vol. 46, pp. 191–202.
Orlov, V.N., Kozlovskii, A.I., Nadzhafova, R.S., and Bulatova, N.Sh., Chromosomal diagnoses and the place of genetic taxa in the evolutionary classification of wood mice of the subgenus Sylvaemus (Apodemus, Muridae, Rodentia) in Europe, Zool. Zh., 1996a, vol. 75, no. 1, pp. 88–102.
Pavlinov, I.Ya., Sistematika sovremennykh mlekopitayushchikh (Systematics of Modern Mammals), Moscow: Mosk. Gos. Univ., 2003.
Pavlinov, I.Ya. and Lisovskii, A.A., Mlekopitayushchie Rossii. Sistematiko- geograficheskii spravochnik (Mammals of Russia: A Taxonomic and Geographic Handbook), Moscow: Tov. Nauchn. Izd. KMK, 2012.
Pavlinov, I.Ya., Yakhontov, E.L., and Agadzhanyan, A.K., Mlekopitayushchie Evrazii. I. Rodentia (Mammals of Eurasia. I. Rodentia), Moscow: Mosk. Gos. Univ., 1995.
Reutter, B., Hausser, J., and Vogel, P., Discriminant analysis of skull morphometric characters in Apodemus sylvaticus, A. flavicollis, and A. alpicola (Mammalia; Rodentia) from the Alps, Acta Theriologica, 1999, vol. 44, no. 3, pp. 299–308.
Rubtsov, N.B., Karamysheva, T.V., Bogdanov, A.S., Likhoshvai, T.V., and Kartavtseva, I.V., Comparative FISH analysis of C-positive regions of chromosomes of wood mice (Rodentia, Muridae, Sylvaemus)), Russ. J. Genet., 2011, vol. 47, no. 9, pp. 1096–1110.
Stakheev, V.V., West Palaearctic wood mouse (Sylvaemus, Muridae) of the Don region steppe: species composition, distribution, and ecology, Cand. Sci. (Biol.) Dissertation, Makhachkala, 2009.
Stakheev, V.V., Bogdanov, A.S., and Vodolazhsky, D.I., Revision of the species composition of the wood mice from the genus Sylvaemus from the territory of Rostov oblast using karyological, allozyme and molecular genetic analysis, Russ. J. Genet, 2011, vol. 47, no. 5, pp. 579–589.
Tarasov, M.P., Data on the ecology of the house mouse of the Northern Caucasus, in Domovaya mysh’ (House Mouse), Moscow, 1989, pp. 209–222.
Vorontsov, N.N., Evolyutsiya pishchevaritel’noi sistemy gryzunov (Evolution of the Digestive System of Rodents), Novosibirsk: Nauka, 1967.
Vorontsov, N.N., Mezhzherin, S.V., Lyapunova, E.A., Akhverdyan, M.R., and Boeskorov, G.G., Genetic differentiation of sibling species of wood mice (Sylvaemus) of the Caucasus and their diagnostics, Dokl. Akad. Nauk SSSR, 1989, vol. 309, no. 5, pp. 1234–1238.
Vorontsov, N.N., Boeskorov, G.G., Mezhzherin, S.V., Lyapunova, E.A., and Kandaurov, A.S., Systematics of wood mice of the subgenus Sylvaemus of the Caucasus (Mammalia, Rodentia, Apodemus), Zool. Zh., 1992, vol. 71, no. 3, pp. 119–131.
Zagorodnyuk, I.V., Identification of Eastern European forms of Sylvaemus (Rodentia) and their geographical distribution, Vestn. Zool., 1993, vol. 27, no. 6, pp. 37–47.
Zagorodnyuk, I.V., Boeskorov, G.G., and Zykov, A.E Variability and taxonomic status of steppe forms of mice of the genus Sylvaemus (falzfeini–fulvipectus–hermonensis–arianus), Vestn. Zool., 1997, vol. 31, nos. 5–6, pp. 37–56.
ACKNOWLEDGMENTS
The authors are grateful to T.E. Ryabova and L.E. Vasilenko, zoologists of the Sochi branch of the Novorossiisk Plague Control Station, and to A. Danilova, a student of Ivanovo University, for their help in collecting the material.
Funding
This work was supported by Russian Foundation for Basic Research, project no. 16-04-00032 а.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by T. Kuznetsova
Rights and permissions
About this article
Cite this article
Okulova, N.M., Bogdanov, A.S., Baskevich, M.I. et al. Skull Sizes and Proportions in Western Palearctic Wood Mice (Sylvaemus, Muridae, Rodentia) from Eastern Europe: 1. Interspecific Variability. Biol Bull Russ Acad Sci 46, 973–987 (2019). https://doi.org/10.1134/S1062359019080107
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359019080107