Abstract
The impacts that environmental conditions generate on the physiological performance of benthic marine invertebrates are specific and involve several levels of structural organization. The central component is related to the perception of temperature, which in turn produces the dissipation of oxygen in the mitochondria and triggers oxidative stress. Several studies have evaluated the effect of temperature on oxidative stress, however, few studies have examined their interaction with other environmental factors. This review analyzes the results of antioxidant activity that have been reported for benthic marine invertebrates, considering the combined effects of temperature and pH. The evidence shows that the activity increases with the reduction of pH and that the differences between the optimal values and the exposure define the scope of the responses. The results are used to suggest a generalized pattern of the mechanism and discuss the hypotheses that have been proposed to explain the interaction of temperature and pH on stress oxidative response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Marine invertebrates respond to temperature by adjusting mitochondrial electron transport (Handy et al., 2009). The response generates superoxide anions (\({\text{O}}_{2}^{{ - \,\centerdot }}\)) as minors by-products (Davidson and Schiestl, 2001), while the antioxidants influx in the mitochondria stimulate the protection of proteins, and avoid lipid peroxidation (Candas and Li, 2014). The enzymatic antioxidant activity increase as response of imbalances of production and scavenging of reactive oxygen species (ROS). This response is the indirect form in which the effect of the environmental condition over oxidative stress has been investigated (Tomanek, 2015).
The ability to adjust the production of antioxidants enzymes to avoid the increase of ROS in response to acute local temperature fluctuations is not exclusive of marine invertebrates as it generally occurs in all eukaryotic cells (Davidson and Schiestl, 2001). It is known that the enzyme activity gradually increases as environment temperature does, and as the exposure to high temperature continues, the rate of enzymatic reaction falls rapidly due to heat energy denatures or inactivates the enzymes. The reaction which also occurs in a significant temperature reduction (Tattersall et al., 2012), becomes complex when interaction between oxidant species and energy metabolism is considered (Quijano et al., 2016).
It stands to reason that tricarboxylic acid cycle (TCA), enzyme α-ketoglutarate dehydrogenase and glycerophosphate deshidrogenase are important sources of ROS (Tomanek, 2015), and that even when ROS production are part of normal cell metabolism, the overproduction of \({\text{O}}_{2}^{{ - \,\centerdot }}\) and H2O2 contribute to cellular DNA damage and promote apoptosis (Handy et al., 2009). The cellular mechanism that reduce the levels of ROS are the antioxidant enzymes, which eliminate hydrogen peroxide, and thus the cellular response is regulated and maintain in homeostasis. Antioxidant activity occurs by dismutation of \({\text{O}}_{2}^{{ - \,\centerdot }}\) to H2O2, and the reduction of H2O2 to H2O happens by activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalases (CAT), and thioredoxins-peroxiredoxin (Trx-Prx) (Vives-Bauza et al., 2007, Tomanek, 2015).
The pH of environment also plays a key role in the control of antioxidant production in aquatic organisms, particularly when they are exposed to changing temperatures (Wang et al., 2009; Chen et al., 2015; Ramírez-Duarte et al., 2016). The importance of pH in the dynamic interaction of antioxidants and the effects of prolonged exposure to acidic conditions have been reported (Wang et al., 2009; Chen et al., 2015). The generalized pattern with the exception of CAT is the increase in the activity of antioxidant enzymes as the pH decreases. It is possible that the increase of GPx activity in reduced pH scenarios is not only related to oxidative stress, but also to other metabolic pathways that may be affected in acidic conditions
Overexpression of GPx could attenuate protein kinase B a serine/threonine-specific protein that plays a key role in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription and cell migration (Handy et al., 2009). Even when increasing the production of the enzymes at low pH might constitute a mechanism to cope with stress, which becomes important to avoid oxidative and microbial impairment (Wang et al., 2009; Chen et al., 2015), the possibility that such response might play a first step towards apoptosis brings the need for a new interpretation of the results of thermal tolerance and oxidative stress under the combined effects of pH.
Some authors have addressed both, temperature and pH effects on the antioxidant activity on marine animals. Matozzo et al. (2013) reported that gills and digestive gland of the clam Chamelea gallina and the mussel Mytilus gallopronvincialis exposed to 22°C at pH 7.7 had higher activity of SOD and glutathione S‑transferase (GST) than those of pH 8.1. Recently, Gullian and Terrats (2017) described a negative correlation between GPx and SOD of coelomic fluid of Isostichopus badionotus exposed to suboptimal temperatures and low pH values; they reported that GPx activity was maximal at the extremes of the cold and warm temperatures, and that the activity of SOD increased between 28 and 30°C. These results showed that in the short time, pH rather than temperature were more important controlling the activity of the antioxidants, and that multiple process could be involved. To distinguish these effects, it is necessary to generalized single and combined impacts of these parameters, and to establish generalized patterns for the description of both.
MATERIALS AND METHODS
The generalized pattern that temperature and pH exert on the antioxidant activity of marine benthic organisms was obtained by comparing experiments that have been carried out. The direction and magnitude of the antioxidant responses were determined in relation to the values recorded for optimum pH and temperature. Whenever possible, the data were modeled with different mathematical expressions and their adjustments were defined by residual analysis (Ratkowsky, 2004). If possible, regression analyses of the transformed data were run to determine correlations among antioxidants. All analyses were performed at p < 0.05, using the package STATISTICS (Statsoft Inc.). For parsimony, antioxidants ratios were verified by generalized linear regression models supported on the relation of O2– and H2O2. The differences between enzymatic activities obtained with different pH values were analyzed to verify structural changes of slopes using the test of Chow (Gujarati and Porter, 2009).
RESULTS
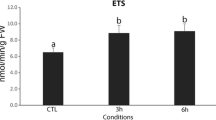
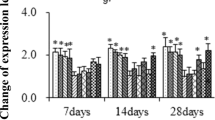
The effects that the reduction of pH produced in the antioxidant activity of octocorals, ascidians, crabs, prawns, bivalves and sea cucumbers were significant. In all cases, the activity of SOD and GPx increased with the reduction of pH. The antioxidant activity of SOD at low pH values was adjusted to a quadratic model with vertex in the maximum antioxidant activity. In the parabola, the antioxidant activity reduced as the temperature increased above the optimum (26°C). The same pattern was observed when temperature reduced below this point. Unlike the low pH, the quadratic relationship did not occur in the alkaline treatments, where the antioxidant activity did not show significant differences as the temperature moved away from the optimum (Table 1, Fig. 1). Opposed to the SOD activity and considering again a low pH environment, the GPx activity presented an inverted parabola. There was greater GPx activity in treatments whose temperatures differed considerably from the optimal temperature (Table 1, Fig. 1). In most cases, the maintenance of constant values of temperature over time, from any tangent of the parabola, determined the behavior that was best adapted to the physiological condition of the organism. In this sense, if the tangent was found to be well below the physiological optimum, decadent expressions of antioxidant activity could be observed; while tangents close to the physiological optimum, were prone to produce oscillating synodal responses of antioxidant activity (Table 1). In comparison with SOD and GPx patterns, CAT activity was less consistent and did not present a defined scheme. In some species, the decrease in temperature increased the activity of the enzyme (Palaemon spp.) and presumably I. badionotus; while in other organisms the antioxidant activity of CAT did not present modifications that could be associated with changes in temperature and pH. Previous studies have shown that the interaction between the antioxidant activities of GPx and SOD were significantly affected by pH and time of exposure. GPx activities were negatively associated with the activities of SOD (Table 1, Fig. 2). For the specific case of I. badionotus, the level of SOD explained 48% of the activity of GPx under alkaline condition, and reducing the pH increased the predictor power of SOD up to 58% (Fig. 2).
Adjustment quadratic models for antioxidant activity of SOD and GPx considering simple and combined effects of pH and temperature. Data were taken from Gullian and Terrats (2017). SOD f(x) = –1.1089x2 + 56.434x – 506.35, R2 = 0.5683, F(1,35) < 6.27, p = 0.02; GPx f(x) = 0.005x2 – 0.024x + 0.3457, R2 = 0.5212, F(1,35)< 7.80, p = 0.00.
Antioxidant activity interaction of superoxide dismutase (SOD) and glutathione peroxidase (GPx) based on catalyzed dismutation of \({\text{O}}_{2}^{{ - \,\centerdot }}\) to H2O2, and the reduction of H2O2 to H2O. Lineal regression models adapted from Gullian and Terrats (2017). Significant with R2 at p ≤ 0.05. Residuals normality for pH 8.00 and pH 7.50: W = 0.973, p ≥ 0.74, and W = 0.948, p ≥ 0.09.
DISCUSSION
It was observed that the change of temperature from optimal values (24 to 28°C) to extremes (16 and 36°C) increased the antioxidant activity of SOD and GPx; and that these effects were greater when the pH was reduced. The results were reported for the oyster Crassostrea virginica (Tomanek et al., 2011), shrimp Litopenaus vannamei (Wang et al., 2009), clam C. gallina (Matozzo et al., 2013), mussels M. gallopronvincialis (Matozzo et al., 2013) and M. coruscus (Hu et al., 2015), and sea cucumber I. badionotus (Gullian and Terrats, 2017). The hypothesis that has been proposed to explain the increased antioxidant activity at low pH, includes, the direct or indirect effect of pH via the Fenton reaction or by CO2 (Trujillo et al., 2008). Ramirez-Duarte et al. (2016) observed an increased antioxidant activity in Oryzias latipes which were exposed to low pH, and considered three possible causes for this response: (i) a decreased hemoglobin affinity for oxygen at low pH, (ii) enhanced iron-mediated ROS production, and (iii) an increased metabolism. Another hypothesis suggests the effect of temperature as a mechanism of perception with oxidative stress. The increased temperatures could activate fluxes of calcium (Ca2+), which acts as a mediator in the mechanism of thermal detection in eukaryote cells (Clapham and Miller 2011; Sengupta and Garrity, 2013). If that occurred, then the rise of Ca2+ within the cell, could sustain for a longer period the ionic balance under hypercapnia conditions, which under the reduction of pH, would results in a higher tolerance to temperature at the costs of increased activity of enzymatic antioxidant. Furthermore, since GPx activity relates poorly with SOD, the increased action of the first need to be related with additional mechanisms to respond to acidity.
Using the data of Gullian and Terrats (2017) to calculated the interaction SOD-GPx, we could identify low coefficients of determination (generally no greater than R2 = 0.58). The low relationship between SOD and GPx, agreed with Kalyanaraman (2013), in the sense that no strong interaction occurred between these enzymes. Thus other types of antioxidants, rather than GPx could be more important in the antioxidant response (Cox et al., 2010; Tomanek, 2015). Increased GPx activity at pH 8.00, even in the absence of SOD, could indicate that peroxides from lipid degradation could have been incorporated into the action of the enzymatic activity (Nikolic et al., 2006). Therefore, such increase, instead of being considered as an antioxidant response related with temperature, should be considered as a cell death signaling.
Another feature of the experiments was the fact that a high variability of antioxidant activity occurred even among the same tissues of organisms of the same species. Madeira et al. (2015) found a high variability in antioxidant activity in octocorals collected at different seasons (Table 1). The response has also been reported in bivalves exposed to thermal stress at different pH (Matozzo et al., 2013), and in sea cucumbers exposed to different temperatures and pH (Gullian and Terrats, 2017). It is possible that the differences on activity could indicate that another antioxidant system might be involved in the removal of H2O2 (Tomanek et al., 2015). That correlates with the fact that 90% of ROS produced in mitochondria are eliminated by the reaction of thioredoxin-peroxiredoxin (Cox et al., 2010), which is congruent with the low correlation that we identified between SOD and GPx.
Finally, changing temperature from optimum and increasing time of exposure could affect the energy of the enzymes and shifted protein aminoacids towards abnormal action kinetic (Tattersall et al., 2012). It is known that values above and below optimum reduce the use of energy, and that such response is critical when time of exposure increased (Dong et al., 2006; An et al., 2007; Lavitra et al., 2010). Therefore, the longer of the activity depends on the difference that exists between optimum and tested conditions.
CONCLUSIONS
All species exhibited a broad antioxidant activity when subjected to the simple and combined effects of pH and temperature. The appearance of greater enzymatic activities in the presence of suboptimal temperatures and reduced pH conditions could be related to the perception of temperature and the control of hypercapnia conditions. The increase of GPx activity and the reduction of the action of SOD constituted a deleterious condition that could affect the survival of these organisms.
REFERENCES
An, Z., Dong, Y., and Dong, S., Temperature effects on growth-ratio relationship of juvenile sea cucumber Apostichopus japonicus (Selenka), Aquaculture, 2007, vol. 272, pp. 644–648.
Candas, D. and Li, J., MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx, Antioxid. Redox Signalling, 2014, vol. 20, no. 10, pp. 1599–1617.
Chen, M., Yang, H., Delaporte, M., and Zhao, S., Immune condition of Chlamys farreri in response to acute temperature challenge, Aquaculture, 2007, vol. 271, pp. 479–487.
Chen, Y., Chen, J., Tseng, K., Lin, Y., and Huang, C., Activation of immunity, immune response, antioxidant ability, and resistance against Vibrio alginolyticus in white shrimp Litopenaeus vannamei decrease under long-term culture pH, Fish Shellfish Immunol., 2015, vol. 46, pp. 192–199.
Clapham, D. and Miller, C., A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels, Proc. Natl. Acad. Sci. U.S.A., 2011, vol. 6, pp. 19492–19497.
Cox, A., Winterbourn, C., and Hampton, M., Mitochondrial peroxiredoxin involvement in antioxidant defense and redox signaling, Biochem. J., 2010, vol. 425, pp. 313–325.
Davidson, J. and Schiestl, R., Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae, Mol. Cell Biol., 2001, vol. 21, no. 24, pp. 8483–8489.
Dong, Y., Dong, S., Tian, X., Wang, F., and Zhang, M., Effects of diel temperature fluctuations on growth, oxygen consumption and proximate body composition in the sea cucumber Apostichopus japonicus Selenka, Aquaculture, 2006, vol. 255, pp. 514–521.
Gujarati, D. and Porter, D., Basic Econometrics, New York: McGraw-Hill, 2009, 5th ed.
Gullian, M., Physiological and immunological condition of the sea cucumber Isostichopus badionotus (Selenka, 1867) during dormancy, J. Exp. Mar. Biol. Ecol., 2013, vol. 444, pp. 31–37.
Gullian, M. and Terrats, M., Effect of pH on temperature controlled degradation of reactive oxygen species, heat shock protein expression, and mucosal immunity in the sea cucumber Isostichopus badionotus, PLoS One, 2017, vol. 12, no. 4.
Handy, D., Lubos, E., Yang, Y., Galbraith, J., Kelly, N., Zhang, Y., Leopold, J., and Loscalzo, J., Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular response, J. Biol. Chem., 2009, vol. 284, no. 18, pp. 11913–11921.
Hu, M., Li, L., Sui, Y., Li, J., Wang, Y., Lu, W., and Dupont, S., Effect of pH and temperature on antioxidant responses of the thick shell mussel Mytilus coruscus, Fish Shellfish Immunol., 2015, vol. 46, pp. 573–583.
Ji, T., Dong, Y., and Dong, S., Growth and physiological response in the sea cucumber Apostichopus japonicus Selenka: aestivation and temperature, Aquaculture, 2008, vol. 283, pp. 180–187.
Kalyanaraman, B., Teaching the basic of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms, Redox Biol., 2013, vol. 1, pp. 244–257.
Lavitra, T., Fohy, N., Gestin, P., Rasolofonirina, R., and Eeckaut, I., Effects of water temperature on the survival and growth of endobenthic Holothuria sacabra (Echinodermata: Holothuroidea) juveniles reared in outdoor ponds, SPC Beche-de-Mer Inf. Bull., 2010, vol. 30, pp. 25–28.
Madeira, C., Madeira, D., Vinagre, C., and Diniz, M., Octocorals in a changing environment: seasonal response of stress biomarkers in natural populations of Veretillum cynomorium, J. Sea. Res., 2015, vol. 103, pp. 120–128.
Matozzo, V., Chinellato, A., Munari, M., Bressan, M., and Marin, M., Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis?, Mar. Pollut. Bull., 2013, vol. 72, no. 1, pp. 34–40.
Meng, X., Liu, P., Li, J., Gao, B.-Q., and Chen, P., Physiological response of swimming crab Portunus trituberculatus under cold acclimation: antioxidant defense and heath shock proteins, Aquaculture, 2014, vol. 434, pp. 11–17.
Nikolic, M., Vranic, D., Spiric, A., Batas, V., Nikolic-Kokic, A., Radetic, P., Turubatovic, L., Blagojevic, D., Jones, D., Niketic, V., and Spasic, M., Could cholesterol bound to hemoglobin be a missing link for the occasional inverse relationship between superoxide dismutase and glutathione peroxidase activities?, Biochem. Biophys. Res. Commun., 2006, vol. 348, pp. 265–270.
Quijano, C., Trujillo, M., Castro, L., and Trostchansky, A., Interplay between oxidant species and energy metabolism, Redox Biol., 2016, vol. 8, pp. 28–42.
Ramírez-Duarte, W., Jin, J., Kurobe, T., and Teh, S., Effects of prolonged exposure to low pH on enzymatic and non-enzymatic antioxidants in Japanese medaka (Oryzias latipes), Sci. Total Environ., 2016, vol. 568, pp. 26–32.
Ratkowsky, D., Model fitting and uncertainty, in Microbial Responses in Foods, CRC Series in Contemporary Food Sciences, McKelllar, R. and Lu, X., Eds., Boca Raton, Fl.: CRC Press, 2004, pp. 151–196.
Rodrigues, T., Moreno, A., Mendes, T., Palmeira, C., and Pardal, M., Biochemical and physiological response of Carcinus maenas to temperature and the fungicide azoxystrobin, Chemosphere, 2015, vol. 132, pp. 127–134.
Sengupta, P and Garrity, P., Sensing temperature, Curr. Biol., 2013, vol. 23, no. 8, pp. 304–307.
Shao, Y., Li, C., Chen, X., Zhang, X., Li, Y., Li, T., and Jiang, J., Metabolomic responses of the sea cucumber Apostichopus japonicus to thermal stresses, Aquaculture, 2015, vol. 435, pp. 390–397.
Tasselli, S., Ballin, F., Franchi, N., Fabbri, E., and Ballarin, L., Expression of genes involved in oxidative stress response in colonies of the ascidian Botryllus schosseri exposed to various environmental conditions, Estuarine, Coastal Shelf Sci., 2017, vol. 187, no. 5, pp. 22–27.
Tattersall, G., Sinclair, B., Withers, S., Fields, P., Seebacher, F., Cooper, C., and Maloney, S., Coping with thermal challenges: physiological adaptations to environmental temperatures, Compr. Physiol., 2012, vol. 2, pp. 2151–2202.
Tomanek, L., Proteomic response to environmentally induced oxidative stress, J. Exp. Biol., 2015, vol. 218, pp. 1867–1879.
Tomanek, L., Zuzow, M., Ivanina, A., Beniash, E., and Sokolova, I., Proteomic response to elevated PCO2 level in eastern oyster, Crassostrea virginica: evidence for oxidative stress, J. Exp. Biol., 2011, vol. 214, pp. 1836–1844.
Trujillo, M., Ferrez-Sueta, G., and Radi, R., Peroxynitrite detoxification and its biological implications, Atioxid. Redox Signaling, 2008, vol. 10, pp. 1607–1620.
Vinagre, C., Madeira, D., Mendoca, V., Dias, M., Roma, J., and Diniz, M., Effect of temperature in multiple biomarkers of oxidative stress in coastal shrimp, J. Therm. Biol., 2014, vol. 41, pp. 38–42.
Vives-Bauza, C., Starkov, A., and Garcia-Arumi, E., Measurement of the antioxidant enzyme activities of superoxide dismutase, catalase, and glutathione peroxidase, in Methods in Cell Biology, Vol. 80: Mitochondria, Pon, L. and Schon, E., Eds., Amsterdam: Elsevier, 2007, pp. 379–393.
Wang, W., Wang, A., Liu, Y., Xiu, J., Liu, Z.-B., and Sun, R.-Y., Effect of temperature of growth, adenosine phosphates, ATPase, and cellular defense response of juvenile shrimp Macrobachium nipponense, Aquaculture, 2006, vol. 256, pp. 624–630.
Wang, W., Zhou, J., Wang, P., Tian, T., Zheng, Y., Liu, Y., Mai, W., and Wang, A., Oxidative stress, DNA damage, and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress, Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol., 2009, vol. 150, pp. 428–435.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
González Durán, E., Cuaya, M.P., Gutiérrez, M.V. et al. Effects of Temperature and pH on the Oxidative Stress of Benthic Marine Invertebrates. Biol Bull Russ Acad Sci 45, 610–616 (2018). https://doi.org/10.1134/S1062359018660019
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359018660019