Abstract
Two methods are proposed for assessing the total concentration (cAr) of the most toxic hydrocarbons (arenes) in wastewaters. The procedures include the extraction of petroleum products with n-hexane, the adsorption purification of the extract from phenols, and the measurement of the absorbance of arenes in the UV region. No separation of alkanes and cycloalkanes is required. The first method enables the rapid estimation of cAr in terms of the standard substance Xst (o-xylene or m-xylene) for screening purposes. Absorbance is measured at 250 nm, which decreases the effect of intragroup selectivity and phenols remaining in the extract. The total concentrations of arenes in n-hexane solutions are estimated by this method rather accurately (error δc < 7% at RSD < 5%). However, the analysis of aqueous solutions (wastewater simulant solutions) in the range of cAr from 0.1 to 50 mg/L leads to δc > 50%. The results of cAr estimation in simulations and wastewaters are underestimated, which is mainly due to the loss of arenes during sample preparation. Using the second (more accurate) method, the absorbance of the extract is measured at seven wavelengths in the region 240–280 nm. The cAr value is found by multiple linear regression without recalculation to Xst. Multivariate calibrations are constructed using extracts from 25 simulated solutions (training set) and passing them through all sample preparation operations, which decreases the loss of analytes. At cAr > 1 mg/L, the error in the analysis of simulants from the test set does not exceed 20% at RSD < 12%. The duration of analysis is 1 h. The procedure was tested in the analysis of wastewaters from enterprises of various profiles. The results agree with the results of chromatographic analyses of the same samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Technogenic pollution of water bodies with petroleum products is controlled by determining total hydrocarbons (HCs) without taking into account their toxicity [1]. Experts recommend an additional determination of the most toxic hydrocarbons, predominantly arenes С6–С9 [2, 3]. For this purpose, after the extraction of petroleum products, the extract is purified from polar compounds, fractionated, evaporated, and chromatographed. Using a flame ionization detector, the C6–C9 arenes are determined at the level of their MPC (approximately 0.1 mg/L) [4]. In addition to conventional extraction, solid-phase microextraction [5] or headspace method [6] are used. Unfortunately, individual arenes are rarely determined in the course of environmental monitoring because of their loss during sample preparation and because of the long duration and laboriousness of the procedures. As different arenes have rather similar MPCs and similar chemical-analytical properties, their total concentration (cAr) can be controlled, which simplifies and speeds up the analysis. An example is provided by the extraction–chromatographic analysis of wastewaters in the range of cAr from 0.1 to 100 mg/L [7]. The results are well reproducible (RSD < 10%). The loss of arenes during sample preparation is taken into account in constructing a calibration dependence. As a result, the error (δc, rel. %) does not exceed 15%. However, a random coincidence of the retention times of arenes and other hydrocarbons (alkanes, cycloalkanes) can lead to much larger δc values.
We propose to determine cAr by an extraction–spectrometric method. In this case, alkanes and cycloalkanes do not interfere with each other, because, unlike arenes, they do not absorb in the near UV region. The direct measurement of the total arene signal eliminates identification errors and the resulting quantitative errors. Ultraviolet spectrometry is a more rapid, precise, and simple method, although it is inferior to gas−liquid chromatography (GLC) in sensitivity and selectivity. Ultraviolet spectrometry is successfully used in the group analysis of technical petroleum products. There were attempts to use this method for determining the concentration of petroleum products in waters [8], but the results turned out to be inaccurate [9]. Ultraviolet spectrometry has not previously been used to determine total concentration of arenes in waters; no publications in this area are known except for a small article [10].
The specific light absorption coefficients of different arenes at the same wavelength (K, L/(mg cm)) differ, in contrast to sensitivity coefficients for their flame-ionization detection. This complicates the direct calculation of cAr, but the total concentration of arenes can be estimated in terms of a standard substance Xst. The corresponding total indices CAr is similar to hydrocarbon or phenolic indices [11]. Naturally, the incompleteness of the extraction of arenes and the intragroup signal selectivity leads to some uncertainty of the new indicator (“arene index”), that is, to unpredictable differences between CAr and cAr. However, methods for reducing the uncertainty of total indices are known: in spectrometry, both the analytical wavelength (AWL) and the nature of the standard are optimized [12], or multi-wave measurements and multivariate calibration are used [13].
We believe that the estimation of cAr should include the following stages: (a) the extraction of petroleum products from a water sample under study, (b) the adsorption purification of the extract from polar components, (c) the measurement of the absorbance of the purified extract in the UV region; and (d) the calculation of CAr based on a previously constructed one-dimensional or multivariate calibration dependence. In the first two stages, the analysis can be carried out in the same way as in determining the “petroleum products” indicator.
The goal of this work was to test a possibility of an accurate determination the total concentration of arenes in waters by the extraction–spectrometric method and to develop and verify an appropriate procedure for the analysis of wastewaters.
EXPERIMENTAL
Arenes of the C6–C9 group were used as model substances: benzene (B), toluene (T), o-xylene (o-X), m-xylene (m-X), p-xylene (p-X), ethylbenzene (EB), and cumene (CM). Multicomponent solutions of arenes in n-hexane were prepared using accurately weighed portions of reagents (cp grade), as described in [7]. The total concentrations of arenes in the model solutions (“mixtures”) ranged from 40 to 1500 mg/L; the mass ratios of different arenes in the mixture did not exceed 10 : 1. In total, more than 30 model mixtures were prepared and analyzed. They were used to prepare simulated wastewater samples, that is, aqueous solutions with the known cAr values. To do this, distilled water, sulfuric acid, or an alkali was added to an accurately measured volume (usually 10.0 mL) of an arene-containing model solution to the desired pH value, thoroughly mixed, and the volume of the mixture was adjusted to 1000 mL with distilled water. The total concentrations of arenes in the simulants were in the range 0.1–50 mg/L, which approximately corresponds to the composition of treated wastewaters and does not exceed the equilibrium solubility of C6–C9 arenes in water. Examples of simulant solutions are given in Table 1. Some simulants were prepared from water-soluble standard reference materials (SRM, GSO) with a known total HC concentration and an approximately known proportion of arenes; an example would be GSO 7117-94. Other simulant solutions were supplemented with the known amounts of phenols (phenol, p-cresol, o-cresol). Simulants were used (a) to assess the recovery of arenes and the degree of purification of the extract from phenols, (b) as calibration solutions in constructing multivariate calibration, and (c) to check the accuracy of the developed methods. The training set included 25 simulant solutions, and the test set consisted of 10 other simulants.
Wastewater samples (1.0 L) were collected at various enterprises by employees of factory analytical laboratories, guided by standard recommendations for determining the “petroleum products” indicator. Heavily contaminated samples were diluted with distilled water before an analysis. The collected samples were analyzed triply (if possible, in the day of sampling). Both samples and simulants were kept cold for no more than 3 days. In the course of analysis, the sum of arenes contained in 250 mL of simulated or test water was extracted with 25 mL of n-hexane in the presence of a salting-out agent (NaCl). The phase contact time was 5 min. To remove polar compounds, the dehydrated extract was passed through a column with Al2O3. The volume of the purified extract was brought up to 25.0 mL with n-hexane, and then the extract was not fractionated or evaporated. The extraction technique was described in detail in the previous communication [7], and the adsorption purification of the extracts was described in the standard [14]. The absorption spectra of model solutions and the extracts were recorded on an SF-2000 spectrophotometer using quartz cells (l = 10.0 mm). The spectra were recorded against n-hexane. The purity of the solvent was controlled by measuring its absorbance at 220 nm against an empty cell. The significance of deviations from the additivity of absorbances (ΔA) was checked according to the procedure [15] using the 3s-criterion.
Using single-wavelength UV spectrometry, the absorbance of the extracts was measured at the selected AWL (usually, at 250 nm). The total concentration of arenes in the extract in terms of Xst was found using a rectilinear calibration graph plotted in advance from the measurement results for Xst solutions. The result of analysis (CAr) was calculated taking into account the sample and extract volumes.
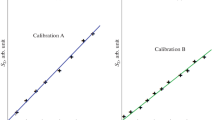
Analyzing simulants and wastewater samples by multi-wave UV spectrometry, we used the method of multiple linear regression in the version of indirect calibration (MLR-2). Typically, optical measurements were performed at seven AWLs (for example, at 243, 250, 260, 265, 270, 275, and 280 nm). In some cases, the absorbance of the extract was automatically measured at a step of 1 or 0.2 nm. MS Excel [13] or Optic-MLR [16] software was used to construct multivariate calibrations and calculate the results of analysis. Calibration A was constructed by the data on absorbances of ten hexane solutions with the known cAr values. Calibration B was built by the data on absorbances of 25 extracts from simulants passed through all stages of sample preparation. In both cases, the overdetermined system of linear equations of the type
was solved by the least-squares method (LSM), where ci was the total concentration of arenes expressed in mg/L in the ith model solution (calibration A) or in the ith simulant (calibration B); Аij was the absorbance of the ith model solution or extract from the ith simulant at the jth AWL; kj was the jth regression coefficient (in mg/L). Substituting the estimates of the coefficients found by the least-squares method into Eq. (1), we obtained the desired calibrations. To analyze the certain sample, the absorbance values of the measured solution at the selected AWLs were substituted into the resulting calibration. The concentration of arenes in the extract was found by calibration A, and then it was recalculated to the initial sample. They, calibration B was used to find the concentration of arenes in the investigated water, without calculating the concentration of arenes in the extract. The data were statistically processed according to the Student’s algorithm (n = 3, P = 0.95). The errors in the analysis of simulants (δc, rel. %) were calculated using the equation
To check the accuracy of the results of wastewater analysis, the standard addition method was used. Mixtures of arenes were used as additions. Some samples were additionally analyzed by extraction–chromatography [7].
RESULTS AND DISCUSSION
Single-wavelength spectrometry: selection of analytical wavelength. When selecting the conditions for the spectrometric assessment of cAr, one should take into account several factors: the sensitivity and precision of measurements at different AWLs, the fulfillment of the Bouguer–Lambert–Beer law, the intragroup and intergroup selectivity of the signals, and the additivity of absorbances of mixtures. Arenes absorb most strongly in the region 180–220 nm, but the results of the corresponding measurements are not reproducible enough and poorly obey the Bouguer–Lambert–Beer law. At λ > 280 nm, some monocyclic arenes (for example, benzene) do not absorb UV radiation, which makes it difficult to estimate cAr accurately. For such an assessment, the region 240–280 nm should be used, where all arenes absorb well, the calibration curves are linear (r > 0.99), and the absorbance measurements are characterized by good repeatability (RSD < 2%) and are approximately additive (ΔA < 3s). Unfortunately, the sensitivity of the determination of monocyclic arenes in the wavelength region 240–280 nm is low [17], and at any fixed AWL, the specific absorption coefficients of various arenes are different (Fig. 1). This leads to the formation of a set of calibrations (Fig. 2). The intragroup signal selectivity was characterized by a dimensionless parameter T, as is customary in the study of total indices [18],
In Eq. (3), Kmax and Kmin denote the maximum and minimum values of specific absorption coefficients of individual arenes at a fixed AWL. The parameter T depends on the selection of the AWL. For the set of arenes we studied, T ≈ 4 at 263 nm and T ≈ 2 at 254 nm. The intragroup selectivity turned to be minimal at λ = 250 nm (T ≈ 1.5). The limits of detection for different arenes in model solutions at this AWL were quite close and amounted to 1–2 mg/L. Consequently, at a tenfold preconcentration of arenes, their limits of detection in water approximately corresponded to the MPC values, and the lower limits of determination (LLDs) were close to 0.5 mg/L. For comparison, let us point out that, in determining arenes according to the procedure [7], the LLD values were five times lower. Nevertheless, the sensitivity of UV spectrometry is sufficient for the determination of arenes in the treated wastewaters of many industrial plants.
An additional advantage of the selected AWL is in a decreased effect of phenols; phenols often accompany arenes in wastewater, and they are not completely separated in the adsorption purification of the extract. The absorbance of phenols in nonaqueous solutions is maximum at 210–230 and 270–280 nm, while in the region of 240–250 nm, their absorption is significantly lower [19]. The test confirmed that, at 250 nm, phenol mixtures absorbed UV radiation weaker than mixtures of monocyclic arenes. At this AWL, heavy arenes (for example, naphthalene and phenanthrene) absorb strongly [17].
Selection of a standard substance. For a correct spectrometric assessment of the total concentration of similar analytes in terms of Xst, the specific absorption coefficient of the standard substance (Kst) must meet the condition
Many substances meet condition (4). The selection of the best standard is facilitated if the concentrations of all analytes of the desired group are of the same order of magnitude, and the sensitivity coefficients for each analyte are known [12]. In this case, the following empirical relationship is suitable for selecting the optimal standard:
The substitution of Kmax and Kmin values found at 250 nm into Eq. (5) leads to a conclusion that the best cAr estimates should be obtained in terms of o-xylene or m-xylene. At 250 nm, these arenes are detected with average and approximately equal sensitivities. The use of other standards should lead to systematically overestimated or systematically underestimated results for cAr, which was confirmed experimentally. The results of analysis of the same model solutions, expressed in terms of different Xst (Table 2) are characterized by good repeatability (RSD < 5%). If the total concentration of arenes in hexane solutions is expressed in terms of o-xylene or m-xylene, then δc < 7 rel. % (modulo). If Xst is selected improperly, the errors are much higher. For example, in using toluene, the errors are negative and reach 20 rel. %; in the case of cumene, the errors are positive and reach 40 rel. %.
Single-wavelength spectrometry in the analysis of simulants. A transition to the analysis of dilute aqueous solutions (in particular, simulated wastewater) leads to a significant decrease in the accuracy of estimating cAr. As the analysis includes additional operations, the repeatability of the results deteriorates (RSD < 12%). The results of the analysis turn out to be strongly underestimated (Table 3); the δc values were predominantly systematic and exceeded 50% (modulo). We believe that these errors are mainly due to the loss of arenes in extraction. Monocyclic arenes are much better soluble in water than other hydrocarbons, this factor lowers the degree of their recovery. According to our data, the loss of arenes in a single extraction with a small volume of n-hexane ranges from 40 to 70%, depending on the nature of the extracted arene. This is consistent with the data [20]. In addition, up to 10% mass of the recovered arenes is lost in the adsorption purification of the extract.
The quantitative recovery of arenes with n-hexane can be achieved by increasing the volume of the extractant in a single extraction and/or by performing numerous repeated extractions and combining the extracts. However, these operations impede the efficient preconcentration of arenes. We believe that it is impractical to achieve the complete recovery of arenes, because the corresponding loss can be taken into account in calculating the results of analysis. We are talking about the method of constructing a calibration dependence rather than about the introduction of empirical correction factors (although in some cases this technique may also be useful). As calibration solutions, aqueous solutions with a known concentration of Xst can be used after passing through the same sample preparation operations as the analyzed samples. According to our data, the use of this technique in the construction of a one-dimensional calibration decreases the systematic errors in the analysis of simulants but does not eliminate them. The loss of arenes during sample preparation is not the only source of systematic errors. Another source may be the difference in the extractability of individual arenes; this factor was not taken into account in selecting AWL or Xst. To decrease its effect, it is desirable to carry out multi-wave measurements and build multivariate calibrations (see the next section).
The effect of phenols turned out to be a less severe problem. Their recoveries from aqueous solutions are lower than the recoveries of arenes, especially in an alkaline medium [19]. After the adsorption puri-fication of the extract, only 5–10 wt % of previously extracted phenols remain in it. At 250 nm, the remaining phenols absorb UV radiation weaker than the C6–C9 arenes. If the initial simulant contains approximately equal amounts of phenols and arenes, the result of determining arenes in terms of Xst increases by only 3–5% compared to the simulant containing no phenols. The results of determining arenes remain underestimated (see Table 3, bottom rows). With a large excess of phenols, overestimates of cAr can be obtained too. In particular, high cph/cAr ratios are characteristic for wastewaters from enterprises producing phenols or phenol–formaldehyde resins. The interfering effects of foreign substances on the results of the extraction–spectrometric determination of arenes in waters of different types require a special study and will be considered in the next communication.
Use of multi-wave measurements and multivariate calibrations. As already noted, in the extraction–spectrometric analysis of wastewaters (or wastewater simulants), intragroup selectivity should be more pronounced than in the analysis of model solutions. Differences in the extractability of these substances are added to the differences in the reference UV spectra of individual arenes. As was shown in previous studies by our group [13, 17], to correctly estimate total concentrations, it is desirable to measure absorbances at several wavelengths and calculate the results of analysis using multivariate calibrations without conversion to Xst. A schematic diagram of such an analysis in relation to the determination of the total concentration of arenes is presented in Fig. 3.
A comparison Tables 3 and 4 shows that the transition to multi-wave measurements and multivariate calibrations significantly improves the results of analysis of the simulants. Multivariate calibrations should be based on the results of measurements carried out on the same spectrophotometer that is used further to analyze real samples; it is impossible to use the published data in such cases. In this article, therefore, we do not present cumbersome equations for multivariate calibrations A and B.
Table 4 compares the results of an extraction–spectrometric analysis of simulants from the test set, obtained using different calibrations. For calculations, the same initial data were used (values of the absorbance of the hexane extract at seven wavelengths). In using calibration A, the errors did not exceed 40 rel. %. Even better results were obtained using calibration B constructed for set of simulants from a training sample. In this case, δc < 20% at RSD < 12%. The errors in the analysis of different simulant solutions differed both in the absolute value and in the sign. In determining arenes at a level of cAr < 1 mg/L, the errors sometimes increased up to 30%. Nevertheless, the accuracy of the results meets the standards of errors specified in the official records of the Russian Federation for the determination of trace impurities in waters [21].
An additional examination of the developed procedure was carried out by analyzing aqueous solutions of GSO 7117-94 with a known concentration of petroleum products. The total concentration of arenes, found using the Optic-MLR software (from 1 to 10 mg/L), was in good agreement with the data on the group composition of this SRM [10].
An increase in the number of AWLs to several tens (or even hundreds) and the subsequent use of the PLS chemometric algorithm further increased the accuracy of determining cAr. However, we do not consider it expedient to complicate the new procedure, because this may complicate its implementation in the practice of analytical laboratories.
Results of analysis of wastewaters. Samples of treated and untreated wastewaters from four industrial enterprises were taken in the Omsk region in 2017–2019; sampling and analysis of some samples were repeated at intervals of several months. The profiles of enterprises, the composition of their wastewaters, and water treatment technologies differed significantly, which affected the total concentration and concentration ratio of different arenes. We used single-wavelength UV spectrometry for screening: if the found cAr values were lower than the LLD, the samples were not analyzed further. The other samples were analyzed in addition by multi-wave measurements using multivariate calibration B. The duration of a single analysis was 1 h. For most samples, the relative standard deviation under repeatability conditions (RSD) did not exceed 12%. For slightly polluted waters (CAr < 0.5 mg/L), RSD < 20%. The results of analyses of 11 samples are given in Table 5. For comparison, the results of analyses of the same samples by the chromatographic method [7] were presented.
The developed procedure ensures an analysis of both treated and untreated wastewaters (Table 5), which is essential for assessing the effectiveness of treatment. The CAr values found by spectrometric and chromatographic methods correlate with each other; the linear correlation is statistically significant (r > 0.99). Differences in the results for some of the samples are statistically insignificant (these results are shown in italics in Table 5). The results of the determination of cAr by the spectrometric method were slightly higher than those obtained for the same samples by gas–liquid chromatography. Probably, this is because the results of extraction–spectrometric analysis yield the total concentration of all compounds absorbing UV radiation in the region 240–280 nm rather than the concentration of monocyclic arenes С6–С9, as in the method [7]. In particular, phenanthrene, anthracene, and other polyarenes, which were found in wastewaters and were also extracted with n-hexane, absorbed UV radiation in this region [17]. According to the method [7], polyarenes were not determined, because, in the chromatographic analysis of the extract, only the peaks of the C6–C9 arenes were recognized and taken into account.
The spectra of extracts from wastewaters are similar to the spectra of extracts from simulants containing mixtures of C6–C9 arenes and strongly differ from the absorption spectra of polyarenes. Apparently, the contribution of polyarenes to the absorbance of the extracts from wastewaters is insignificant. It is known that the concentration of polyarenes in natural and waste waters is much lower than the concentration of monocyclic arenes: because of their low solubility in water, polyarenes quickly pass to bottom sediments [22]. Nevertheless, for some wastewaters, the contribution of polyarenes to the absorbance of extracts turned out to be statistically significant.
To examine the accuracy of the results of analyses of wastewaters, we used the standard addition method (Table 6). Aqueous solutions with the known cAr were added as additives. The error in determining the total concentration of arenes in the additives did not exceed 25% (modulo).
CONCLUSIONS
Thus, UV spectrometry can be used for an approximate (δc > 50%) estimate of the total concentration of arenes in waters in terms of the standard substance Xst, for example, for screening. For an accurate (δc < 20%) and metrologically correct (without conversion to Xst) determination of the total concentration of arenes in waters, multi-wave measurements of absorbance in the region 240–280 nm should be carried out and appropriate multivariate calibration should be used. In constructing calibrations, extracts from aqueous solutions with the known cAr values passed through all sample preparation operations should be used. The results of the UV-spectrometric analysis of several wastewaters slightly exceed the results of analysis of the same samples by GLC, although the data obtained correlate with each other, and the discrepancies in the mean values are not always significant. The discrepancies are explained by the fact that the spectrometric method determines a wider group of hydrocarbons, including both monocyclic and polycyclic arenes, while the chromatographic method determines only monocyclic C6–C9 arenes (the main but far from the only aromatic compounds in the corresponding hydrocarbon mixtures). The developed procedure for the extraction–spectrometric determination of the total concentration of arenes in the range 0.5–50 mg/L is suitable for the analysis of wastewaters and can be recommended for analytical laboratories of environmental protection. It is unsuitable for analyzing slightly polluted natural waters or controlling the composition of drinking water. Additional studies are needed to increase the sensitivity and intergroup selectivity of the proposed procedure.
REFERENCES
Leonenko, I.I., Antonovich, V.P., Andrianov, A.M., Bezlutskaya, I.V., and Tsymbalyuk, K.K., Metody Ob”ekty Khim. Anal., 2010, vol. 5, no. 2, p. 58.
Analysis of Petroleum Hydrocarbons in Environmental Media, Weisman, W., Ed., Total Petroleum Hydrocarbon Criteria Working Group Series, Amherst: Amherst Sci., 1998, vol. 1.
Drugov, Yu.S. and Rodin, A.A., Ekologicheskie analizy pri razlivakh nefti i nefteproduktov (Environmental Analyzes for Oil and Petroleum Product Spills), St. Petersburg: Anatoliya, 2000.
Desideri, P.G., Lepri, L., Heimler, D., Oianessi, S., and Checchini, L., J. Chromatogr. A, 1984, vol. 284, no. 10, p. 167.
Bianchin, J.N., Nardini, G., Merib, J., Dias, A.N., Martendal, E., and Carasek, E., J. Chromatogr. A, 2012, vol. 1233, p. 22.
RD.52.24.473-2012: Mass Concentration of Volatile Aromatic Hydrocarbons in Waters. Gas Chromatographic Measurement Technique Using Equilibrium Vapor Analysis, Rostov-on-Don: Virazh, 2012.
Vershinin, V.I. and Usova, S.V., J. Anal. Chem., 2021, vol. 76, no. 3, p. 337.
Gomelya, N.D., Kalabina, L.V., and Khokhotva, A.P., Khim. Tekhnol. Vody, 1999, vol. 21, no. 6, p. 611.
Klenkin, A.A., Pavlenko, L.F., and Temerdashev, Z.A., Zavod. Lab., Diagn. Mater., 2007, vol. 73, no. 2, p. 31.
Buryukina, P.A., Vlasova, I.V., and Mirgaleeva, R.R., Vestn. Omsk. Univ., 2016, no. 3, p. 54.
Baena, J.R. and Valcárcel, M., TrAC, Trends Anal. Chem., 2003, vol. 22, no. 10, p. 641.
Vershinin, V.I., J. Anal. Chem., 2017, vol. 72, no. 9, p. 947.
Vershinin, V.I. and Petrov, S.V., Talanta, 2016, vol. 148, p. 163.
GOST (State Standard) 51797-2001: Drinking Water. Method for Determination of Oil Products Content, Moscow: Standartinform, 2010.
Vershinin, V.I., Tsyupko, T.G., and Vlasova, I.V., Metody Ob”ekty Khim. Anal., 2010, vol. 5, no. 4, p. 226.
Vlasova, I.V., Tsyupko, T.G., and Shelpakova, A.S., Metody Ob”ekty Khim. Anal., 2012, vol. 7, no. 1, p. 18.
Clar, E., Polycyclic hydrocarbons, New York: Academic, 1964, vol. 1.
Vershinin, V.I., Talanta, 2015, vol. 131, no. 1, p. 293.
Aluker, N.L., Lavrentieva, A.-L., and Suzdaltseva, Ya.M., Opt. Spectrosc., 2020, vol. 128, no. 3, p. 422.
Antonova, T.V. and Usova, S.V., Analitika Kontrol’, 2017, no. 4, p. 307.
GOST (State Standard) 27384-2002: Water. Rates of Measurement Error of Characteristics of Composition and Properties, Moscow: Standartinform, 2010.
Adeniji, A.O., Okoh, O.O., and Okoh, A.I., in Recent Insights in Petroleum Science and Engineering, El-Sayed Abdul-Raouf, Ed., InTech, 2018, p. 3475.
ACKNOWLEDGMENTS
The authors are grateful to S.M. Dobrovolsky and S.V. Usova for valuable advices and comments. Students T.P. Domanina, O.A. Kazakova, A.V. Mamontova, and P.A. Buryukina took part in the experiments.
Funding
The work was supported by the Russian Foundation for Basic Research (project no. 16-03-550479).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Antonova, T.V., Vershinin, V.I. & Vlasova, I.V. UV-Spectrometric Determination of Total Concentration of Arenes in Wastewaters. J Anal Chem 76, 815–823 (2021). https://doi.org/10.1134/S1061934821070042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934821070042