Abstract
A method has been developed for the separation of lanthanides, Ce, Nd, Gd, La, Pr, Sm, Dy, Eu, Er, Ho, Yb, Tm, Tb and Lu, using oxalate form of Dowex-1, a reactive ion-exchanger. Lanthanides present in such samples as monazite sand, coal fly ash and sediment were separated from matrix at pH 2.5 in a column containing oxalate form of Dowex-1 resin. The samples have been decomposed in H2SO4 and taken in 0.36 M H2SO4 prior to separation. The lanthanides in the column were eluted with 2 M HNO3. Lanthanides present in the eluent were determined by inductively coupled plasma optical emission spectrometry (ICP−OES) and the recovery of analytes ranged from 92 to 105%. Matrix free solutions were analyzed for lanthanides by ICP−OES. The relative standard deviation was in the range of 4–7% and limits of detection were between 0.015–0.16 mg/kg. The developed procedure was applied to the separation and determination of lanthanides in a standard reference material NIST Coal Fly Ash 1633b, a lake sediment and monazite sand. The results obtained by the present method are in close agreement with certified values in case of certified reference material and microwave digestion method for other samples. Accuracy of other samples is ensured based on standard addition recoveries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Lanthanides are an important group of elements available in the Earth’s crust. They have many similar properties, which often leads to their joint presence in geologic deposits. They have been widely used in numerous industrial processes such as the production of superconductors, super magnets, catalysts, pigments in glasses, plastics, additives, medicines and cosmetics as well as fertilizers [1–5]. Major application of rare earths is in the manufacture of magnets which are utilized in electric motors to produce greater power and torque, and owing to the power of the magnets, less material is required such that engines can be considerably smaller and lighter. The importance of lanthanides in industrial applications and geological and environmental studies demands their determination from percentage levels to ultra trace levels. Monozite and xenotime which occur in small amounts in heavy mineral concentrates obtained from placer deposits are the most important minerals of lanthanides. Coal fly ash is also a source of lanthanides that can be processed to extract lanthanides [6–8]. Levels of lanthanides in monazite and coal fly ash will let us know their commercial value. Another reason for lanthanides determination in environmental samples is pollution. As a result of their use in various industries, lanthanides would be increasingly released to the environment entering food chain as they are taken up by aquatic micro-organisms and scavengers. Although lanthanides are not considered as priority environmental contaminants unlike ubiquitous toxic elements (e.g., As, Cd and Pb), they are known to induce adverse health effects [9, 10], and therefore require sensitive methods for accurate monitoring in complex environmental samples such as soils and sediments. Overall, determination of lanthanides is necessary both with respect to toxicity and also commercial point of view.
Inductively coupled plasma based techniques are used for the determination of lanthanides in a wide variety of samples due to the high temperature of plasma that ensures complete atomization of refractory elements such as lanthanides. Inductively coupled plasma mass spectrometry is a more sensitive and extensively used technique for specialized analysis. Though less sensitive, inductively coupled plasma optical emission spectrometry (ICP−OES) is a commonly used technique with lower operational costs and is suitable for preliminary scanning of samples for lanthanides. Direct determination of lanthanides in environmental samples has been reported using ultrasonic nebulizer in combination with axial viewing [11]. Fisher and Kara reviewed separation methods applicable to lanthanides in natural waters [12]. Kala has reported a review on online and offline separation procedures developed for lanthanides from environmental, geological and biological samples and explained the occurrence, demand and economic importance, country wise distribution of lanthanides [13]. Another review was published on the determination of lanthanides using spectroscopic techniques [14]. Separation methods are based on solvent extraction using toxic organic solvents, cloud point extraction, solid phase extraction mainly using ion exchange resin or chemically modified resins with suitable complexing agents that aid the separation of lanthanides together [15–19]. Sana Kutun Sungur and Aycicek have studied the separation and determination of rare earth elements by Dowex 2-X8 resin using sodium trimetaphosphate as eluent, qualitative and quantitative determinations were conducted by spectrofluorometry [20].
In this paper, we present a method for the selective separation of lanthanides using Dowex-1 modified with oxalate, a reactive ion-exchanger, for their determination in a variety of samples. Conditions for quantitative adsorption and elution have been discussed. Application of the developed analytical methodology for the analysis of coal fly ash, monazite and sediment has also been described.
EXPERIMENTAL
Instrumentation. Lanthanides were measured based on their emission intensities using an inductively coupled plasma optical emission spectrometer Model ULTIMA 2 (Horiba Jobin Yvon, France) equipped with a cross flow nebulizer and a cyclonic spray chamber. The spectrometer was equipped with a Czerny-Turner monochromator using a 2400 grooves/mm holographic grating. Plasma conditions shown in Table 1 are those that yielded best sensitivity viewed in radial mode. Wavelengths selected (Table 1) are based on sensitivity and lack of spectral interference.
Reagents and samples. Analytical grade reagents were used throughout the experiment. All acids used, HCl, HNO3, H2SO4, were procured from Merck, India. Potassium oxalate was from S.D. Fine chemicals, India. Milli-Q water (Millipore Milli-QTM, Beckford, MA, USA) with 18 MΩ cm resistivity was used for the preparation of all solutions. Stock standard solutions (1000 mg/L) of Lu, Tm, Yb, La, Gd, Er, Ho, Tb, Eu, Dy, Th, Nd, Pr, Sm, Ce were procured from Merck, Germany.
Monozite sand was collected from a beach in Kerela, India, and analyzed as obtained. A sediment was collected from Hussain Sagar Lake (Hyderabad, India) polluted with industrial effluents. Sediments were collected in precleaned polythene bags, air dried, ground and sieved to 80 mesh, and then used for analysis.
Preparation of resin. A column of 40 mm inner diameter was taken and was filled with 10 g of chloride form of Dowex-1 (50–100 mesh, Sigma, USA), washed with 100 mL of 2 M HNO3 followed by Milli-Q water till acid removal. The resin was washed with 50 mL of 6 M HCl to convert it into chloride form, thoroughly washed with Milli-Q water free from acid and loaded with 100 mL of 10% potassium oxalate. The resin was again thoroughly washed with Milli-Q water, air dried and then used for the studies.
Separation of lanthanides. Lanthanides were separated together as oxalates on oxalate form of Dowex-1. However, adsorption and elution conditions were optimized to ensure quantitative recoveries.
Adsorption. Studies were carried out using 25 mL of a solution containing analytes at 500 ng/mL. A column of 10 mm i.d. was prepared with 2 g of oxalate form of Dowex-1 and mixed standard solution was passed through the column at various pH in the range of 1.0–2.5 with a flow rate of 1 mL/min using gravitational flow controlled using stopper of the column.
Elution. After adsorption, lanthanides need to be eluted with a suitable reagent. Elution has been carried out using HNO3. To optimize concentration required for quantitative elution, the experiment was carried out with 1.5–2.5 M HNO3. Elution volume was 20 mL, and flow rate was 1 mL/min.
Analytical method for coal fly ash, monazite and sediment. Samples (100 mg) were accurately weighed into a dry 100 mL beaker, and 10 mL of concentrated sulphuric acid was added. The beaker was covered and heated on a sand bath for 2–3 h (frequent stirring) until the evolution of dense fumes ended. The beaker was then cooled in an ice bath, and 50 mL of Milli-Q water was added to dissolve the residue. The supernatant liquid was transferred into a 50 mL standard flask. Any remaining insoluble residue was treated with 5 mL of 18 M sulfuric acid and heated on a sand bath until the evolution of dense fumes ended. The residue was cooled, dissolved in 20 mL of Milli-Q water, and the solution was filtered. This filtrate was transferred to the above standard flask and made up to 50 mL. Procedural blank solutions were also prepared concurrently following the same procedure. Lanthanides present in above sample solutions were separated at pH 2.5 on the oxalate form of Dowex-1 resin column. The adsorbed lanthanides were eluted with 2 M HNO3 and analyzed by ICP−OES. The recovery of lanthanides was in the range of 92–105%. Matrix free solutions were analyzed for lanthanides by ICP−OES.
Microwave digestion method. Monozite and sediment samples (100 mg) were accurately weighed and placed into the reaction vessel (100 mL) and digested with HNO3 (4 mL), H2O2 (2 mL) and HF (2 mL) in the microwave digestion system. Insoluble fluorides were decomposed by fuming with H2SO4 after evaporating the acids used for dissolution. After cooling and dissolution in Milli-Q water, the final solution was made up to 25 mL with Milli-Q water.
RESULTS AND DISCUSSION
Monozite, an important mineral of lanthanides, constitutes the toughest task in analytical chemistry, as it is a highly resistant mineral and extremely difficult to completely decompose. Coal fly ash and sediments are also known to contain detectable concentrations of lanthanides. In the present study, separation of lanthanides has been attempted using oxalate form of Dowex-1 which is a reactive ion-exchanger. Adsorption on a reactive ion-exchanger is more favorable than ion exchange as there is involvement of chemical reaction. Rare earth elements are known to form insoluble oxalates at acidic pH. However, optimization of such parameters as pH and concentration of eluting agent is necessary.
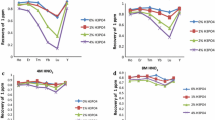
Studies have been carried out using a 500 ng/mL mixed aqueous standard solution of lanthanides. A column was prepared with 2 g of oxalate form of Dowex-1 as mentioned in the previous section. The pH of the loading solution was made in the range of 1.0–2.5 and passed through the resin. The adsorbed lanthanides were eluted with 2 M HNO3. The results are shown in Fig. 1. At pH 1.0 the recovery of lanthanides was very low (below 40%) and increased with pH and reached quantitative recoveries at pH equal to 2.5. The adsorption of lanthanides could be due to the complexation reaction of lanthanides with oxalate on the resin.
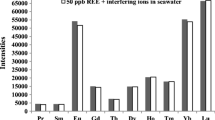
Similarly, HNO3 concentration required for complete elution of lanthanides was also studied in the range of 1.5–2.5 M for 20 mL of eluting solution. As can be seen in Fig. 2, recoveries were increased with HNO3 concentration till 2 M and then reached a plateau. The optimized conditions for separation of lanthanides from complex matrices are: loading solution pH value of 2.5 and 2 M HNO3 eluting solution. As the concentration of lanthanides varied from percentage to few mg/kg in monazite sand, spike recoveries of lanthanides were evaluated on a sediment sample and recoveries were found to be nearly quantitative.
The sorption of lanthanides onto oxalate form of Dowex-1 could be due to the formation of lanthanide oxalates on the resin as described in our previous paper [21]. During the sorption of lanthanides, anions present in the sample solution can partially replace oxalate present on the resin. In the elution process, lanthanide oxalates were desorbed from the resin. The above phenomena did not affect the recovery of lanthanides from various sample solutions analyzed.
To verify the accuracy of the method, it has been applied to the determination of lanthanides in a NIST SRM Coal fly ash 1633b. The values obtained are close to the recommended values (Table 2). The method has been applied to a monazite sand and a lake sediment sample. The values obtained were validated with microwave decomposition method followed by oxalate separation. As can be seen in Table 3, the values are in close agreement. All determinations were carried out using external calibration. The percentage relative standard deviation values were in the range of 4–7% and limits of detection (LODs) calculated as the signal equivalent to three times the standard deviation were in the range of 0.015–0.16 mg/kg and LODs of individual elements are included in Table 3.
CONCLUSIONS
A set of 14 lanthanides has been separated and determined in monazite and lake sediments. The method has been validated by the analysis of a NIST SRM coal fly ash 1633b. Separation of lanthanides has been carried on a reactive ion-exchanger, oxalate form of Dowex-1. The procedure has been demonstrated to be suitable for the detection of lanthanides present in coal fly ash, monazite sand and sediment samples. The methodology is simple, easy to adopt with quantitative recoveries. The same resin can be prepared again with used Dowex-1. The method is useful for the screening of environmental samples for lanthanides content.
REFERENCES
Bahramifar, N. and Yamini, Y., Anal. Chim. Acta, 2005, vol. 540, p. 325.
Cyber Riden. http://cyberraiden.wordpress.com/ 2012/04/22/rare-earth-elements-and-their-uses. Accessed February 2, 2018.
Rao, T. and Kala, R., Talanta, 2004, vol. 63, p. 949.
Shan, X.Q., Lian, J., and Wen, B., Chemosphere, 2002, vol. 47, p. 701.
Hubicki, Z. and Olszak, M., J. Chromatogr. A, 2002, vol. 955, p. 257.
Franus, W., Wiatros-Motyka, M.M., and Wdowin, M., Environ. Sci. Pollut. Res. Int., 2015, vol. 22, p. 9464.
Peterson, R., Heinrichs, M., Glier, J., Lane, A., and Taha, R., Proc. 2017 World of Coal Ash (WOCA) Conf., Lexington, 2017.
Peiravi, M., Ackah, L., Rajesh, G., Mohanty, M., Liu, J., Xu, B., Zhu, X., and Chen, L., Miner. Metall. Process., 2017, vol. 34, p. 170.
Pagano, G., Guida, M., Tommasi, F., and Oral, R., Ecotoxicol. Environ. Saf., 2015, vol. 115, p. 40.
Li, X., Chen, Z., Chen, Z., and Zhang, Y., Chemosphere, 2013, vol. 93, p. 1240.
Betlin, F.R.S. and Pozebon, D., J. Braz. Chem. Soc., 2010, vol. 21, p. 627.
Fisher, A. and Kara, D., Anal. Chim. Acta, 2016, vol. 935, p. 1.
Rao, T. and Kara, D., Talanta, 2004, vol. 63, p. 949.
Zawisza, B., Pytlakowska, K., Feist, B., Polowniak, M., Kita, A., and Sitko, R., J. Anal. At. Spectrosc., 2011, vol. 26, p. 2373.
Xie, F., An Zhang, T., Dreisinger, D., and Doyle, F., Miner. Eng., 2014, vol. 56, p. 10.
dos Santos Depoi, F., Bentlin, F.R.S., Flores Ferrao, M., and Pozebon, D., Anal. Methods, 2012, vol. 4, p. 2809.
Zereen, F., Yilmaz, V., and Arslan, Z., Microchem. J., 2013, vol. 110, p. 178.
Karadas, C. and Kara, D., Water, Air, Soil Pollut., 2014, vol. 225, p. 2192.
Karadas, C., Kara, D., and Fisher, A., Anal. Chim. Acta, 2011, vol. 689, p. 184.
Sungur, S. and Akseli, A., J. Chromatogr. A, 2000, vol. 874, p. 311.
Venkateswarlu, G., Sahayam, A.C., Chaurasia, S.C., and Mukherjee, T., Talanta, 2006, vol. 68, p. 748.
ACKNOWLEDGMENTS
The authors wish to acknowledge constant support and encouragement from Dr. Sunil Jai Kumar, Head, NCCCM, BARC, Hyderabad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gumma Venkateswarlu, Mamatha, P.R., Thangavel, S. et al. Determination of Lanthanides in Coal Fly Ash, Sediment and Monazite Sand by Inductively Coupled Plasma Optical Emission Spectrometry After Separation Using Oxalate form of Ion-Exchange Resin . J Anal Chem 76, 180–184 (2021). https://doi.org/10.1134/S1061934821020155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934821020155