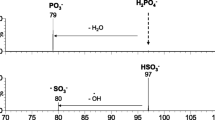
Abstract
The decay paths of negative molecular ions of phosphate esters, formed under the conditions of resonance electron capture, are of the same type and, therefore, predictable. A combination of mass spectra of the studied substances obtained by this method with the mass spectra of positive ions recorded in the convential electron ionization mode significantly expands the possibilities of their identification and determination of the characteristics of their molecular structures. In this case, only one mass spectrometer can be used, quickly switched from one mode to another.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The problem of rapid and reliable identification of toxic substances, especially in the field, remains relevant. The difficulties of this identification are due to the fact that, for detecting toxic substances in small quantities in complex matrices (soil, water, etc.), only positive ion electron ionization mass spectrometry (PI–EI–MS) in combination with gas chromatography is useful [1–3]. However, the long-term practice shows that the use of only one spectral method, as a rule, does not allow the identification of an unknown substance with a high reliability if its spectrum is not present in a reference database [4, 5].
The List 1a of the Chemical Weapons Convention [6] (hereinafter referred to as the Convention), presents entire classes of organophosphorus toxic substances to be controlled (nos. 1–3 in the List). The number of compounds in these classes varies from tens to hundreds of thousands, while only a few hundred representatives of each class have been studied. For example, in the case of O-alkylalkyl fluorophosphonates (I),

where R = CH3, C2H5, C3H7, (CH3)2CH and R' = an alkyl or cycloalkyl radical with the number of carbon atoms 1–10, only 300–350 substances out of more than 24 000 substances of this class [7], subject to control according to the Convention [6], are presented in the reference spectral databases.
In this situation, it is advisable to carry out identification in two stages. At the first stage, group identification is performed; that is, the assignment of a test compound to a known taxonomic unit—a class or a homologous series (or some group thereof)—is made without the complete decoding of the molecular structure of this compound [3, 8]. The conclusions drawn at this stage determine the feasibility of the complete determination of the molecular structure of a substance at the second stage. From a practical point of view, the division into two stages is fully justified, because all substances of the same class have identical physiological effect on humans. The results of the group identification of O-alkylalkyl fluorophosphonates (no. 1 in the List 1a of the Convention), as well as the most toxic of all organophosphorus compounds O-alkyl-S-2-(N,N-dialkylamino)ethylalkyl thiophosphonates (V gases) using the combinations of gas chromatography and PI–EI–MS cannot be considered sufficiently reliable. The main reason is the insufficient number of group characteristic features of these compounds, obtained by PI–EI–MS, and the presence of many of these features in compounds of related classes [9].
There is one more problem. The majority of toxic organophosphorus compounds and many of their precursors and conversion products are phosphate esters, that is, esters of phosphoric, alkylphosphonic, alkylphosphonous, and alkylthionphosphonic acids. The length and structure of alkoxyl radicals largely determine the toxicity of substances, as well as their physicochemical properties [10]. Despite some successful attempts to determine these structural characteristics by conventional mass spectrometry [11], in the general case, this is difficult even for monoesters (II). Difficulties even increase in the case of phosphate diesters and triesters with different alkoxyl radicals in one molecule (III and IV).

In this case, conventional electron ionization mass spectrometry is even more efficient than 13C NMR [12]. Nevertheless, the task of determining, for example, the number of carbon atoms in the radicals R, R1, R2 of such molecules, even using the combination of spectral methods recommended by the Organization for the Prohibition of Chemical Weapons (NMR, IR spectroscopy, PI–EI–MS) and gas chromatography, has no general solution. Negative ion resonant electron capture mass spectrometry (NI–REC–MS) can effectively solve this problem.
Academician Zolotov (Russian Academy of Science) wrote about the negative-ion resonance electron capture mass spectrometry [13]:
“To work in this mode, one can upgrade almost any mass spectrometer, but this is not very simple. The complexity of the method and the lack of serial production of the corresponding devices or at least add-ons to common mass spectrometers became the reason that the method was not widely used, despite the accumulated data (approximately 400 publications). Nevertheless, the method is characterized by considerable opportunities.”
We also mention that the method was not widely used, because until recently, experiments using the equipment for such studies were rather complicated and time-consuming, and were not suitable for serial analysis [14, 15]. Terent’ev and Ivanova, the authors of this paper, in close collaboration with experts from the Institute of Physics of Molecules and Crystals, Ufa Scientific Center, Russian Academy of Sciences, modified the serial Crystal 5000.1/DSQ Thermo Finnigan chromatography–mass spectrometry complex with a quadrupole mass analyzer to work with negative ions at low (up to 15 eV) ionization energies [16]. The modification of the instrument resulted in that the NI–REC–MS method can now be used for serial analysis, and the duration of the experiment is no more than in the conventional version of positive-ion electron ionization. Thus, it became possible to study the formation of the NI–REC mass spectra for a wide range of chemical compounds.
Negative-ion resonance electron capture mass spectra are observed in the general case in regions corresponding to different resonance values of the energy of ionizing electrons [17, 18]. We found that the resonance regions of the formation of negative ions of the studied organophosphorus compounds are at approximately 4 eV, and, therefore, the most intense signals of the total ion current are recorded at these energies of ionizing electrons.
The purpose of this work was to study the similarities and differences in the main directions of fragmentation of phosphate esters in electron ionization modes in recording positive and negative ions of resonance electron capture. Another goal was to show a significant increase in the reliability and expansion of possibilities of the group identification of phosphate esters and to determine the structure of alkoxyl radicals in the molecules of these compounds by a combined use of the two above modifications of the mass spectrometric technique.
EXPERIMENTAL
The experiment was carried out using a complex consisting of a Crystal 5000.1 gas chromatograph (Chromatec, Russia) and a DSQ Thermo Finnigan mass spectrometric quadrupole detector. We selected the following gas chromatograph operating conditions: DB-5MS quartz capillary column (30 m × 0.25 mm × 0.25 μm); injector temperature 250°C; thermostat temperature programming mode from 40 to 250°C at a rate of 10 K/min; temperature exposure after sample injection 1 min; flow rate of helium carrier gas 1.1 mL/min; and the interface temperature 255°С. The parameters of the mass selective detector were as follows: temperature of the ion source 257°С; energy of ionizing electrons 70 eV for recording positive ions (PI) and 4 ± 0.5 eV for registering negative ions; scanned mass range 30–500 Da.
Monoalkyl- and dialkyl-substituted chlorophosphates of the purity of not less than 95% were synthesized according to the known procedure [19]. The concentration of test substances in the solution was 0.1 mg/mL.
RESULTS AND DISCUSSION
Comparison of the main directions of the decay of phosphate esters in electron ionization modes with recording positive and negative ions of resonance electron capture. Earlier, we proposed mechanisms for the fragmentation of phosphate esters of the general formula (RO)nP(O)Xn–3, where X = R, halogen, OCH3, NR2, in the PI–EI–MS mode [12].
One of the two main directions of the fragmentation of such compounds is the sequential removal of alkoxyl radicals, which is accompanied by the migration of one or two hydrogen atoms to the phosphorus-containing fragment. This process consists of several stages, the number of which is determined by the number of alkoxyl radicals in the molecule [12].
For (RO)P(O)XY monoesters, where X and Y are an alkyl radical, a halogen, a pseudohalogen (for example, a −CN group), a −OCH3 group, or an amino group, the process under consideration is one-stage, that is,

The cleavage of an alkoxyl radical from the molecular ion is accompanied by the reverse migration of two hydrogen atoms to the phosphorus-containing fragment with the formation of ion V (Eq. (1)). This rearrangement is sometimes called “McLafferty + 1” [18].
The second main direction of the fragmentation of phosphate monoesters (as well as of many other monofunctional compounds [20]) is the break of the same O–C bond, leading to the cleavage of the [R–H]+• radical cation from the molecular ion [21], which is a typical example of McLafferty rearrangement [18] with the charge localization at an olefin fragment, that is

The so-called “alkene subspectrum” of the total mass spectrum of a substance [20], consisting of the peak of this radical cation and the peaks of its decay products, potentially contains information on the number of carbon atoms of the alkoxyl radical and its structure. For monoesters, such information can be obtained in some cases. This question was considered in sufficient detail in [11, 20] and is not discussed further here, because the determination of the structure of alkoxyl radicals in monoesters is not the subject of study in this work. As an example, we cite the positive-ion electron ionization mass spectra of O-(3-methyl)butylmethyl fluorophosphonate and O-(1-methyl)pentylmethyl fluorophosphonate (Fig. 1). The peak of V in the PI mass spectra of O-alkylalkyl fluorophosphonates, as well as other phosphate monoesters, is almost always the main one, which is confirmed by Fig. 1.
Positive ion mass spectra of (a) O-3-(methyl)butylmethyl fluorophosphonate and (b) O-1-(methyl)pentylmethyl fluorophosphonate [22].
For XP(O)(OR’)(OR'') diesters, the decay process in the main direction is two-stage and corresponds to the following mechanism [12]:


At the first stage of the main reaction, one alkoxyl radical is detached with the migration of two hydrogen atoms to the phosphorus-containing fragment, and intermediate ions VIa and/or VIb are formed. In the case of symmetric diesters (R′ = R″), one intermediate ion, VI, is formed. Note that the first stage of the reaction under consideration (Eq. (3)) entirely coincides with the main direction of the decomposition of monoesters, for example, O-alkylalkyl fluorophosphonates [11].
In the second stage (Eq. (4)), the second alkoxyl radical is cleaved, and the main ion VII, very similar to V (Eq. (1)), is obtained. Figure 2 shows a PI mass spectrum of O-decyl-O-pentylmethyl phosphonate [12] as an example. Judging from the obtained experimental data (Fig. 2), a version of the well-known general rule [18] is implemented here: a longer radical is mainly cleaved at the first stage (Eq. (3)).
Positive ion mass spectrum of O-decyl-O-pentylmethyl phosphonate [12].
We do not present the decomposition mechanism of phosphate triesters in the PI–EI–MS mode, because it is generally similar to Eqs. (3) and (4), except that one more stage is added, during which the last third alkoxy radical is cleaved, and a cation [P(OH)4]+ is formed [12].
Next, we consider the negative ion mass spectra of the studied compounds, obtained in the resonance electron capture mode. It should immediately be pointed out that the general (and main) direction is the fragmentation of all the studied phosphate esters (33 substances in total) of the same type. Regardless of the number of ester groups in the molecule, the O–C bond always breaks with cleavage of one of alkoxyl radicals from the molecular ion. In general, the corresponding reaction can be written as follows:

where X and Y are not only an alkyl radical, a halogen, a pseudohalogen (for example, a –CN group), an OCH3 group, or an amino group but also an –OR group.
Let us consider some features of the NI–REC mass spectra of the monoesters, diesters, and triesters. We studied seven O-alkyl dichlorophosphates, two O‑alkylalkyl fluorophosphonates, and one O,O-dialkyldialkyl pyrophosphonate. The NI–REC mass spectra of three monoesters of phosphoric acids: O-(2-ethyl)butyl dichlorophosphate, O-pinacolylmethyl fluorophosphonate (Soman), and O,O-diethyldimethyl pyrophosphonate are presented in Fig. 3. In the case of the first compound (Fig. 3a), the general and main direction of fragmentation leads to the cleavage of the alkoxyl (in this case, 2-ethylbutyl) radical with the formation of ion VIII. The same is observed during the fragmentation of two other compounds, the spectra of which are shown in Figs. 3b and 3c, respectively. The mass number of the resulting VIII ion is always the same as the mass of the molecule minus the mass of the alkoxyl radical (218 – 85 = 133 Da for the first compound and for all O-alkyl dichlorophosphates).
In the case of O-alkyl dichlorophosphates, to which the first compound belongs (Fig. 3a), a further partial decay of VIII proceeds with the removal of the chlorine atom. In this case, the negative charge is localized mainly at the formed phosphorus-containing ion IX but, partially, at the outgoing chlorine atom (ion X).

Similar results were obtained for all seven studied O-alkyl dichlorophosphates. The cluster structure of the IX peak confirms the presence of only one chlorine atom (Fig. 3a).
In the pyrophosphate mass spectrum (Fig. 3c), a rather intense signal of an ion with m/z 229 is observed, which formed by the removal of a hydrogen atom from a molecular ion. There is also a peak of an ion resulting from a simple breaking of one of the P–O bonds (m/z 123), the charge being naturally localized at the fragment containing three oxygen atoms.
The NI–REC mass spectra of 28 phosphate diesters and triesters were studied: ten O,O-dialkyl chlorophosphates, four O,O-dialkylalkyl phosphonates, one O,O-dialkyl fluorophosphate, eight O,O-dialkyl pyrophosphates, and five O,O,O-trialkyl phosphates. The characteristic mass spectra are shown in Fig. 4. The fragmentation of phosphate diesters and triesters obeys the general laws (Eq. (5)); that is, one of the alkoxyl radicals is detached from the negative molecular ion. A feature of the decomposition of diesters (OR)(OR')P(O)X, where R ≠ R', is that the cleavage of various alkoxyl radicals occurs in parallel and at different rates.
The group of diesters can also conditionally include O-alkyl-S-2-(N,N-dialkylamino)ethylalkyl thiophosphonates (V gases), the most toxic of all organophosphorus compounds, the structure of which is described by the general structural formula XI.

We have studied two such compounds. The fragmentation of V gases under conventional PI–EI–MS conditions is unique, and the resulting mass spectra are relatively uninformative. In a first approximation, the appearance of the mass spectra is determined by the structure of the radicals at the nitrogen atom (R, R'), while the effect of the radicals at the phosphorus atom (R'') and ester oxygen (R''') is rather insignificant [7, 9]. In this regard, difficulties arise both with group identification and with the determination of the structure of the alkyl radicals of the molecule, which is discussed in more detail below. However, in the NI–REC mode, these substances are very similar in their behavior to phosphate diesters, apparently because of the similarity of the electronic structure of oxygen and sulfur atoms. The analysis of the spectrum in Fig. 4c shows that the fragmentation of the molecular ions of such compounds is mainly reduced to two competing processes: the simple break of the O–C and S–C bonds with the cleavage of the corresponding neutral fragments. The charge is localized, as is usual for diesters, at a phosphorus-containing fragment [23]. Below is a mechanism of the fragmentation of O‑ethyl-S-2-(N,N-diisopropylamino)ethylmethyl thiophosphonate in the NI–REC–MS mode:

It is seen that an S-2-N,N-dialkylaminoethyl radical is preferably cleaved (Fig. 4c); that is, the “left” direction prevails in the above scheme. Thus, the above spectra altogether confirm the above general pattern: in all cases, the general decomposition process occurs, which consists of breaking the PO–C bond (in this case, the PS–C bond) in the molecule and cleavage of the O-alkyl (S-alkyl) radical. The mass number of the resulting ion (XII or XIII) is always the same as the mass of the molecule minus the mass of this radical. The uniformity of the fragmentation of phosphate esters makes NI–REC–MS a valuable and reliable method for identifying the compounds in question and determining essential characteristics of their molecular structure.
Group identification of phosphate monoesters. The group identification of some highly toxic organophosphorus compounds by PI–EI–MS is not reliable enough. The main reason is the insufficient number of group characteristic features in the PI–EI mass spectra of these compounds and the coincidence of many of these features with compounds of related classes. Let us consider this issue in more detail. Kireev et al. [7] proposed an algorithm for the group identification (classification) of highly toxic O-alkylalkyl fluorophosphonates by PI mass spectra. We restricted ourselves only to the main group of this class, containing almost all of its compounds, in which the number of carbon atoms in the alkoxyl radical is more than 1. We divide this group into four subgroups depending on the type of radical at the phosphorus atom (R is a methyl, ethyl, propyl, or isopropyl group in accordance with the requirements of the Convention). The starting point of group identification is the presence of a peak at m/z = 47 in the mass spectrum, belonging to the group P=O. After that, belonging to each of the four subgroups is determined by the set of characteristic peaks of phosphorus-containing ions [7].
For example, the assignment of the subgroup of O‑alkylmethyl fluorophosphonates with the general formula CH3P(O)(OR)F was made by the presence of peaks at m/z = 47.99 Da (peak V, almost always the main one) and a peak at m/z = 81. Similarly, the assignment of the studied substance to the subgroup of O-alkylethyl fluorophosphonates was done by the presence of peaks at m/z = 47, 113 (peak V) and 84, 95 Da. In many cases, this algorithm works, but not always. It is enough to look at the spectra in Fig. 1 to make sure how unreliable this assignment can be. First, there are no peaks in the spectra of both substances at m/z = 47 (they are always low intense); therefore, the entire identification procedure collapses. In addition, the characteristic peaks at m/z 81, 95, and 84 Da are also of low intensity. Second, the peaks at m/z 81, 95, and 84 Da may belong to peaks of the alkene or cycloalkene subspectrum considered above, which does not contain any information about the group membership of the substance (Eq. (2)) [20], rather than to the characteristic phosphorus-containing ions. For example, the peak at m/z = 84 Da in the spectrum of O-(1-methyl)pentylmethyl fluorophosphonate (Fig. 1b) refers to the intermediate alkene radical cation [C6H12]+• [21]. With an increase in the length of the alkoxyl radical, the peaks of characteristic phosphorus-containing ions are increasingly lost among the peaks of the alkene subspectrum or are superimposed on them [11]. The situation is aggravated to because of the strong dependence of the PI–EI mass spectra of O-alkylalkyl fluorophosphonates on the branching of the alkoxyl radical [11].
Thus, at present, we do not have a sufficiently reliable algorithm of group identification for highly toxic O-alkylalkyl fluorophosphonates (Sarin-type substances) using the positive-ion electron ionization mass spectra, because often, only one feature is reliable among the claimed 3–4 group features [7], that is, the intense peak of V with the structure [RP(OH)2F]+. Surely, this characteristic feature is ideal for group identification. First, the peak of V is very intense, and second, its mass number is uniquely related to the nearest environment of the phosphorus atom in the molecule and does not depend on the structure of alkoxyl radicals. In this respect, it surpasses even the chemical shift of 31P NMR. However, it is apparent that one attribute is not enough for reliable group identification.
The way out is the use of NI–REC mass spectra. In accordance with Eq. (5), the main direction of fragmentation leads to the formation of VIII, which in the case of O-alkylmethyl fluorophosphonates has the structure

and the mass number of 97 Da.
Ion VIII also has an ideal structure for group identification and is very close in structure to ion V; its mass number differs by only 2 Da. In addition, its peak is the most intense or the second most intense in the NI–REC mass spectra of phosphate esters.
Thus, based on the regularities found, it can be argued with a high probability, the main peaks (Table 1) are recorded in the mass spectra of the main group of O-alkylmethyl fluorophosphonates RP(O)(OR′)F; in the positive-ion mode, the peak of V can exceptionally rarely be second in intensity.
Based on the foregoing, we propose an approach to the group identification of O-alkylalkyl fluorophosphonates by chromatography–mass spectrometry data. First, a chromatography–mass spectral study of the sample is carried out in the PI–EI–MS mode. When a substance is detected in the mass spectrum of which there is an intense signal with a mass number of 99, 113, or 127 Da, the corresponding mass spectrum is recorded in the NI–REC mode. If in the spectrum of a substance with the same gas-chromatographic retention time, the mass number of the main peak is two units lower, then the substance is identified as a representative of the corresponding subgroup of O-alkylalkyl fluorophosphonates. In addition to high reliability, this method is much simpler than the sophisticated and multistage algorithm proposed in [7]. The accurate results were obtained for all O-alkylalkyl fluorophosphonates presented in the work. In using this approach, the range of compounds related to O-alkylalkyl fluorophosphonates should be expanded. This means that the radical R' can not only be alkyl or cycloalkyl but also contain a double bond, heteroatoms, or functional groups in the far-end positions. The proposed method is a means for reliable detection of the nearest environment of the phosphorus atom, which corresponds to that in the molecules of O-alkylalkyl fluorophosphonates.
Since the direction of fragmentation described by Eq. (5) is the main and predominant for all studied phosphate esters, similar approaches can be used for the group identification of other monoesters, for example, O-alkyl dichlorophosphates. In the PI mass spectra of these compounds, an intense peak of the [Cl2P(OH)2]+ ion (main for all seven studied substances) with a mass number of 135 Da is observed. Correspondingly, there is an intense peak of the [Cl2P(O)2]– ion in the NI–REC mass spectra with a mass number of 133 Da (Fig. 3a). In our opinion, because of the characteristic cluster structure, only one method is sufficient for the group identification of O-alkyl dichlorophosphates with the above caveat, PI–EI–MS or NI–REC–MS; for other monoesters, both methods must be used. Among such compounds, O-alkyl difluorophosphates, O-alkyl diiodophosphates, O-alkyl dicyanophosphates, O-alkyl alkyl cyanophosphonates, and many others can be mentioned.
It seems very promising to use the approach described above for the group identification of the most toxic organophosphorus substances, O-alkyl-S-2-(N,N-dialkylamino)ethylalkyl thiophosphonates (V gases). The methods for the group identification of these substances by PI mass spectra were proposed in [7] and [9]. Although the method developed in [9] is much simpler and takes less time, in principle, the situation does not change. Because of the lack of characteristic features in the PI–EI mass spectra, it is impossible or challenging to distinguish V gases from a wide range of compounds containing P–S–CH2CH2NR2 or P–O–CH2CH2NR2 fragments, for example, from their precursors with a phosphorus(III) atom [9]. For example, the factors of coincidence of the mass spectrum of O-ethyl-O-2-(N,N-diisopropylamino)ethylmethyl phosphonite

with the mass spectrum of the extremely toxic substance O-ethyl-S-2-(N,N-diisopropylamino)ethylmethyl thiophosphonate, found using the NIST information retrieval program [24], are 833 for the direct search and 860 for the reverse search (Similarity algorithm), that is, quite high. In practice, this circumstance can have far-reaching consequences if the relatively low-toxic precursors are mistaken for extremely toxic V gases. The use of NI–REC mass spectra eliminates, with a very high probability, this uncertainty. In fact, the peak with m/z = 139 (Fig. 4c), belonging to the 13th homological group, is the main peak in the NI–REC mass spectra of V gases [25, 26]. The appearance of an intense peak of this homological group in the NI–REC mass spectra of O-alkyl-O-2-(dialkylamino)ethyl methylphosphonites is improbable if we proceed from the pattern of fragmentation of phosphate esters that we found (Eq. (5)), from which no deviations have been found.
Determination of the number of carbon atoms in alkoxyl radicals of phosphate diesters. The task of determining the number of carbon atoms in the alkoxyl radicals of phosphate diesters and triesters is challenging and has no general solution. The situation is complicated by the fact that with increasing length of the alkoxyl radicals, signals from “internal” carbon atoms in the 13C NMR spectrum tend to merge (see, for example, spectra nos. 362, 455, and 480 in [27]).
Positive-ion mass spectrometry gives some opportunities for obtaining this kind of information. In [12], it was found that in the mass spectrum of dialkylalkyl phosphonates RP(O)(OR') (OR'') with R' ≠ R'', the signals from two different intermediate ions VIa and VIb are observed, the number of carbon atoms in which are easily determined by the equations
where m/z(VIa) and m/z(VIb) are the mass numbers of the first and second intermediate ions (Eq. (3)), n1 and n2 are the numbers of carbon atoms in the first and second alkoxyl radicals, respectively, and VII is the [RP(OH)3]+ ion (Eq. (4)). Apparently, according to the mass spectrum shown in Fig. 2, it is easy to find out that the first alkoxyl radical of the molecule contains five carbon atoms and the second alkoxyl radical contains ten carbon atoms.
Unfortunately, in the case of alkoxyl radicals branched at the α- and β-positions, the reliability of this method for determining the number of carbon atoms decreases sharply because of a decrease in the intensities of the peaks of VI to several percents relative to the peak of VII [12] even in the case of symmetrical compounds. Naturally, when alkoxyl radicals are different, these values are even smaller, and the overall spectral picture is complicated, which prevents the reliable determination of the desired characteristics.
The intensity of the peaks of VI is also very low in the PI mass spectra of other phosphate diesters. The PI mass spectra of O-butyl-O-propyl chlorophosphate [23] and O-heptyl-O-pentyl chlorophosphate are presented in Fig. 5. In the spectra of both compounds, the peaks of putative VIa ions (m/z 159 and 187 Da) are of low intensity, and the peaks of VIb ions (m/z 173 and 217 Da) are practically not observed. Because of this, the determination of the desired structural characteristics is impossible or very unreliable. It is noteworthy that the peak of VII (m/z = 117 Da) prevails over other peaks, as a result of which the PI mass spectra of the substances of this class are rather similar. In general, it can be noted that the PI mass spectra of dialkyl chlorophosphates do not contain enough data for reliable group identification and do not enable determining the number of carbon atoms in the alkoxyl radicals of the molecule.
Both of these problems can be solved using data from NI–REC mass spectra, for example, of O-butyl-O-propyl chlorophosphate (Fig. 4b). In accordance with Eq. (5), it has two peaks of VIIIa and VIIIb with the mass numbers of 157 and 171 Da, respectively. It is easy to verify that the mass numbers of VIIIa and VIIIb are two units less than that of VIa and VIb (Fig. 5) in the PI mass spectra; however, unlike the latter, their peaks are the main ones in the spectrum.
The following method is proposed for the simultaneous group identification of O,O-dialkyl chlorophosphates and determination of the number of carbon atoms in both alkoxyl radicals. First, a chromatography–positive-ion mass spectral study of the sample is carried out. After detecting the main peak with a characteristic cluster structure and a mass number of 117 Da in the spectrum of a substance, a chromatography–mass spectral study of the sample is performed in the NI–REC–MS mode. If the spectrum of a substance with the same gas chromatographic retention time contains an intense peak or two peaks with a mass number corresponding to the third homological group [26] and exceeding the mass number of V (that is, 143, 157, 171 Da, etc.), the belonging of the substance to the class of O,O-dialkyl chlorophosphates with acyclic alkoxyl radicals is concluded. Next, the number of carbon atoms in alkoxyl radicals is determined by the equation
where m/z(V) is the mass number of the main peak V in the PI mass spectrum.
For a substance, the spectrum of which is shown in Fig. 4b (O-propyl-O-butyl chlorophosphate), we obtain
If there is only one peak in the NI–REC mass spectrum, the number of carbon atoms in the alkoxyl radicals in the molecule is the same. After that, one can quickly determine the molecular weight of the substance.
Using the method described above, we obtained accurate results for all the five studied O,O-dialkyl chlorophosphates (O,O-dimethyl chlorophosphate, O,O-diethyl chlorophosphate, O-propyl-O-butyl chlorophosphate, O,O-dibutyl chlorophosphate, and O-propyl-O-(2-methyl)butyl chlorophosphate) and five O,O,O-trialkyl phosphates (O,O,O-triisopropyl phosphate, O,O-dipropyl-O-butyl phosphate, O,O-dibutyl-O-propyl phosphate, O,O,O-tributyl phosphate, and O-propyl-O-butyl-O-pentyl phosphate).
CONCLUSIONS
Thus, a combination of positive ion and negative ion resonance electron capture mass spectrometry is an effective way to the group identification and determination of essential structural characteristics of phosphate esters. The structural information obtained is often complicated if at all possible, to obtain using other instrumental methods. Successful optimization of the recording mode of the NI–REC mass spectra (see Experimental section) makes it possible to apply this method to the study of substances of other classes. Negative ion resonance electron capture mass spectrometry deserves wide implementation in analytical practice.
REFERENCES
Recommended Operating Procedures for Analysis in the Verification of Chemical Disarmament, Vanniner, P., Ed., Helsinki:VERIFIN, 2011.
Brodskii, E.S. and Kireev, A.F., J. Anal. Chem., 1997, vol. 52, no. 8, p. 801.
Lebedev, A.T., Lebedev, K.S., Myasoedov, B.F., Rybal’chenko, I.V., Sigeikin, G.I., and Suvorkin, V.N., Mass-Spektrom., 2006, vol. 3, no. 4, p. 277.
Ioffe, B.V., Kostikov, R.R., and Razin, V.V., Fizicheskie metody opredeleniya stroeniya organicheskikh soedinenii (Physical Methods for Determining the Structure of Organic Compounds), Moscow: Vysshaya Shkola, 1984.
Silverstein, R., Webster, F., and Kiemle, D., Spectrometric Identification of Organic Compounds, New York: Wiley, 2005, 7th ed.
The Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction, GE 9261926, Paris, 1993..
Kireev, A.F., Rybal’chenko, I.V., Savchuk, V.I., Suvorkin, V.N., Tipukhov, I.A., and Khamidi, B.A., J. Anal. Chem., 2002, vol. 57, no. 8, p. 708.
Note by the Director-General. Revised Standard Operation Procedure for Evaluation of the Results of OPCW Proficiency Tests, The Hague, OPCW, Technical Secretariat, 1998.
Morozik, Y.I., Kuchinskii, E.V., Galyaev, G.V., and Smirnov, A.O., J. Anal. Chem., 2013, vol. 68, no. 8, p. 680.
Rozengart, V.I. and Sherstobitov, O.E., Izbiratel’naya toksichnost’ fosfororganicheskikh insektoakaritsidov (Selective Toxicity of Organophosphorus Insectoacaricides), Leningrad: Nauka, 1972.
Lebedev, A.T., Morozik, Yu.I., Myasoedov, B.F., Rybal’chenko, I.V., and Fomenko, P.V., Mass-Spektrom., 2007, vol. 4, no. 4, p. 255.
Smirnov, A.O., Morozik, Yu.I., and Fomenko, P.V., Russ. J. Gen. Chem., 2009, vol. 79, no. 2, p. 203.
Zolotov, Yu.A., Rossiiskii vklad v analiticheskuyu khimiyu (Russian Contribution to Analytical Chemistry), Moscow: Lysenko, 2017.
Mazunov, V.A., Shchukin, P.V., Khatymov, R.V., and Muftakhov, M.V., Mass-Spektrom., 2006, vol. 3, no. 1, p. 11.
Muftakhov, M.V., Khatymova, L.Z., Khatymov, R.V., and Mazunov, V.A., Izv. Ufim. Nauchn. Tsentra Ross. Akad. Nauk, 2014, no. 4, p. 38.
Terent’ev, A.G., Ivanova, M.V., Khatymov, R.V., and Dudkin, A.V., RF Patent 158407, 2015, Byull. Izobret., 2015, no. 36.
Khvostenko, V.I., Mass-spektrometriya otritsatel’nykh ionov v organicheskoi khimii (Mass Spectrometry of Negative Ions in Organic Chemistry), Moscow: Nauka, 1981.
Lebedev, A.T., Mass-spektrometriya v organicheskoi khimii (Mass Spectrometry in Organic Chemistry), Moscow: Binom, 2003.
Kirby, A.J. and Warren, S.G., The Organic Chemistry of Phosphorus, Amsterdam: Elsevier, 1967.
Tkachuk, Yu.V., Morozik, Yu.I., and Dudkin, A.V., Russ. J. Gen. Chem., 2015, vol. 85, no. 3, p. 556.
Morozik, Yu.I., Dudkin, A.V., Rybal’chenko, I.V., and Naumov, A.R., J. Anal. Chem., 2018, vol. 73, no. 13, p. 1253.
OPCW Central Analytical Database, e-OCAD V. 2016, Heulweg, The Netherlands: Technical Secretariat of the Organization for the Prohobition of Chemical Weapons, 2014.
Terentyev, A.G., Morozik, Y.I., Dudkin, A.V., Smirnov, A.O., Galyaev, G.V., and Rybal’chenko, I.V., J. Anal. Chem., 2016, vol. 71, no. 13, p. 1266.
NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectral Library, ver. 2.2, June 10, 2014.
Ioffe, B.V., Russ. J. Org. Chem., 1996, vol. 32, no. 5, p. 623.
Zenkevich, I.G. and Ioffe, B.V., Interpretatsiya mass-spektrov organicheskikh soedinenii (Interpretation of the Mass Spectra of Organic Compounds), Ioffe, B.V., Ed., Leningrad: Khimiya, 1986.
Johnson Le Roy, F. and Jankowski, W.C., Carbon-13 NMR-Spectra: A Collection of Assigned, Coded and Indexed Spectra, New York: Wiley, 1980.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Terent’ev, A.G., Morozik, Y.I., Ivanova, M.V. et al. Identification and Determination of the Molecular Structure of Phosphate Esters by the Joint Application of Positive Ion and Negative Ion Electron Ionization Mass Spectrometry. J Anal Chem 75, 208–218 (2020). https://doi.org/10.1134/S1061934820020161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934820020161