Abstract
A new rapid and simple method for simultaneous determination of azoxystrobin, acetamiprid and metalaxyl in tomatoes has been developed and validated. The separation and quantification of investigated pesticides was performed by reverse-phase HPLC with diode-array detection. Pesticide residues were extracted with acetone and purified by liquid‒liquid extraction and solid-phase extraction. The best results were obtained using acetonitrile‒water (50 : 50, v/v) as a mobile phase at a flow rate of 1 mL/min and UV detection at 220 and 250 nm. The optimal analytical separation was achieved with a LiChrospher 60 RP-select B (250 × 4 mm, 5 µm) column. The developed method was validated for linearity, sensitivity (limits of detection and quantification), accuracy (recovery) and intraday precision (repeatability). This method may find further application in analyses of tomato samples contaminated with residues of investigated pesticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fruits and vegetables contain a lot of components with nutritional values and because of that are essential for human nutrition. One of the most consumable and popular vegetable with multiple uses is tomato (Lycopersicon esculentum Mill.) which belongs to the family Solanaceae. It has nutritive value and contains a substantial quantity of vitamins C and A, as well has a medicinal value [1]. In Macedonia, tomato cultivation covers a significant area due to its adaptability to a wide range of soils and climates. This vegetable is highly susceptible tomany insects, pests and diseases in the field. In order to improve quality, to increase yields and protect tomato from pests, farmers have no other option except to apply excessive amounts of different types of pesticides [2‒4]. Pesticides are sprayed directly on the plants which are able to persistent for a long time in vegetables [5]. At the same time, cultivation of tomato especially in green house conditions demands frequent application of a large number of pesticides to control a variety of insects, diseases and other pests [6]. Tomato is consumed as cooked and fresh manners, so residues that may remain on the harvested product are not removed by processing.
It is well known that pesticides are potentially harmful to the environment, and consequently to human beings, not only by direct contact, but also through the consumption of pesticide contaminated food. To prevent the presence of pesticide residue above the Maximum Residue Levels (MRLs), the time between pesticide application and harvest must be determined, since the pesticide decay time depends on crop type, pesticide and environmental conditions. With the intensive use of pesticides, residues may be accumulated at levels higher than the MRLs [7]. The MRLs of pesticides in tomato are set up by European Union Regulation (EC) No. 396/2005 [8]. There is a wide range of pesticides which are used for tomatoes protection and their residue contents must be accurately monitored to ensure food safety and safe consumption. The pesticides which are extensively used in tomato production in Macedonia are metalaxyl, acetamiprid and azoxystrobin. Concentrations of these pesticides in food commodities, such as tomatoes, are low. Determination of low concentrations of these pesticides in a complex tomato matrix requires an effective tomato extraction procedure, followed by final chromatographic determination in order to separate as much analyte as possible from the matrix interference substances [9]. The wide array of food matrices requires different sample preparation techniques for accurate and reproducible results. The accurate results often depend upon the sample preparation techniques which, in their turn, depend on food matrices. It has been estimated that the sample preparation step in most determinations consumes approximately 60–70% of the total time required for the analysis. It must be able to produce analytically accurate results and be economically efficient for routine analysis. In addition, it must be safe and easy to perform. The frequently used extraction methods are liquid‒liquid extraction (LLE) ad solid-phase extraction (SPE) [10–13]. In the literature there are a lot of papers describing different kinds of techniques and analytical methods for determination of pesticide residues in tomato, but the most commonly used are gas chromatography and HPLC [7, 14–19].
Pesticide residual analysis in tomato has become indispensable in order to guarantee the use of pesticides in field according to good agricultural practices (GAP) as well as to protect consumers’ health. This is the reason why there is a constant need to develop new sensitive, rapid and high-resolution analytical methods for determination and monitoring of pesticides in vegetables, especially in those which are mainly consumed fresh, such as tomatoes.
Hence, the objective of the present study was to perform simultaneous determination of azoxystrobin, acetamiprid and metalaxyl pesticide residues in tomato samples. In order to achieve that, the reverse-phase HPLC with diode-array detection (DAD) was developed and validated. The clean up procedure was performed by LLE and SPE. The developed method was tested on tomato samples collected from different regions of Macedonia. The chemical structures of acetamiprid (N-[(6-chloro-3-pyridyl)methyl]-N’-cyano-N-methyl-acetamidine), azoxy-strobin (Methyl (2E)-2-(2-{[6-(2-cyanophenoxy)pyrimidin-4-yl]oxy}phenyl)-3-metoxyacrylate) and metalaxyl (2-[(2,6-dimethylphenyl)-(2-metoxy-1-oxoethyl) ami-no]pro-panoic) are shown in the Scheme.

The structural formulas of acetamiprid (a), azoxystrobin (b) and metalaxyl (c).
EXPERIMENTAL
Chemicals and instruments. Chromatographic analysis was carried out using an Agilent 1260 Infinity Rapid Resolution Liquid Chromatography (RRLC) system equipped with a vacuum degasser (G1322A), a binary pump (G1312B), an autosampler (G1329B), a thermostatted column compartment (G1316A), a diode array detector (G1316B) and ChemStation software. Successful separation of investigated pesticides was achieved using a reverse-phase column LiChrospher 60 RP-select B (250 × 4 mm, 5 µm). An ultrasonic bath “Elma” was applied to ensure better dissolving of standard and sample solutions. Evaporation of the samples was performed using a vacuum rotary evaporator Buchi. A vacuum manifold Visiprep (Supelco, Sigma Aldrich) was used for solid phase extraction, while the samples were vortexed with IKA Vortex Genius 3 (Germany). The SPE procedure was performed on Supelclean ENVI-18 tubes, 6 mL, 0.5 g (Supelco, Sigma Aldrich, Germany).
Analytical standards of azoxystrobin (99.7%), acetamiprid (99.5%) and metalaxyl (99.8%) were produced by Sigma Aldrich (Germany). The used chemicals (acetonitrile, acetone, ethyl acetate and water) were produced by Sigma Aldrich (Germany). Formic acid with purity 98–100% was obtained from Merck. All chemicals and reagents were of HPLC grade.
Preparation of standard and working solutions. Standard solutions were prepared by dissolving exact amounts of analytical standards of azoxystrobin (3.6 mg), acetamiprid (3.1 mg) and metalaxyl (3.2 mg) in acetonitrile using 10 mL volumetric flasks. The solutions were degassed for 15 min with an ultrasonic bath. Working solutions were prepared daily by appropriate dilution of the standard solutions withacetonitrile‒water (50 : 50, v/v). All solutions were stored in a refrigerator in the dark at 4°C before use.
Preparation of sample solutions. Clean up is an essential step in analysis, greatly influencing the reliability and accuracy of result. Preparation of the samples for analysis was performed in few steps. First step in sample preparation procedure for HPLC determination was homogenization of tomato sample to obtain a uniform matrix. Tomato samples were collected randomly from different regions of Macedonia. After homogenization 100 g was transferred into a 250 mL conical flask with stopper and 150 mL of acetone was added into the flask. The conical flask was shaken for 60 min in the ultrasonic bath. The separation of the extracts from the solid part of the tomato matrix was performed by vacuum filtration using a Buchner funnel with double filter paper. Additional 20 mL of acetone was used for rinsing the conical flask and the filter paper. The obtained extract was transferred into a flask with round bottom and concentrated using a rotary evaporator under vacuum. Approximately 5 mL of extract was obtained and transferred into a separating funnel together with 100 mL of distilled water and 20 g of NaCl. The combined aqueous phases were extracted twice with 40 mL of ethyl acetate. The extracts were dried with sodium sulfate and then evaporated to dryness in a rotary evaporator. The residue obtained from the extract was dissolved in 10 mL mixture of water and methanol in the volume ratio of 9 : 1. The obtained solution was filtrated through Büuchner funnel with double filter paper under vacuum. A clean up step was employed to remove interfering matrix components. The SPE cartridges prior to use were conditioned with 3 mL of methanol and equilibrated using 3 mL of water at a flow rate of 2 mL/min. Subsequently, 9 mL of the samples were passed through the cartridges and then the tubes were washed with 3 mL of water. The drying process of the cartridges was carried out under a vacuum for 10 min. The elution of the cartridges was achieved with 3 mL of methanol‒ethyl acetate (75 : 25, v/v) and the eluates were evaporated to dryness in a nitrogen evaporator. The obtained residues were dissolved in 1 mL of methanol and filtered through 0.45 µm Iso-Disc PTFE syringe filters prior to analysis by HPLC. The blank samples were prepared in the same way but using tomatoes which were not treated with investigated pesticides.
Conditions of HPLC determination. After clean up, the samples were analyzed by HPLC. Several tests were carried out before setting up the best experimental separation of investigated pesticides. The best chromatographic separation was achieved using isocratic elution with a mobile phase consisting of acetonitrile‒water (50 : 50, v/v). The flow rate of the mobile phase was 1 mL/min at ambient column temperature. The investigated pesticides were detected at wavelengths of 220 and 250 nm with the injection volume of 30 μL. The run time of the analysis was 11 min. Identification of the pesticides in the samples was accomplished on the basis of their retention times and by comparison between the UV spectrum of the pesticides in the standard solutions and the UV spectrum of the detected peak in the sample. Quantification was made with a freshly prepared standard curve of the relevant pesticide.
RESULTS AND DISCUSSION
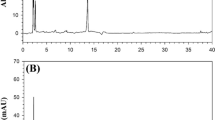
UV spectra of investigated pesticides. The investigated pesticides azoxystrobin, acetamiprid and metalaxyl have been extensively applied in tomato production in Macedonia. Because of the fact that tomato is widely used in every day diet, its intake may cause human exposure to the pesticide residues. Effective separation, identification and quantification of these pesticides were achieved using HPLC with DAD detection. The investigated pesticides showed the best absorbance in the UV region of the spectrum (Fig. 1). From the UV spectra (Fig. 1) it can be seen that the absorption maxima of azoxystrobin, acetamiprid and metalaxyl are placed at wavelengths around 220 and 250 nm. Further chromatographic determination of investigated pesticides was performed at these wavelengths. The obtained UV spectra were used for identification of investigated pesticides [20]. The values of purity index were up to 990 (whereas the maximum value is 1000), which confirmed that the chromatographic peaks were not affected by other compounds.
Chromatograms of investigated pesticides and tomato samples.The best separation of investigated pesticides with symmetrical peak shapes was achieved using a LiChrospher 60 RP-select B (250 × 4 mm, 5 µm) column under chromatographic conditions explained in experimental section. The obtained chromatogram of a standard mixture of acetamiprid, metalaxyl and azoxystrobin is presented in Fig. 2. It can be seen that the retention times for acetamiprid (I), metalaxyl (II) and azoxystrobin (III) are 3.4, 5.4 and 10.2 min, respectively. The retention time values were used for identification of investigated pesticides by comparison with those of the reference standards. The values of retention factors (k') were 2.44, 4.46 and 9.32 for acetamiprid, metalaxyl and azoxystrobin respectively. The separation factor (α) values were 1.829 and 2.087. The values of the retention factor below 20 and the separation factor above 1.2 indicated that the separation of investigated pesticides under the presented chromatographic conditions was successful [21]. The chromatograms of blank tomato sample and tomato sample spiked with investigated pesticides at concentration levels equal to MRLs for azoxystrobin, acetamiprid and metalaxyl are presented in Fig. 3. As it can be seen, the chromatograms were without interfering peaks in the areas of interest. The retention times of investigated pesticides at the spiked samples completely matched with those of the standard samples (see Fig. 2).
Method validation parameters. The developed method was validated for linearity, sensitivity (limits of detection and quantification), accuracy (recovery) and intraday precision (repeatability). Method validation was performed according to EU regulations [22, 23]. The linearity of the method was important to be tested in order to demonstrate a proportional relationship of the peak area/height versus analyte concentration over the working range. Five concentration levels were used from 80 to 120% of the concentration which corresponded to the MRLs of investigated pesticides. Acceptability of linearity data was evaluated by the correlation coefficient (R2) and intercept of the linear regression line obtained from plotting the peak area/height versus the concentration of the analyte. In the present study, linearity was studied in the range between 0.07 and 0.24 mg/kg for metalaxyl, 0.07–0.24 mg/kg for acetamiprid and 1.05–3.59 for azoxystrobin. The correlation coefficient obtained for the regression line demonstrated the good relationship between the peak area/height and the concentrations of investigated pesticides (Table 1). The quantitation was carried out according to calibration curves obtained with different concentrations of the standard solutions.
Sensitivity of the method was evaluated using the limit of detection (LOD) and limit of quantification (LOQ) values. Their determination is significant for the samples containing very low concentrations of target analyte. LOD is defined as the lowest amount of analyte that can be detected above baseline noise, typically, three times the noise level. LOQ is defined as the lowest amount of analyte which can be reproducibly quantified above the baseline noise, that gives S/N = 10. The values of LOQ were lower than MRLs set by European Union for analyzed pesticides [8]. According to the obtained results, the developed method allows to identify and quantify the pesticides in the tested concentration range (Table 1).
The accuracy of an analytical method is the closeness of test results obtained by that method to the true value. Accuracy is often determined by recovery studies which can be estimated by spiking known amounts of the analyte to test samples with further measurements by the method being evaluated. This was performed by the method of standard additions in tomato samples. In the present study spiked samples were prepared in triplicate at three different concentration levels over the range of MRL – 30%, MRL and MRL + 20%. The percent recoveries were then calculated. Recoveries obtained for tomato sample spiked with pesticides at three concentration levels are shown in Table 2. It is evident from their values that this method is accurate within the desired recovery range from 95.56 to 106.11%.
Precision is the measure of the degree of repeatability of an analytical method under normal operation and is normally expressed as the percent relative standard deviation for a statistically significant number of samples. In this study, precision of the method was evaluated through the repeatability of the method (intra-day precision) by assaying eight replicate (n = 8) injections of pesticide at the same concentration, during the same day, under the same experimental conditions. The obtained standard deviation (SD) and relative standard deviation (RSD) values demonstrated satisfactory precision (Table 3). A precision criterion for an assay method is that the instrument precision estimated from the RSD values will be ≤1%.
Tomato samples.The developed method was applied to the monitoring of azoxystrobin, acetamiprid and metalaxyl in tomato samples grown in different regions of Macedonia. For this purpose, six samples were randomly collected from agricultural fields in different locations. The obtained result showed that none of the pesticides analyzed were detected in any of the tested samples. The reason for that could be attributed to the storage period and conditions that influence the rate of degradation of investigated pesticides in tomatoes. According to literature data, under field conditions the half-life values for degradation of acetamiprid, metalaxyl and azoxystrobin were 1.04, 1.81 and 4.07 days in tomato [24]. Therefore, the tomato fruits should not be used for human consumption until the residues reach the MRLs. A protection average period of about 7 days for metalaxyl and acetamiprid and 20 days for azoxystrobin were predicted [25].
CONCLUSIONS
The development of effective analytical methods to monitor pesticide residues in vegetables is of significant importance. In this work we described a simple, rapid, low-cost and selective reverse-phase HPLC method for simultaneous determination of azoxystrobin, acetamiprid and metalaxyl widely used in tomato production.Effective separation and quantification was achieved in 11 min, at a flow rate of 1 mL/min using isocratic elution. The pesticide residues from the tomato samples were extracted with acetone and purified by both LLE and SPE. Analytical characteristics of the separation, such as linearity, sensitivity (LOD and LOQ), accuracy and repeatability were evaluated. According to the results presented in this study, reverse-phase HPLC method is sufficient and accurate enough for the detection and quantification of investigated pesticides in tomatoes. The developed method was applied for determination of pesticide residues in tomatoes. The obtained results indicated that analysed samples did not contain detectable residues of analyzed pesticides.
REFERENCES
Ware, G.W. and Whitacre, D.M., An Introduction to Insecticide, Willoughby, OH: MeisterPro, 2004, 4th ed.
Alyaseri, I.I., Ali, M.A.S., Ali, A.K.J., and Bahi, N.K., J. Agric. Sci. Technol. A, 2012, vol. 2, p. 65.
Kim, M.R., Na, M.A., Jung, W.Y., Kim, C.S., Sun, N.K., Seo, E.C., Lee, E.M., Pak, Y.G., Byun, J.A., Eom, J.H., Jung, R.S., and Lee, J.H., Korean J. Pestic. Sci., 2008, vol. 2, p. 323.
Goto, T., Ito, Y., Oka, H., Saito, I., Matsumoto, H., and Nakazawa, H., Anal. Chim. Acta, 2003, vol. 487, p. 201.
McCauley, L.A., Anger, W.K., Keifer, M., Langley, R., Robson, M.G., and Rohlman, D., Environ. Health Perspect., 2006, vol. 114, p. 953.
Kansaye, L., Zhang, J., Wu, H., Gao, B., and Zhang, X., J. Agric. Sci., 2013, vol. 5, p. 235.
Melo, A., Aguiar, A., Mansilha, C., Pinho, O., and Ferreira, I.M.P.L.V.O., Food Chem., 2012, vol. 130, p. 1090.
Regulation (EC) no. 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending, Council Directive 91/414/EEC Text with EEA relevance, 2005.
Cserháti, T. and Szőgyi, M., Eur. Chem. Bull., 2012, vol. 1, p. 58.
Żwir-Ferenc, A. and Biziuk, M., Pol. J. Environ. Stud., 2006, vol. 15, p. 677.
Fernandes, V.C., Domingues, V.F., Mateus, N., and Delerue-Matos, C., J. Chromatogr. Sci., 2011, vol. 49, p. 715.
Singh, S.K., Dwevedi, R., Deopa, P., and Krishna, V., J. Environ. Sci. Toxicol. Food Technol., 2014, vol. 8, p. 113.
Hailemariam, T. and Bekele, T., Int. J. Modern Chem. Appl. Sci., 2015, vol. 2, p. 92.
Tahir, M.U., Naik, S.M., Rehman, S., and Shahzad, M.A., Biomedica, 2009, vol. 25, p. 171.
Sundravadana, S., Alice, D., Samiyappan, R., and Kuttalam, S., J. Braz. Chem. Soc., 2008, vol. 19, p. 60.
Malhat, F.M., Arabian J. Chem., 2013, vol. 1, p. 32.
Obana, H., Okihashi, M., Akutsu, K., Kitagawa, Y., and Hori, S., J. Agric. Food Chem., 2002, vol. 50, p. 4464.
Lazić, S., Šunjka, D., Grahovac, N., Guzsvány, V., Bagiland, F., and Budakov, D., Pestic. Phytomed., 2012, vol. 27, p. 321.
Topuz, S., Özhan, G., and Alpertunga, B., Food Control, 2005, vol. 16, p. 87.
Jenkie, D.R., J. Liq. Chromatogr. Relat. Technol., 1996, vol. 19, p. 737.
Dong, M.W., Modern HPLC for Practicing Scientists, Hoboken, NJ: Wiley, 2006.
Document no. SANCO/12495/2011: Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed, 2011.
Guidance Document no. SANCO/825/00 on Pesticide Residue Analytical Methods, rev. 8.1, European Commission, Directorate General Health and Consumer Protection, 2010.
Ali, M., Shams, E.D., Azab, M.M., Tahany, R., El-Zaher, A., Zidan, Z.H.A., and Morsy, A.R., Am.-Eurasian J. Toxicol. Sci., 2012, vol. 4, p. 103.
Abdelhadi, A.I., Ashour, M.-B.A., Tohamy, M.R.A., and Ragheb, D.A., Zagazig J. Agric. Res., 2015, vol. 42, p. 1547.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Mirjana S. Jankulovska, Velkoska-Markovska, L., Petanovska-Ilievska, B. et al. Application of High Performance Liquid Chromatography for Determination of Metalaxyl, Acetamiprid and Azoxystrobine in Tomato Samples. J Anal Chem 74, 339–344 (2019). https://doi.org/10.1134/S1061934819040075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934819040075