Abstract
Products of the interaction of 1,1-dimethylhydrazine with nitrogen dioxide in an aqueous solution are characterized by high-resolution mass spectrometry with electrospray ionization. It was found that more than 200 compounds of CHO, CHN, and CHNO classes formed in the reaction; the main component is extremely dangerous N-nitrosodimethylamine. Among the products of the transformation of 1,1-dimethylhydrazine, N-nitrosodibutylamine is identified for the first time. The results obtained are of importance for understanding processes of the transformation of rocket fuel in surface and ground waters at the places of impact of spent rocket stages containing both the propellant and the oxidant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In view of the extremely high toxicity of rocket fuels based on 1,1-dimethylhydrazine (unsymmetrical dimethylhydrazine, UDMH) and its ability to oxidative transformations [1], much attention in an assessment of the ecological consequences of the space-rocket activity should be paid to problems of the identification and quantification of dangerous products of the oxidation of UDMH in environmental samples.
By now a considerable volume of information on the pathways of transformation of unsymmetrical dimethylhydrazine under the action of various oxidants, such as, air oxygen, hydrogen peroxide, potassium permanganate, hypochlorites, and Fenton’s reagent, has been accumulated in the literature [2]. It is known that the most important nitrogen-containing products of the degradation of UDMH are hydrazine, methylhydrazine, dimethylamine, 1-methyl-1H-1,2,4-triazole, tetramethyltetrazene, N,N-dimethylformamide, dimethylguanidine, formaldehyde dimethylhydrazone, formic acid dimethylhydrazide, and N-nitrosodimethylamine (NDMA) [1, 3–6].
In [7], using orbitrap high-resolution mass spectrometry, we showed that because of the proceeding radical reactions of the oxidation of alkylhydrazine, including those involving methyl radicals, the range of the products formed can include hundreds of compounds from various classes. Among them one can find very complex structures, and also numerous heterocyclic compounds (pyridines, pyrazines, triazoles, tetrazoles, etc.). The results obtained became a basis for the improvement of highly efficient methods for the control of the products of environmental transformations of rocket fuels by chromatography–mass spectrometry [8].
An important feature of environmental pollution in the case of an impact of spent stages of carrier rockets containing unburned fuel is the simultaneous spilling of UDMH fuel itself and the rocket oxidant (liquid nitrogen dioxide occurring mainly as the N2O4 dimer), used together with the fuel. We can expect that, in natural reservoirs and ground waters, dissolved nitrogen dioxide, forming nitric and nitrous acids, will also remain an active agent capable of forming specific products of the oxidation of unsymmetrical dimethylhydrazine. It is obvious that the probability of the formation of nitrosamines, possessing high carcinogenic, teratogenic, and mutagenic activity and related to superecotoxicants [9, 10] in this case will increase. For example, it is well known that one of the products of UDMH degradation, dimethylamine, readily reacts with nitrous acid solutions giving NDMA [11]. Despite this fact, we could not find in the literature any data on the mechanism and products of the reaction of alkylhydrazines with nitrogen oxides in aqueous solutions.
The aim of this study was to fill this gap by characterizing the products of oxidation of UDMH with nitrogen dioxide using the previously developed approaches based on the use of high-resolution mass spectrometry [7].
EXPERIMENTAL
Reagents and materials. 1,1-Dimethylhydrazine (98%, Aldrich, United States) was used without additional purification.
Nitrogen dioxide was synthesized by dissolving metallic copper (reagent grade, Neva-Reaktiv, Russia) in concentrated nitric acid (70%, high-purity grade, Khimmed, Russia). The reaction was conducted in a 250-mL round-bottom glass flask with ground joints and a splash head adapter, the released gas was delivered through a silicone pipe to a reception vessel (Polezhayev absorber) cooled by a mixture of ice with sodium chloride to a temperature of about –10°C. The obtained liquid NO2 (N2O4) was used immediately for the preparation of the reaction mixture.
Standard solutions of nitrosamines for calibrating the high-performance liquid chromatography/mass spectrometry (HPLC/MS) system and recording MS/MS spectra were prepared from a certified solution (EPA 8270, Supelco, United States) containing N-nitrosodimethylamine, N-nitrosomethylethylamine, N-nitrosodiethylamine, N-nitrosodi-N-propylamine, N-nitrosodibitylamine, N-nitrosodiphenylamine, nitrosomorpholine, 1-nitrosopyrrolidine, and 1-nitrosopiperidine with the concentration of each component 2000 µg/mL.
The solutions were prepared using type I ultrapure water, which was obtained using the Milli-Q system (Millipore, France).
The solvent for the injection of samples into the mass spectrometer was methanol (MS-grade, Merck, Germany).
Carrying out the reaction. A 10-mL portion of a freshly prepared 1,1-dimethylhydrazine solution of the concentration 1 mg/mL (16.7 mM) was placed in a glass test tube with a ground joint and a tight stopper and liquid nitrogen dioxide was added to the concentration 23 mg/mL (504 mM) with a precooled syringe, which gave its 30-fold excess with respect to the oxidized substance. The precise amount of the added NO2 was determined by the weight method. The obtained solution was thoroughly stirred and allowed to stand in a light-proof place at room temperature (18–20°C) with periodically (once an hour) sampling 100-μL portions for the study by mass spectrometry. The total duration of the experiment was 6 h.
Analysis by mass spectrometry. Mass spectra were recorded on a hybrid Q Exactive Plus mass spectrometer (Thermo Scientific, United States) with an orbitrap mass analyzer with the resolution 70 000 FWHM (for m/z 200) and an Ion Max HESI II ion source of electrospray ionization. The calibration of the mass scale was performed using a 10 mM solution of sodium formate by the peaks of the formed ion clusters [12] in the m/z range 50–750. Reaction mixture of the volume 1 μL was injected into the mass spectrometer with a flow (200 μL/min) of methanol using a chromatographic LC-30 Nexera system (Shimadzu, Japan). Mass spectra were recorded in the positive ion registration mode by averaging the results of not less than 10 scans and the subtraction of the solvent background. The peaks were marked using the threshold value of relative intensity equal to 0.1%.
We used the following parameters of the ion source, found in preliminary experiments: sheath gas pressure 30 psi; aux and sweep gas pressure 8 and 3 arbitrary units, respectively; desolvation line temperature 320°C; heater temperature 120°C; and radio-frequency voltage on the S-lens 55 arbitrary units.
The control of the mass spectrometer and data acquisition and processing were performed using the Xcalibur software (Thermo Scientific, United States).
In the determination of the elemental composition of compounds corresponding to the peaks in mass spectra, the admissible relative deviation of the calculated molecular mass from the measured one was accepted equal to 3 ppm (at m/z <90, admissible deviation was 5 ppm). We used the mMass [13], Microsoft Excel 2013, and Origin Pro 8.5 software for the exclusion of isotope peaks and the construction of diagrams.
The quantification of nitrosamines by chromatography–mass spectrometry was performed using a TripleTOF 5600+ quadrupole time-of-flight mass spectrometer (ABSciex, Canada) with a TurboIon Spray electrospray ionization source. Chromatographic separation was carried out on a Zorbax SB Aqua reversed-phase column (Agilent, United States) with enhanced retention of polar analytes [14, 15], 150 × 2.1, 1.8 μm, at the temperature 40°C using an LC-30 Nexera HPLC system (Shimadzu, Japan) which consisted of two LC-30AD pumps, a SIL-30A autosampler, and a CTO-20A column thermostat. The mobile phase was a mixture of water (A) and methanol (B) with an admixture of 0.1% HCOOH in the gradient mode. The program of gradient elution was as follows: 0–5 min 2% B, from 5 to 30 min increase of the percentage of B to 100%, 30–35 min 100% B. The flow rate of the mobile phase was 0.3 mL/min. The volume of the injected sample was 10 μL.
Detection was performed by the selected [M + H]+ ions of nitrosamines with m/z 75.0553 (N-nitrosodimethylamine), 89.0709 (N-nitrosomethylethylamine), 103.0866 (N-nitrosodiethylamine), 131.1179 (N-nitrosodi-N-propylamine), 159.1492 (N-nitrosodibitylamine), 199.0866 (N-nitrosodiphenylamine), 117.0659 (nitrosomorpholine), 115.0866 (1-nitrosopiperidine), 101.0709 (1-nitrosopyrrolidine). The width of the detected mass range was 5 mDa. For each of the specified precursor ions, we recorded MS/MS spectra at a collision energy of 30 eV. The collision gas was nitrogen. The system for quantitative analysis was calibrated by the areas of chromatographic peaks in the mode of selected ion ([M + H]+) monitoring using standard solutions of nitrosamines at five concentration levels in the range 100–50 000 μg/L (for N-nitrosodibitylamine and N-nitrosodiphenylamine, 1–1000 μg/L).
The results obtained were processed using the MultiQuant and LibraryView software (AB Sciex, Canada).
The gas phase over the reaction mixture was analyzed by gas chromatography/mass spectrometry using a QP-2010 Ultra GC/MS system (Shimadzu, Japan). Separation was conducted on an HP-5ms column, 30 m × 0.25 mm (Agilent, United States). Sampling was done with a gas-tight syringe directly over the surface of the solution under air.
RESULTS AND DISCUSSION
Characterization of reaction products. In contrast to reactions with such oxidants as oxygen or hydrogen peroxide, the reaction of UDMH with nitrogen dioxide even in a dilute aqueous solution proceeds extremely rapidly. Immediately after mixing the reagents, we observed the release of a small amount of gaseous products, which, according to GC/MS, consisted of nitrogen(I) oxide and, probably, molecular nitrogen. In 2 h after the beginning of the reaction, no considerable changes in the composition of the reaction mixture were observed.
The mass spectrum of the products formed (Fig. 1) contained about 200 peaks, the vast majority of which were of low relative intensity (<1%) and belonged to compounds bearing no nitrogen (Table 1). Among these, of the highest intensity were peaks of C4H8O and C4H6O2, which probably correspond to butanone and butandione. The formation of relatively complex organic compounds on the oxidation of UDMH provides additional confirmation for the radical mechanism of the process, accompanied by the generation of free alkyl radicals [7]. Fifty-nine peaks of nitrogen-containing compounds of CHN and CHNO classes were observed, but only seven of them were characterized by relative intensity higher than 1%. The elemental compositions of the corresponding compounds, the degree of unsaturation (ring and double bond equivalent, RDBE), and their presumable identification are presented in Table 2. In general, the composition of the transformation products of unsymmetrical dimethylhydrazine drastically differs from that obtained previously using hydrogen peroxide as an oxidant [7]. Thus, the mass spectrum of the obtained reaction products contained no intense peaks of nitrogen-containing heterocyclic compounds. An exception was only provided by the compound C8H14N2, forming the [M + H]+ ion with m/z 139.1233 and, in our assumption, relating to the class of imidazoles or pyrazoles. The absolute domination of the peak of the protonated C2H6N2O molecule (m/z 75.0552) in the mass spectrum corresponding, probably, to N-nitrosodimethylamine, attracted our attention as well as the total absence of the initial UDMH in the reaction mixture. The presence of the compound with the gross formula C2H6O2N2, which we assigned to N-nitrodimethylamine, can be, possibly, considered an indirect confirmation of the formation of NDMA. According to the published data [16], in the reaction of alkylamine with N2O4, the nitrosation and nitration processes occur simultaneously.
The obtained evidence for the formation of NDMA as the main product of the oxidation of 1,1- dimethylhydrazine is of great importance because of the extreme hazard of this compound and raises a question about its quantitative determination and also about a possibility of detection of other nitrosamines in a reaction mixture.
Identification and determination of nitrosamines. Because of a possibility of the presence of isomeric compounds with identical elemental compositions in a test solution, we used chromatographic separation with high-resolution mass-spectrometric detection for the determination of nitrosamines.
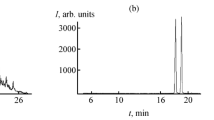
A comparison of the chromatograms of a model mixture of nitrosamines and a solution of reaction products (Fig. 2) pointed to the presence of NDMA and small amounts of N-nitrosodibitylamine in the last case. In addition to the coincidence of retention times (tR) and exact masses (found value m/z 159.1495, Δ = 1.9 ppm), an additional criterion of the reliability of identification is the coincidence of the recorded MS/MS spectra of these compounds with the corresponding standards (Fig. 3). For N-nitrosodibitylamine, a relatively bad agreement between the ratio of the peak intensities of product ions and the standard sample is explained by the very low concentration of the analyte, close to the lower limit of the analytical range, and, correspondingly, by the low signal intensity in the mass spectrum.
Comparison of MS/MS spectra of the products of reaction of 1,1-dimethylhydrazine with nitrogen dioxide (a, upper) m/z 75.0552 and tR = 2.2 min. and (b, upper) m/z 159.1495 and tR= 20.2 min. and of the standards, (a, lower) N-nitrosodimethylamine and (b, lower) N-nitrosodibitylamine. Collision energy 30 eV.
The results of the quantitative analysis of a solution of reaction products showed that the concentration of N-nitrosodimethylamine was equal to 32 ± 1 mg/L, which is 3.2% of the amount of the initial UDMH. N‑nitrosodibitylamine was present the mixture in the concentration 4 orders of magnitude lower and equal to 1.1 ± 0.2 μg/L. Taking into account that the limits of detection we found for the other nitrosamines are of the order 50 μg/L, they can also present among the products of UDMH oxidation in trace amounts, comparable with those for N-nitrosodibitylamine. An exception is provided by N-nitrosodiphenylamine, to which the sensitivity of the method is very high (limit of detection about 0.1 μg/L).
CONCLUSIONS
The interaction of 1,1-dimethylhydrazine with nitrogen dioxide in aqueous solution gives a specific range of oxidation products of CHO, CHN, and CHNO classes, the main of which is extremely dangerous N-nitrosodimethylamine. The presence of N‑nitrosodibitylamine among the products of UDMH transformation suggests a possibility of the formation of other highly toxic nitrosamines in surface and ground waters at the places of impact of spent stages of carrier rockets containing unburned fuel and an oxidant. This circumstance should be taken into account in the development of methods of analytical control and the assessment of the ecological consequences of the space-rocket activity.
REFERENCES
Kenessov, B., Alimzhanova, M., Sailaukhanuly, Y., Baimatova, N., Abilev, M., Batyrbekova, S., Carlsen, L., Tulegenov, A., and Nauryzbayev, M., Sci. Total Environ., 2012, vols. 427–428, p. 78.
Abilev, M.B., Kenessov, B.N., Batyrbekova, S.Y., and Grotenhuis, T., Chem. Bull. Kazakh Natl. Univ., 2015, vol. 77, no. 1, p. 20.
Rodin, I.A., Moskvin, D.N., Smolenkov, A.D., and Shpigun, O.A., Russ. J. Phys. Chem. A,2008, vol. 82, no. 6, p. 911.
Smolenkov, A.D., Rodin, I.A., Shpak, A.V., and Shpigun, O.A., Int. J. Environ. Anal. Chem., 2007, vol. 87, no. 5, p. 351.
Kosyakov, D.S., Ul’yanovskii, N.V., Bogolitsyn, K.G., and Shpigun, O.A., Int. J. Environ. Anal. Chem., 2014, vol. 94, p. 1254.
Kosyakov, D.S., Ul’yanovskii, N.V., Pokryshkin, S.A., Lakhmanov, D.E., and Shpigun, O.A., Int. J. Environ. Anal. Chem., 2015, vol. 95, p. 1321.
Ul’yanovskii, N.V., Kosyakov, D.S., Pikovskoi, I.I., and Khabarov, Yu.G., Chemosphere, 2017, vol. 174, p. 66.
Bakaikina, N.V., Kenessov, B., Ul’yanovskii, N.V., Kosyakov, D.S., Pokryshkin, S.A., Derbissalin, M., and Zhubatov, Z.K., Chromatographia, 2017, vol. 80, no. 6, p. 931.
Loeppky, R.N. and Michejda, C.J., Nitrosamines and Related N-Nitroso Compounds: Chemistry and Biochemistry, Washington, DC: Am. Chem. Soc., 1994.
Goto, Y., Matsuda, T., Ito, K., Huh, N.H., Thomale, J., Rajewsky, M.F., Hayatsu, H., and Negishi, T., Mutat. Res., 1999, vol. 425, no. 1, p. 125.
Xu, L., Sun, Z., Liu, Q.M., Liu, Y.D., Zhong, R.G., and Wu, F., J. Chem., 2013, vol. 2013, 818943.
Zhou, S. and Hamburger, M., Rapid Commun. Mass Spectrom., 1996, vol. 10, no. 7, p. 797.
Strohalm, M., Hassman, M., Kosata, B., and Kodicek, M., Rapid Commun. Mass Spectrom., 2008, vol. 22, no. 6, p. 905.
Ul’yanovskii, N.V., Kosyakov, D.S., Bogolitsyn, K.G., Falev, D.I., Smolenkov, A.D., and Shpigun, O.A., Moscow Univ. Chem. Bull. (Engl. Transl.), 2015, vol. 70, no. 2, p. 63.
Ripollе́s, C., Pitarch, E., Sancho, J.V., López, F.J., and Hernández, F., Anal. Chim. Acta, 2011, vol. 702, no. 1, p. 62.
Lv, C.L., Liu, Y.D., and Zhong, R., J. Phys. Chem. A, 2008, vol. 112, no. 30, p. 7098.
ACKNOWLEDGMENTS
This work was performed using instrumentation of the Core Facility Center “Arktika” under financial support of the Russian Foundation for Basic Research (grant No. 16-33-60159-mol-a-dk) and Ministry of Education and Science of the Russian Federation (state assignment, project no. 4.2518.2017/4.6).
We are grateful to S. A. Pokryshkin for his help in carrying out GC/MS analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Rykova
Rights and permissions
About this article
Cite this article
Ul’yanovskii, N.V., Kosyakov, D.S., Pikovskoi, I.I. et al. Study of the Products of Oxidation of 1,1-Dimethylhydrazine by Nitrogen Dioxide in an Aqueous Solution by High-Resolution Mass Spectrometry. J Anal Chem 73, 1223–1228 (2018). https://doi.org/10.1134/S1061934818130130
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934818130130