Abstract
Prospects for using synthetic and modified nanostructured mesoporous adsorbents for the adsorption, preconcentration determination, and chromatographic separation of biologically active substances (BAS) are shown. The need in the account of the effect of the structure of nanocomposites on their adsorption capacity is noted. The structuredness of mesoporous materials such as MCM-41 and the high rate of delivery and removal of biologically active substances (adsorption–desorption) upon contact with the material increase the efficiency of chromatographic columns. Procedures for the preconcentration and separation of phospholipids on silicon-containing materials, such as MCM-41, are developed. The number of stages in the isolation, preconcentration, and determination of BAS was decreased by increasing the recovery of the target component by ordered composites based on MCM-41. Dynamic preconcentration of phosphatidylcholine on mesoporous adsorbents is described using the dynamic model for convex adsorption isotherms. An increased efficiency of adsorption preconcentration on nanostructured adsorbents is achieved through the more complete use of the adsorption capacity while reducing the loss of the analyte under dynamic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The strategy for the determination of biologically active substances (BAS) in real samples depends on the type of the sample and the nature and structure of the test compound. The following stages of the investigation are distinguished: extraction of substances from the sample, purification of accompanying components, separation, and identification [1–3]. Adsorption preconcentration is an alternative to liquid–liquid extraction [1–3]. In sorption preconcentration, the selectivity of mesoporous materials can be changed by varying the nature and strength of adsorbate interactions both with the adsorbent and the eluent [4, 5]. The variety of materials applied in chromatography and solid-phase extraction as the most widely used methods for separation and preconcentration ensures the account of the nature of the analytes and the presence of complex matrices. Sorption preconcentration is widely used in the determination of inorganic and organic substances. An integrated approach to the determination of BAS in mixtures with similar structures of components and, consequently, adsorption properties supposes the selection of an effective adsorbent by varying the structure of the material, functional groups, and methods of their immobilization.
Silicas as adsorbents are the most often used materials for determination of BAS [1–3]. The ability to vary the affinity for the analyte, taking into account the availability of active sites and the sufficient degree of development of the surface (specific surface area), makes it promising to use structured materials, such as MCM-41, with a controlled pore size for the determination of biologically active substances, in particular, phospholipids.
The adsorption preconcentration of substances is carried out under batch or dynamic conditions [6]. In the batch mode, the adsorption system can be characterized by a distribution coefficient under the conditions of attaining an equilibrium, while in the dynamic regime, it is necessary to take into account adsorption kinetics and the completeness of using the adsorption capacity of the material. To increase the efficiency of adsorption, it is necessary to achieve the maximum possible concentration coefficient in a minimum of time [6–10]. The effect of some equilibrium and kinetic parameters significantly complicates the selection of the adsorbent and the optimal extraction conditions, for example, the height of the adsorbent layer, flow rate of the solution, etc. The conditions of the sorption preconcentration of BAS are often selected empirically or by analogy with extraction preconcentration techniques. Usually, the distribution coefficients of the adsorbate and the recovery are not determined [2]. This significantly complicates the selection of effective adsorption systems for the preconcentration of substances.
To extract and preconcentrate phospholipids, such adsorbents as silica, modified silica, and mesoporous materials of the MCM-41 type are used [11]. However, there is no data on the efficiency of the adsorption process for various silicon-containing materials.
The goal of this work was to evaluate and compare the efficiencies of the dynamic preconcentration of phosphatidylcholine on silicon-containing materials with different degrees of order.
EXPERIMENTAL
The test sample was individual phospholipid phosphatidylcholine (Sigma-Aldrich, Germany; the chemical structure is given below) containing 95% of the main substance, isolated from soya beans. Phosphatidylcholine is one of the most important biologically active substances in the body’s vital functions. It is an ester of glycerol and two fatty acids containing phosphoric acid residues and a nitrogenous base.

Chemical structure of phosphatidylcholine (bipolar form)
KSK silica (IMID, Russia), MCM-41 mesoporous nanostructured material (Süd-Chemie, Germany), and its analog synthesized by the procedure [12] (hereinafter referred to as MMC-1) were used as adsorbents. The MMC-1 material was synthesized from a reaction mixture consisting of an alcohol–ammonia solution containing tetraethoxysilane (TEOS), cetyltrimethylammonium bromide (CTAB), with a further hydrothermal treatment at 120°C with the continuous rotation of the autoclave [12]. The surface area, pore volume and pore size distribution were determined for all samples from nitrogen adsorption–desorption isotherms at 77 K; a relative pressure p/p0 was varied in the range of 10–5–0.99, using a Tristar II 3020 specific surface analyzer (Micromeritics, United States). Before each measurement, the samples were degassed under vacuum at 120°C for 20 h. Specific surface was calculated using the Brunauer–Emmett–Teller (BET) equation [13]; pore size distribution was determined by the Barrett–Joyner–Halenda (BJH) method [14, 15]. The surface and bulk properties of the adsorbents are presented in Table 1.
The adsorption of phospholipids by nanostructured materials under dynamic conditions was studied using a chromatographic unit, including a HPLC pump with a limiting working pressure of 30 MPa, a container for analytical solutions and eluents, regulators of the flow direction, columns (dcol = 0.5 cm, h = 2.5 cm) with the adsorbent, and the receiver flask.
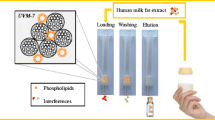
The flow rate of the solution was varied in the range 0.1–1.0 mL/min. To obtain elution curves, a hexane solution of phosphatidylcholine (c = 2.00 × 10–3 M) was passed through a column with an adsorbent (silica, MCM-41, or MMC-1), mads = 0.3500 g (Fig. 1). The volume of the eluate fraction was 3.00 mL. The breakthrough curves of phosphatidylcholine in adsorption on silicon-containing materials were plotted on the coordinates c/c0–V/V0. The amount of the adsorbed substance E ' (mmol/g) was calculated from the data of the breakthrough curves by the equation
where \(Q = \frac{{\left( {{{{s}}_{0}} - {s}} \right)V}}{m} \times 1000\) is the amount of adsorbed substance in each eluate fraction, mmol/g; c0 is the initial concentration of the solution, M; c is the equilibrium concentration of the solution, M; V is the volume of the passed solution, L; m is the weight of the adsorbent sample, g; and V0 is the volume of the adsorbent in the column, L.
The analyte was then desorbed with a 96% ethanol, passing through a column with the adsorbent (Fig. 1). The amount of the desorbed substance in the ith fraction of the eluate (mmol/g) was calculated by the equation
where c and V are the concentration (mM) and volume (L) of the eluate, respectively, and m is the adsorbent weight, g.
To quantify the phospholipids, a Shimadzu UV-1800 (Japan) spectrophotometer was used with λmax = 273 nm; optical path length was l = 1.00 cm. Hexane (HPLC grade, Aldrich) was used as the solvent.
RESULTS AND DISCUSSION
The adsorption chromatographic separation of BAS can be used for their extraction and preconcentration; it can also precede the determination of the target components by one of physicochemical methods. The use of adsorption methods of preconcentration and separation is promising at the stage of sample preparation; this decreases the number of stages and reduces the consumption of toxic solvents. Currently, the use of highly ordered mesoporous materials (such as MCM-41) as adsorbents for extracting BAS is being developed [12, 16, 17], but there is little data available. The features of the structure and surface density of silanol groups, the ability to adsorb solvent molecules and participate in the solvation and fixation of analytes enable us to take into account processes occurring during the separation of closely related compounds. It becomes possible to consider both the kinetic and thermodynamic parameters of the adsorption of substances.
To select the conditions for adsorption preconcentration, the following parameters should be optimized: the surface and bulk properties of the adsorbent, the volume of the column packed with the adsorbent, and the flow rate of the solution [6, 8].
A comparison of adsorption systems under dynamic conditions supposes the selection of the following criteria characterizing the effectiveness of such systems (Table 2): preconcentration factor (PF), the recovery of the target component (R, %), dynamic breakthrough capacity (E), fraction of the adsorbent capacity expended before the breakthrough (α), and preconcentration efficiency (PE).
In working with silica gels with a low adsorption capacity, only highly dilute solutions of BAS can be used, while for mesoporous materials with a highly developed surface, their significant adsorption capacity and the availability of adsorption sites contribute to the value of the preconcentration factor (PF) and the total adsorbent capacity (η) used at the end of preconcentration. MMC-1 (mesoporous analog of MCM-41) exhibits a 20- to 50-fold preconcentration of phospholipid in comparison with the initial concentration c0 of phosphatidylcholine in solution (Table 2). Desorption time becomes significantly shorter, which is associated with a rapid mass transfer of the analyte in the mesopore system of nanostructured adsorbents. The latter fact is especially important since the overall duration of the determination of BAS by their preliminary solid-phase extraction with subsequent determination by one of the physicochemical methods is decreased.
The highest values of the recovery of the analyte, preconcentration factor, and preconcentration efficiency are achieved in using nanostructured silicon-containing materials compared to silica (Table 2) and supercrosslinked polystyrenes (SPS). The adsorption capacity of polymeric materials, with the pore size and surface area, comparable with those of MCM-41 and its analogs, is much lower, which is due to the lack of nanostructuring in SPS [11]. Comparing the adsorption on silica gel and MCM-41, it should be noted that, having an identical silicate matrix, the distribution coefficients of these materials differ by more than twofold. This is explained by the ordered structure of MCM-41 in comparison with the chaotic distribution of pores and channels in silica. Low values of the dynamic breakthrough capacity and the fraction of the adsorbent capacity expended before the breakthrough for silica are associated, first of all, with a small specific surface area of the material. Under the conditions of analysis in columns packed with nanostructured mesoporous materials such as MCM-41, a higher preconcentration efficiency is achieved compared to conventional polymeric adsorbents and silicas (Table 2).
Adsorption under dynamic conditions and the breakthrough of the analyte in the elution curves are determined mainly by the kinetics of the adsorption process (film diffusion or intraparticle diffusion). Venitsianov [8] and Tsyzin et al. [18] noted that the models for the adsorption dynamics could be used to optimize the dynamic preconcentration process taking into account the kinetics and equilibrium. Adsorption dynamics is often described taking into account the linearity of the isotherms. In optimizing adsorption processes under dynamic conditions, significant difficulties arise in the case of selective adsorption with nonlinear isotherms.
Limitations imposed on solving optimization problems in dynamic preconcentration can be the fraction of the used capacity of the adsorption layer at the time of flow termination (η). It is shown [18] that the preconcentration factor
functionally depends on the fraction of the used capacity of the adsorption layer at the time of flow termination, that is,
It was noted in [18] that η could be calculated as a function of dimensionless variables of time (T) and coordinate (X). It is necessary to consider such systems for preconcentration, for which the distribution coefficient (Kd) would reach the maximum values, and the process kinetics would allow the adsorption capacity to be used to the fullest degree to reach the maximum value of η.
The second limitation caused by the characteristics of preconcentration is associated with the level of breakthrough, that is, the allowable loss of the target component, the fraction of the total amount of substance entering the adsorption layer, described by the equation [18]
where Qb is the amount of breakthrough, and Q0 is the amount of the preconcentrated component added to the adsorption layer (g).
Function χ can be calculated from the solution of equation of adsorption dynamics for one of the types of kinetics and a linear isotherm and can be represented in the form of theoretical lines [18].
In the case of mixed-diffusion kinetics, the intradiffusion scales of the arguments are usually selected [18]. In this case, a set of curves is plotted for each specific value of the Biot number. The scales of length and time are described by the equations
and the dimensionless variables of time and coordinate for intradiffusion limitation are
There are no data for intradiffusion kinetics in publications; therefore, we plotted functions χ and η for convex isotherms as isolines in the X–T plane (Fig. 2). A solution of the problem was found in dimensionless coordinates under the limitations indicated above. The optimum values of Xopt and Topt were determined by combining two graphs of η and χ in the X–T plane at the intersection of lines for given metrological requirements (at the breakthrough level of χ = 0.05 and χ = 0.1). The efficiency of preconcentration as an optimality criterion [18] is related to the flow rate υ and the distribution coefficient Kd and is determined by the equation
Analytical solutions to the problems of adsorption dynamics under diffusion kinetics are most conveniently obtained for linear and rectangular isotherms [19–21]. The latter version is close to the patterns of selective adsorption of nonpolar BAS (phospholipids) by mesoporous materials such as MCM-41. As soon as a particle comes into contact with a solution containing an adsorbate, the full saturation of the adsorbent surface is reached.
The adsorption option that takes into account both the mass transfer resistance in the case of external diffusion in the film and during diffusion inside the particle is the simplest in the mathematical description and adequate for the coincidence of the experimental and calculated elution curves. In this work, we used the diffusion parameter \(\delta = \frac{{{{k}_{f}}}}{{{{k}_{p}}}}\frac{{{{c}_{0}}}}{{\overline {{{q}_{0}}} }},\) where kf (s–1) is the mass transfer coefficient in the external solution, \({{k}_{p}} = \frac{{15\bar {D}}}{{{{R}^{2}}}}\) is the kinetic coefficient for diffusion in the solid phase, c0 (M) is the concentration of the substance in the solution, \({{\bar {q}}_{0}}\) (mol/dm3) is the concentration in the adsorbent phase at saturation, \(\bar {D}\) (cm2/s) is the effective coefficient of internal diffusion, and R (cm) is the radius of the adsorbent particle. Diffusion parameter δ (the Helferich criterion) is equivalent to the Biot criterion (Bi) and takes into account the contribution of the intradiffusion (\(\delta \to 0\)) or the external-diffusion (δ → ∞) kinetics of the process. It characterizes a possibility of changing the kinetic regime (from intradiffusion to external-diffusion and vice versa), which involves the use of poorly studied models in the mixed-diffusion dynamics of adsorption.
To plot the dependences on the dimensionless coordinates of length and time, we used the solution of the inverse problem of adsorption dynamics in the form of analytic solutions of the system of asymptotic equations (11) [20]. The presented system of equations (11) was obtained on the basis of the approach described by Yoshida et al. [20]; it takes into account the mixed-diffusion kinetics of the process for adsorption systems with convex isotherms. The solution of the inverse problem of adsorption dynamics and the calculation of the kinetic (diffusion) parameters of adsorption (\(\bar {D},\) δ) in this paper are based on the type of the elution curves using the system of asymptotic equations for mixed-diffusion control under \(\delta \to 1\,:\)
Asymptotic relations (11) were obtained by the method of characteristics using the analytical solutions given by Yoshida [20], with the equation z = x – υt in the absence of longitudinal effects. With a long adsorption time \((t \to \infty )\) and boundary conditions \(z \to \infty \,\,(x \to \infty ),\)\(n = 0,\)\(\bar {N} = 0;\)\(z \to - \infty \)\((x = 0,\,\,t \to \infty ),\) and \(n = {{n}_{0}},\,\,\bar {N} = {{N}_{\infty }}\), Eqs. (11) satisfactorily describe the adsorption of BAS in the columns for that external-diffusion and intradiffusion kinetics are more typical. Here n, n′, \(\bar {N}\), and \({{N}_{\infty }}\) are the molar fractions of the adsorbate in the bulk solution, at the interface of the adsorbent, in the adsorbent, and at \((t \to \infty )\), respectively.
It is essential for the selective adsorption of BAS that δ \( \gg \) 1 (for example, δ = 10); then, a significant decrease in the loss of the analyte, corresponding to χ = 0.05–0.1, can be achieved. Such adsorbents are promising for dynamic adsorption preconcentration with the full use of adsorption capacity up to 0.6–0.8. It is important to use materials with δ = 1.0 (for mixed-diffusion kinetics), for which the difference in the mass transfer rate in solution and in the adsorbent phase is minimal. For nanostructured materials, such as MCM-41, with minimal kinetic limitations, the adsorption capacity of the material is most fully utilized, and the maximum values of the concentration factor are reached.
The breakthrough curves (Fig. 3) represent multiparameter dependencies, taking into account both kinetic and equilibrium adsorption parameters of substances. The curves in Fig. 3 significantly change with the variation of the isotherm type (the distribution coefficient Kd). Phospholipids under dynamic conditions are adsorbed on nanostructured MCM-41 with comparable contributions of intradiffusion and external-diffusion kinetics at the Biot number Bi close to 4 [21], which corresponds to δ → 1.
The calculations carried out using the system of equations (11) show the highest efficiency of the dynamic preconcentration of BAS on nanostructured materials of the MCM-41 type with a narrow pore size distribution. Apparently, this is achieved due to the higher adsorption capacity of ordered materials in comparison with conventional silicas, higher availability of adsorption sites, and, as a result, more complete use of adsorption capacity.
The use of mesoporous adsorbents (MCM-41 and its synthetic analog MMC-1) as solid-phase carriers offers the achievement of significant extraction rates and increases the accuracy and rapidness of analysis, while decreasing the total costs of implementing the analytical process. The widening of the adsorption front of phosphatidylcholine, especially at c/c0 > 0.5 (Fig. 3), indicates a decrease in the efficiency of chromatographic separation in the transition from MCM-41 (and its analogue MMC-1) to silica, which can cause loss of the target component and the error in determination of the phospholipid during its adsorption preconcentration.
The desorption stage plays a vital role in the adsorption extraction and preconcentration of phosphatidylcholine, which ensures the evaluation of the degree of adsorbent regeneration and a possibility of its further use. Desorption was studied specifically for the hexane systems because the maximum adsorption capacity of silicon-containing materials with respect to phospholipids is obtained for extraction from hexane solutions. Desorption was carried out with 96% ethanol because phospholipids have a greater affinity for this solvent compared to less polar hexane.
The curves of phosphatidylcholine desorption (Fig. 4) from silicon-containing adsorbents differ in their fronting compared to conventional disordered adsorbents (for example, polymeric SPS [11]). The maximum concentration in the desorption curve compared with the initial concentration of phosphatidylcholine increases several times, which indicates the possibility of preconcentrating phospholipids using silicon-containing adsorbents. The preconcentration factor is determined as the ratio of the concentration of the component in the preconcentrate (cpreconc, M) to its concentration in the initial sample (c0, M) [7], that is,
The greatest efficiency of phosphatidylcholine desorption is observed when the first 2-mL portion of the solvent is passed through the adsorbent layer of the height h = 2.5 cm at the adsorbent weight m = 0.35 g. The preconcentration factor of the phospholipid increases in the series of silica < MCM-41 < MMC-1 (Table 3), which enables the use of the synthesized ordered MMC-1 material at the stages of sample preparation as an analog of preconcentration cartridges.
The volumes of ethanol required to achieve 80% and 95% desorption (V80% and V95%, respectively) and the preconcentration factors (PFs) are presented in Table 3. The desorption rate reaches 80% with the passage of 3–9 mL of ethanol and 95% with the passage to 20 mL of ethanol, which shows the economic use of solvents in the extraction and preconcentration of phospholipids on silicon-containing adsorbents.
CONCLUSIONS
The use of nanostructured mesoporous materials, such as MCM-41, in adsorption chromatographic processes increases the efficiency of the separation and preconcentration of biologically active substances. Increasing the availability of pore space in the bulk adsorbent ensures an increase in the proportion of adsorption sites involved in the retention of analytes (up to 80–90%) in comparison with conventional adsorbents (less than 30%) in the adsorption chromatographic determination of BAS. The most significant increase is in the dynamic breakthrough capacity, which enables the separation of the analytes similar in nature and adsorption properties.
REFERENCES
Khimiya privitykh poverkhnostnykh soedinenii (Chemistry of Grafted Surface Compounds), Lisichkin, G.V., Ed., Moscow: FIZMATLIT, 2003, p. 592.
Chu, B.S., Baharin, B.S., Che Man, Y.B., and Quek, S.Y., J. Food Eng., 2004, vol. 62, p. 105.
Olieman, C., Sedlick, E., and Voskamp, D., J. Chromatogr. A, 1981, vol. 207, p. 421.
Solid Phase Extraction Applications Guide and Bibliography. A Resourse for Sample Preparation Methods Development, McDonald, P.D. and Bouvier, E.S.P., Eds., Milford, MA: Waters, 1995.
Filippov, O.A., Tikhomirova, T.I., Badun, G.A., and Tsizin, G.I., J. Phys. Chem. A, 2003, vol. 77, no. 6, p. 981.
Zolotov, Yu.A., Sorbtsionnoe kontsentrirovanie mikrokomponentov iz rastvorov. Primenenie v neorganicheskom analize (Adsorption Preconcentration of Trace Components from Solutions: Application in Inorganic Analysis), Moscow: Nauka, 2007, p. 320.
Kokotov, Yu.A. and Pasechnik, V.A., Ravnovesie i kinetika ionnogo obmena (Equilibrium and Kinetics of Ion Exchange), Leningrad: Khimiya, 1970, p. 336.
Venitsianov, E.V. and Rubinshtein, R.N., Dinamika sorbtsii iz zhidkikh sred (Dynamics of Adsorption from Liquid Media), Moscow: Nauka, 1983, p. 237.
Dmitrienko, S.G., Kosyreva, O.A., Runov, V.K., and Zolotov, Yu.A., Mendeleev Commun., 1991, no. 2, p. 75.
Zolotov, Yu.A., Ross. Khim. Zh., 2005, vol. 49, no. 2, p. 6.
Sinyaeva, L.A., Karpov, S.I., Belanova, N.A., Roessner, F., and Selemenev, V.F., Russ. J. Phys. Chem. A, 2015, vol. 89, no. 12, p. 2278.
Belousov, O.V., Parfenov, V.A., Solov’ev, L.A., and Kirik, S.D., RF Patent 2287485, Byull. Izobret., 2006, no. 32.
Brunauer, S., Emmet, P.H., and Teller, E., J. Am. Chem. Soc., 1938, vol. 60, p. 309.
Barrett, E.P., Joyner, L.G., and Halenda, P.P., J. Am. Chem. Soc., 1951, vol. 73, p. 373.
Chen, J., Li, Q., Xu, R., and Xiao, F., Angew. Chem., Int. Ed. Engl., 1996, vol. 34, p. 2694.
Liu, F., Wang, J., Li, L., Shao, Y., Xu, Z., and Shourong, Z., J. Chem. Eng. Data, 2009, vol. 54, p. 3043.
Sinyaeva, L.A., Belanova, N.A., Karpov, S.I., and Selemenev, V.F., Sorbtsionnye Khromatogr. Protsessy, 2017, vol. 17, no. 2, p. 291.
Venitsianov, E.V., Kovalev, I.A., Tsizin, G.I., Teor. Prakt. Sorbtsionnykh Protsessov, 1998, vol. 23, p. 24.
Karpov, S.I. and Korabel’nikova, E.O., Russ. J. Phys. Chem. A, 2015, vol. 89, no. 6, p. 1096.
Yoschida, H., Kataoka, T., and Ruthven, D.M., Chem. Eng. Sci., 1984, vol. 39, no. 10, p. 1489.
Sinyaeva, L.A., Belanova, N.A., Karpov, S.I., Selemenev, V.F., and Roessner, F., Russ. J. Phys. Chem. A, 2016, vol. 90, no. 11, p. 2254.
ACKNOWLEDGMENTS
The work was supported by the Ministry of Education and Science of the Russian Federation in the framework of the State Task Assignment to Universities in the Field of Scientific Activity for 2017–2019, project no. 1.4539.2017/8.9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Sinyaeva, L.A., Belanova, N.A., Karpov, S.I. et al. Adsorption Preconcentration of Phosphatidylcholine on Nanostructured Mesoporous Materials under Dynamic Conditions. J Anal Chem 73, 847–854 (2018). https://doi.org/10.1134/S1061934818090149
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934818090149