Abstract
Nanoemulsions are promising disperse systems that can be used as carriers of hydrophobic drugs and biologically active compounds. In this work, nanoemulsions, which contain oleic acid as a dispersed phase and are stabilized with mixtures of nonionic surfactants, Tween 80/Span 80 and Tween 60/Span 60, have been studied. Nanoemulsions stabilized with Tween 80 and Span 80 are unstable. Dispersed phase droplets of such systems undergo intense flocculation and coalescence. Nanoemulsions stabilized with Tween 60 and Span 60 have appeared to be much more stable. The average particle diameter of the dispersed phase remains almost unchanged for a long time at 25°C, as well as in two cycles of heating to 60°C and subsequent cooling to 5°C. Microcalorimetry has been employed to determine the temperatures of phase transitions in such nanoemulsions. The obtained thermograms have two peaks due to melting of oleic acid and a mixed adsorption layer consisting of Tween 60 and Span 60 molecules. Since a solid shell is formed on the surface of oleic acid droplets, such systems can be considered to be nanocapsules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
Recently, interest has increased in lipid disperse systems, including nanoemulsions (NEs) [1, 2], which are promising to be used as carriers for transport of active compounds into the human body. As a component of the dispersed phase of an NE, oleic acid can be used, which is a biocompatible compound [3] exhibiting antimetastatic and anti-inflammatory properties [4]. In this case, dispersed phase droplets will not only provide the biologically active effect of oleic acid itself, but also will serve as containers for encapsulating hydrophobic drugs.
Oleic acid NEs stabilized with various surfactants have been studied. For example, NEs with an average droplet diameter of 72 nm were prepared by ultrasonic dispersing. At a weight ratio between Tween 80 and oleic acid of 2 : 1 and a dispersed phase fraction of 7 wt %, such NEs were stable for more than a year [5]. At the same time, in emulsions also prepared by ultrasonic dispersing and stabilized with Tween 80, the average diameter of the dispersed phase droplets increased from 147 to 175 nm in 28 days, when being stored at 25°C [6].
Emulsions stabilized with a mixture of nonionic surfactants, Tween 80 and lipophilic mannide monooleate taken in a volume ratio of 5.25 were produced by high-pressure homogenization. After the NEs were stored at 4°C for 6 weeks, the average droplet diameter of the dispersed phase (154 nm) remained unchanged. At 25 and 37°C, the diameter increased to 161–162 nm over the same period of time [7]. Oleic acid NEs stabilized with Tween 80 and small amounts of cetyltrimethylammonium bromide or a carbamate-based surfactant were more stable than those stabilized with Tween 80 alone [8].
In most publications on emulsions and NEs with oleic acid as a dispersed phase, the attention was focused on the prospects for their biomedical applications rather than the colloid-chemical properties of such disperse systems. NEs with active components possessing a wide spectrum of action were studied. For example, emulsions with a mixture of oleic and linoleic acids used as a dispersed phase and with encapsulated lutein were prepared by ultrasonic dispersing followed by solvent evaporation. Tween 20 was used to stabilize the NEs. The NEs with an average droplet diameter of 110 nm were stable at 4°C; however, at 25°C, the droplet size grew to 350 nm over 30 days [9].
NEs of oleic acid droplets 96 nm in diameter, which were loaded with antibacterial components, such as lactic acid, nisin, and lauryl alginate, were investigated. These NEs were produced by ultrasonic dispersing and stabilized with Tween 80 [10].
Emulsions stabilized with Tween 80 and PEG 400 were prepared by intense dispersing. In emulsions loaded with diacerein, the average droplet diameter was 174 nm [11]. When insulin was loaded, the droplet diameter was 460 nm [12]. When atorvastatin was encapsulated and Tween 80/PEG 400 and Tween 20/PEG 400 mixtures were used as stabilizers, the diameter of the dispersed phase droplets varied from 142 to 472 nm [13].
When using Tween 80 as a stabilizer, the average droplet size of the dispersed phase was 108 nm in NEs prepaed by ultrasonic dispersing and loaded with paclitaxel [14]. The combination of temperature phase inversion and ultrasonic dispersing methods led to the formation of NEs with a droplet size of 32–43 nm, when they were loaded with loratadine [15].
Emulsions, which contained a mixture of myrrh oil and oleic acid in the dispersed phase and were loaded with atorvastatin, were studied. Upon stabilization with Tween 40, Tween 60, or Tween 80, the diameter of the dispersed phase droplets varied from 130 to 274 nm [16]. In emulsions stabilized with Capmul MCM EP, Span 85, cetyltrimethylammonium bromide, and phosphatidic acid, oleic acid droplets were 123–167 nm in size. When such emulsions were exposed at 4, 25, and 37°C for 21 days, the droplet size remained almost unchanged [17].
In NEs stabilized with Cremophor RH 40/Transcutol HP mixtures and loaded with emodin, the droplet size was 10–30 nm [18], while stabilization with a Transcutol P/Labrasol mixture and loading with doxepin or imipramine gave oleic acid droplets 17–20 nm in size [19].
Emulsions and NEs with oleic acid were used to produce nano- and microcapsules with polyelectrolyte shells 81–117 nm in size [20, 21], while microcapsules with shells formed from hyaluronic acid dodecyl ester had a diameter of 365 nm [22]. Microcapsules with sizes of 265–710 nm were prepared from emulsions with droplets of a mixture of tricaprylin and oleic acid that were stabilized with Tween 80 and soy lecithin. Microcapsules loaded with hyperlongumin were produced by creating shells of chitosan or sodium alginate [23]. Tocopherol-containing microcapsules 233 nm in size were formed by creating shells from chitosan oleate [24].
Other lipid systems with oleic acid were also actively studied as drug carriers. For example, liposomes were obtained, the phospholipid bilayer of which included a complex of oleic acid and human α-lactoalbumin [25]. Nanostructured carriers were prepared from glyceryl behenate and oleic acid and loaded with astaxanthin [26], telmisartan-containing microcapsules were produced in the form of oleic acid emulgel coated with a chitosan shell [27], and lyotropic liquid crystals with solubilized oleic acid were formed from CETETH-10 and loaded with donepezil [28].
Thus, there are limited data in the literature on the colloid properties of NEs with oleic acid as a dispersed phase. In this work, we studied the influence of mixtures of nonionic surfactants, Tween 80/Span 80 and Tween 60/Span 60 mixtures, on the dispersity and stability of such NEs. It has been shown that, when an NE is stabilized with a mixture of Tween 60 and Span 60, a solid shell is formed on the surface of dispersed phase droplets; i.e., nanocapsules containing oleic acid are formed.
2 EXPERIMENTAL
2.1 Reagents and Materials
NEs were prepared using oleic acid (Stanchem Sp. z o.o. Przedsiębiorstwo Chemiczne), polyoxyethylene sorbitan monooleate (Tween 80, ≥95%), polyoxyethylene sorbitan monostearate (Tween 60), sorbitan monooleate (Span 80, ≥95%), sorbitan monostearate (Span 60, ≥95%), and NaCl (≥99.5%) produced by Sigma-Aldrich. The reagents were used as received. Bidistilled water was used to prepare NEs and nanocapsules.
2.2 Preparation of Nanoemulsions and Nanocapsules
NEs and nanocapsules were prepared by the method of temperature phase inversion. A vessel containing oleic acid (2.5 mL), a Tween 80/Span 80 or Tween 60/Span 60 mixture (1.25 mL), and an aqueous 0.15 M NaCl solution (6.25 mL) was heated to the phase inversion temperature. The mixture was then cooled in an ice bath under intense stirring. The molar ratio of the surfactants in the mixtures was 0.76 [29].
2.3 Study of Nanoemulsions and Nanocapsules
Particle sizes were measured with a Zetasizer Nano ZS laser analyzer (Malvern Instruments) operating at a detection angle of 173°. The analyzer was equipped with a He–Ne laser operating at a wavelength of 633 nm. Particle size distributions were determined using the Multiple Narrow Modes algorithm.
The stability of the NEs and nanocapsule dispersions to flocculation and sedimentation was determined with a MultiScan MS 20 instrument (DataPhysics Instruments GmbH). The intensities of light transmission and backscattering were measured by scanning a sample along its height with steps of 20 µm; the scanning velocity was 12.5 mm/s. The wavelength of monochromatic radiation was 880 nm. The data obtained were used to plot the dependences of light backscattering on sample height, i.e. on the distance from the bottom of a vessel containing an NE or a dispersion of nanocapsules, expressed in relative units from 0 to 1.
Melting and crystallization thermograms were obtained with a VP-DSC microcalorimeter (MicroCal Inc., United States) using cells made of a tantalum alloy. A nanocapsule-containing sample was diluted tenfold and placed into a measuring cell (0.5 mL); a 0.15 M NaCl solution (0.5 mL) was poured into a reference cell. The thermograms were recorded in two cycles of heating and cooling (5–60–5–60–5°C) at a scanning velocity of 60°C/h. The intervals between each heating and cooling were 15 min.
3 RESULTS AND DISCUSSION
3.1 Temperature-Related Characteristics of Nanoemulsions Stabilized with Tween 80/Span 80 and Tween 60/Span 60 Mixtures
NEs were produced using the temperature phase inversion method [30]. According to this method, a mixture of components was heated to a temperature above the phase inversion temperature. Upon stirring under such conditions, a water-in-oil (w/o) macroemulsion was formed. The system was then quenched in an ice bath under intense stirring. As a result of the phase inversion, the w/o macroemulsion was transformed into an oil-in-water (o/w) NE.
The temperature of phase inversion in an emulsion stabilized with a Tween 80/Span 80 or Tween 60/ Span 60 mixture was determined by conductometry. Figure 1 presents the temperature dependences for the electrical conductivities of the emulsions. A sharp change in electrical conductivity indicates the transformation of a w/o emulsion existing at a high temperature into an o/w emulsion at a lower temperature. The phase inversion occurs in narrow temperature ranges of 79–81°C and 76–78°C in the emulsions stabilized with Tween 80/Span 80 and Tween 60/Span 60 mixtures, respectively.
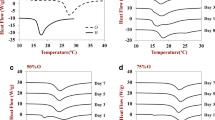
According to manufacturers’ data, the melting temperature of oleic acid is in a range of 13–14°C. Nonionic surfactants Tween 80 and Span 80 melt at ‒(21–25)°C and 1°C, respectively. The melting temperatures of Tween 60 and Span 60, which are equal to 24.5 and 52°C, were determined in our previous work [31]. Thus, at 25°C, all components in the NEs stabilized with Tween 80 and Span 80 were in the liquid state. The phase transition temperatures in the NEs stabilized with the Tween 60/Span 60 mixture were determined by microcalorimetry. Figure 2 shows the thermograms obtained in two cycles of heating and cooling within a temperature range of 5–60°C.
The first heating curve has two endothermic peaks at 14.3 and 41.3°C (Table 1). The peak at 14.3°C apparently corresponds to the melting of oleic acid. The second maximum at 41.3°C is due to the melting of the mixed adsorption layer composed of Tween 60 and Span 60 molecules [31]. During the second heating, these peaks remain in the curves, but their shapes are less pronounced. The first peak becomes wider and is extended from 17.5 to 27.0°C, whereas the second peak shifts toward lower temperatures down to 38.4°C.
The curve corresponding to the first cooling process exhibits exothermal peaks. The first of them (bifurcated) starts at 13.6°C and ends with a clearer minimum at 18.9°C. The second exothermal peak is clearly pronounced and corresponds to a temperature of 30.6°C. During the second cooling process, the shape of the curve remains almost unchanged, the shifts in the positions of the maxima are actually within the measurement error.
When preparing NEs stabilized with the Tween 60/Span 60 mixture, the systems were heated to a temperature exceeding the melting point of all components, and then cooled rapidly. A phase inversion occurred, and an adsorption layer of Tween 60 and Span 60 solidified. Oleic acid, which has surface-active properties, did not have time to be incorporated into the adsorption layer and solidified, being located in the cores of the droplets. The curve of the first heating reflects, in fact, the state of the emulsion after its preparation. At 25°C, the disperse system consisted of liquid oleic acid droplets surrounded by solidlike shells of surfactant molecules. Since the shell formed on the droplet surface in such NEs was solid, these systems will be referred to as nanocapsules below.
In the course of the microcalorimetric analysis, the systems were heated and cooled relatively slowly, at a rate of 1°C/min. Therefore, during the first cooling process, a certain amount of oleic acid had time to be incorporated into the adsorption layer, and a small part of nonionic surfactant molecules could penetrate into the cores of the nanocapsules. As a result, upon reheating, the first peak became more diffuse and shifted toward higher temperatures. The second maximum shifted toward lower temperatures. Such a redistribution of the components in the nanocapsules had a weak effect on the crystallization curves. Almost coincidence of their shapes during the first and second cooling processes indicates that the nanocapsules consisting of oleic acid cores and shells formed from the Tween 60/Span 60 mixture are stable in the studied temperature range, with its upper boundary exceeding the melting point of the shell covering the surface of the nanocapsules.
3.2 Stability of Nanoemulsions and Nanocapsules at 25°C and Elevated Temperatures
Since NEs are kinetically stable systems, the processes of flocculation, creaming, coalescence, and Ostwald ripening can occur in them. NEs stabilized with Tween 80 and Span 80 were unstable. The histograms for these NEs were bimodal after their preparation (Fig. 3). The first maximum corresponded to individual droplets of oleic acid 33–35 nm in diameter. The second maximum was, most likely, due to the formation of flocs [32] with sizes of 300–330 nm by dispersed phase droplets.
The size of individual droplets increased rapidly with time. The dependences presented in Fig. 4 (r is the droplet radius) were plotted to find out which process: the coalescence or the Ostwald ripening was dominating. The time dependence of r3 turned out to be nonlinear; therefore, the growth in the size of individual droplets with time was not due to the predominant effect of the Ostwald ripening [33]. The time dependence of 1/r2 could be approximated by a linear function with a rather high correlation coefficient (R2 = 0.9637). Therefore, it could be concluded that the increase in the size of individual droplets occurred as a result of their coalescence [32].
The light backscattering profile for an initial NE stabilized with the Tween 80/Span 80 mixture was an almost falt horizontal line, thereby indicating that the nanoemulsion structure was uniform. Quite soon, a zone of lower backscattering appeared in the lower part of the sample, with this zone expanding during the first several hours after the nanoemulsion preparation (Fig. 5). At the same time, a zone of higher light scattering was formed in the upper part of the sample. This indicated the occurrence of flocculation of oleic acid droplets and creaming of flocs, which gradually slowed down and almost stopped in 24 h. The light transmission of the samples was very low and remained unchanged along the height of the NE column for 24 h (the data have not been shown); i.e., the aqueous phase was not separated from the NE stabilized with Tween 80 and Span 80 for this time.
In contrast to the NEs stabilized with Tween 80 and Span 80, dispersions of nanocapsules coated with Tween 60 and Span 60 were stable. The size distribution of nanocapsules remained monodisperse for 28 days at 25°C, and their average diameter did not change significantly (Fig. 6a). The light backscattering profiles were represented by almost horizontal lines, and the light scattering value slightly diminished with time (Fig. 6b). This suggested that the nanocapsules flocculated very slowly in these dispersions, while the structure of the samples remained to be uniform along their heights.
When using lipid carriers containing encapsulated drugs, they must remain stable within a relatively wide temperature range. Therefore, the stability of nanocapsules, which contained oleic acid and were stabilized with Tween 60 and Span 60, was studied in a temperature range of 25–55°C. During heating and cooling, the average diameter of the nanocapsules varied within the measurement error (Fig. 7). This indicated the high stability of the nanocapsules with shells of Tween 60 and Span 60. Moreover, the nanocapsules remained stable in the temperature range exceeding the melting temperature of the shell.
4 CONCLUSIONS
Nanoemulsions containing oleic acid as a dispersed phase are promissing as carriers of drugs and biologically active compounds. For successful use, such disperse systems must be stable for a long time even at elevated temperatures. In this work, we studied NEs, which contained oleic acid as a dispersed phase and were stabilized with mixtures of nonionic surfactants, namely, Tween 80/Span 80 and Tween 60/Span 60 mixtures.
The temperatures of the phase transitions in NEs stabilized with Tween 60 and Span 60 were determined by microcalorimetry. During the first heating from 5 to 60°C, the thermogram showed two endothermic peaks at temperatures of 14.3 and 41.3°C due to the melting of oleic acid and the mixed adsorption layer consisting of Tween 60 and Span 60 molecules, respectively. Upon repeated heating, the first peak widened, while the second peak shifted toward lower temperatures, because a certain amount of oleic acid was incorporated into the adsorption layer and a small fraction of nonionic surfactant molecules could penetrate into the core of the nanocapsules.
NEs stabilized with Tween 80 and Span 80 were unstable. In such systems, dispersed phase droplets were subjected to intense flocculation and coalescence. Unlike NEs stabilized with Tween 80 and Span 80, nanocapsules coated with Tween 60/Span 60 shells were highly stable. Being stored for 28 days at 25°C, the nanocapsules retained their monomodal distribution, while their average diameter did not change significantly. In two cycles of heating to 60°C and subsequent cooling to 5°C, the average diameter of the nanocapsules varied within the measurement error. Thus, nanocapsules with Tween 60/Span 60 shells remained stable even at temperatures above the melting point of the shell.
REFERENCES
Zhang, R., Zhang, Z., and McClements, D.J., Colloids Surf., B, 2020, vol. 194, p. 111202.
Koroleva, M.Yu. and Yurtov, E.V., Russ. Chem. Rev., 2012, vol. 81, p. 21.
Cury-Boaventura, M.F., Pompéia, C., and Curi, R., Clin. Nutr., 2004, vol. 23, p. 721.
Rinaldi, F., Forte, J., Pontecorvi, G., Hanieh, P.N., Carè, A., Bellenghi, M., Tirelli, V., Ammendo-lia, M.G., Mattia, G., Marianecci, C., Puglisi, R., and Carafa, M., Int. J. Pharm., 2022, vol. 613, p. 121391.
Pérez-González, M.L.L., Rosa, C.H.G., Pérez-Hernández, G., and Beltrán, H.I., Colloids Surf., B, 2001, vol. 187, p. 110758.
Branco, I.G., Sen, K., and Rinaldi, C., Chem. Eng. Process., 2020, vol. 153, p. 107942.
Sravanthi, V., Pallavi, M.C.P., Bonam, S.R., Sathyabama, S., and Kumar, H.M.S., J. Drug Deliv. Sci. Technol., 2015, vol. 28, p. 56.
Mirgorodskaya, A.B., Koroleva, M.Yu., Kushnazarova, R.A., Mishchenko, E.V., Petrov, K.A., Lenina, O.A., Vyshtakalyuk, A.B., Voloshina, A.D., and Zakharova, L.Ya., Nanotechnology, 2021. https://doi.org/10.1088/1361-6528/ac467d
Toragall, V., Srirangam, P., Jayapala, N., and Vallikanana, B., Mater. Today Commun., 2021, vol. 28, p. 102522.
Sánchez-Ortega, I., Garía-Almendárez, B.E., Santos-López, E.M., Reyes-González, L.R., and Regalado, C., Food Hydrocoll., 2016, vol. 52, p. 906.
Chattopadhyay, H. and Datta, S., Mater. Today: Proc., 2018, vol. 5, p. 9690.
Chakraborty, T., Gupta, S., Nair, A., Chauhan, S., and Saini, V., J. Drug Deliv. Sci. Technol., 2021, vol. 64, p. 102601.
Shaker, D.S., Ishak, R.A.H., Elhuoni, M.A., and Ghoneim, A.M., Int. J. Pharm., 2020, vol. 578, p. 119073.
Cao, S.-q., Zhang, K.-y., Yan, X., and Ma, Y., Chin. Herbal Med., 2018, vol. 10, p. 310.
Sarheed, O., Shouqair, D., Ramesh, K.V.R.N.S., Khaleel, T., Amin, M., Boateng, J., and Drechsler, M, Int. J. Pharm., 2020, vol. 576, p. 118952.
Shehata, T.M., Khalil, H.E., Elsewedy, H.S., and Soliman, W.E., J. Drug Deliv. Sci. Technol., 2021, vol. 61, p. 102277.
Zaichik, S., Steinbring, C., Jelkmann, M., and Bernkop-Schnürch, A., Int. J. Pharm., 2020, vol. 581, p. 119299.
Shi, Y., Li, H., Li, J., Zhi, D., Zhang, X., Liu, H., Wang, H., and Li, H., J. Drug Deliv. Sci. Technol., 2015, vol. 27, p. 46.
Sandig, A.G., Campmany, A.C.C., Campos, F.F., Villena, M.J.M., and Naveros, B.C., Colloids Surf., B, 2013, vol. 103, p. 558.
Bazylińska, U., Skrzela, R., Szczepanowicz, K., Warszyński, P., and Wilk, K.A., Soft Matter, 2011, vol. 7, p. 6113.
Bazylińska, U., Warszyński, P., and Wilk, K.A., Colloids Surf., A, 2012, vol. 413, p. 266.
Janik-Hazuka, M., Szafraniec-Szczesny, J., Kamiński, K., Odrobińska, J., and Zapotoczny, S., Int. J. Biol. Macromol., 2020, vol. 164, p. 2000.
Giacone, D.V., Dartora, V.F.M.C., Matos, J.K.R., Passos, J.S., Miranda, D.A.G., Oliveira, E.A., Silveira, E.R., Costa-Lotufo, L.V., Maria-Engler, S.S., and Lopes, L.B., Int. J. Biol. Macromol., 2020, vol. 165, p. 1055.
Bonferoni, M.C., Riva, F., Invernizzi, A., Dellera, E., Sandri, G., Rossi, S., Marrubini, G., Bruni, G., Vigani, B., Caramella, C., and Ferrari, F., Eur. J. Pharm. Biopharm., 2018, vol. 123, p. 31.
Jung, S., Lee, S., Lee, H., Yoon, J., and Lee, E.K., Colloids Surf., B, 2020, vol. 146, p. 585.
Tamjidi, F., Shahedi, M., Varshosaz, J., and Nasirpour, A., Innovative Food Sci. Emerging Technol., 2014, vol. 26, p. 366.
Chin, L.Y., Tan, J.Y.P., Choudhury, H., Pandey, M., Sisinthy, S.P., and Gorain, B., J. Drug Deliv. Sci. Technol., 2021, vol. 62, p. 102341.
Souza, I.F.F., Santos, T.Q., Placido, R.V., Mangero-na, B.A., Carvalho, F.C., Boralli, V.B., Ruela, A.M., and Pereira, G.R., Colloids Surf., B, 2021, vol. 203, p. 111721.
Koroleva, M.Yu., Nagovitsina, T.Yu., and Yurtov, E.V., Mendeleev Commun., 2015, vol. 25, p. 389.
Izquierdo, P., Esquena, J., Tadros, T.F., Dederen, C., Garcia, M.J., Azemar, N., and Solans, C., Langmuir, 2002, vol. 18, p. 26.
Koroleva, M., Portnaya, I., Mischenko, E., Abutbul-Ionita, I., Kolik-Shmuel, L., and Danino, D., J. Colloid Interface Sci., 2022, vol. 610, p. 61.
Koroleva, M., Nagovitsina, T., and Yurtov, E., Phys. Chem. Chem. Phys., 2018, vol. 20, p. 10369.
Koroleva, M.Yu. and Yurtov, E.V., Russ. Chem. Rev., 2021, vol. 90, p. 293.
Funding
This work was supported by the Russian Foundation for Basic Research, project nos. 19-53-06014 and MOST 3-16496.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of inte-rest.
Rights and permissions
About this article
Cite this article
Mishchenko, E.V., Timofeeva, E.E., Artamonov, A.S. et al. Nanoemulsions and Nanocapsules with Oleic Acid. Colloid J 84, 64–70 (2022). https://doi.org/10.1134/S1061933X22010082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X22010082