Abstract
It has been shown that adsorption of 3-phenylpropenic (cinnamic), 3-(4-hydroxyphenyl)propenic (coumaric), and 3-(3-methoxy-4-hydroxyphenyl)propenic (ferulic) acids on the surface of highly dispersed alumina is characterized by L-type isotherms according to the Giles classification. The maximum adsorption values (~3.3 × 10–4 mol/g), as well as dissociation constants (pKCOOH = 4.5 ± 0.1), are almost equal for all acids, thereby suggesting that their molecules are identically bound to the adsorbent surface via carboxyl groups. The pH dependences of the adsorption of cinnamic, coumaric, and ferulic acids are described by dome-shaped curves, and the positions of their maxima in the pH scale coincide with the thermodynamic constants of carboxyl group dissociation. A linear correlation found between the adsorption values of 3-phenylpropenic acids and their hydrophobicity parameters log P indicates a noticeable contribution of hydrophobic interactions to the adsorption of the acids on the surface of highly dispersed alumina.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
At present, a necessity arises more and more often to isolate phenylpropenic acids (PPAs) of vegetable origin, in particular 3-phenylpropenic (cinnamic), 3‑(4-hydroxyphenyl)propenic (coumaric), and 3-(3-methoxy-4-hydroxyphenyl)propenic (ferulic) acids and identify them in native medicinal plants, drugs, foodstuffs, etc. [1, 2]. The increasing interest in natural compounds of this class is, primarily, due to their antioxidant activity, which ensures the high efficiency of treating and preventing various diseases [3, 4]. Moreover, their qualitative composition and quantitative content may be used as criteria of goodness for standardization of foodstuffs and beverages [5]. These 3-phenylpropenic acids represent structural units of lignin and are used as model compounds for studying the profound processing of vegetable biomass and lignin-containing materials with the purpose to produce biofuel and other products with desired consumer properties [6]. Since PPAs are specific indicators for the decomposition of organic substances contained in vegetable raw materials, it is of importance to analyze their content in process water.
3-Phenylpropenic acids are commonly concentrated and identified using the methods of sorption and high-performance liquid chromatography [7]. Chemically modified silica gels [8, 9], polystyrene–divinylbenzene copolymers [10, 11], and zeolites [12, 13] are used as sorbents. Solid phase extraction with the help of these sorbents is a long-term and multistage process, which involves large amounts of toxic organic solvents (methanol, acetone, and acetonitrile). In recent years, the principles of “green” chemistry are increasingly developed in scientific researches devoted to the elaboration of environmentally safe methods for the complex isolation of biologically active substances from vegetable raw materials [14] and the quantitative determination of these substances [15] with the help of environmentally friendly solvents (water or water–ethanol mixtures). Inorganic oxides, in particular, silica and alumina, may be the most promising industrial sorbents for isolating and concentrating individual PPAs or mixtures thereof from obtained aqueous and water–ethanol extracts.
For example, we have previously shown [16–18] that the values of adsorption of ferulic and caffeic acids from aqueous solutions by highly dispersed silica are very low (1–2 µmol/g), because it occurs on its surface ≡SiOН groups exclusively via hydrogen bonds involving carboxyl and/or phenol hydroxyl groups and, therefore, essentially depends on the competing solvation process. The value of PPA sorption on silica can be increased only with the use of inert toxic solvents having low polarity and electron-donor ability (hexane, carbon tetrachloride, chloroform, and dichloroethane).
Alumina may appear to be more efficient for the adsorption of acids from aqueous solutions, because, in contrast to silica, Al2O3 surface is positively charged in a wide pH range [19]. Indeed, it has been shown that 4,5-dihydroxybenzene-1,3-disulfonic acid disodium salt [20, 21] and salicylic and sulfosalicilic acids [21] are efficiently adsorbed on alumina primarily via the electrostatic interaction with its positively charged surface.
We have failed to find in the literature data on the adsorption of structurally similar 3-phenylpropenic acids, which contain carboxyl and phenol hydroxyl groups but have different reactivities in proton-transfer processes, as well as strengths and structures of hydrogen bonds in their associates and complexes with electron donors. These peculiarities suggest the possibility of different mechanisms for their binding to alumina surface.
The goal of this work is to study the regularities of adsorption and changes in the spectral characteristics of structurally analogous 3-phenylpropenic acids, namely, cinnamic, coumaric, and ferulic acids, on a highly dispersed alumina surface as depending on the chemical structure of the acids and the acidity of their aqueous solutions within a wide pH range.
EXPERIMENTAL
In this work, highly dispersed pyrogenic alumina Aeroxide Alu C with a particle size of 13 nm and a specific surface area of 100 m2/g (Evonik Degussa AG) was used in the form of an aqueous 0.2% dispersion (ionic strength of 0.01 N), which was prepared by stirring weighed portions of the sorbent (1.00 g) and NaCl (0.29 g) in water (500 mL) on a magnetic stirrer for 2 h.
Initial solutions of cinnamic, coumaric, and ferulic acids (Sigma-Aldrich) were prepared by dissolving weighed portions thereof in hot distilled water. The working solutions were prepared immediately before the experiments. Solutions of hydrochloric acid and an alkali were obtained from a concentrated HCl solution and NaOH (analytical grade). The acidity of the solutions before and after adsorption was controlled using a Hanna Instruments HI 221 universal ionometer equipped with a glass electrode.
Electronic spectra of PPA solutions were measured in quartz cells before and after mixing with alumina dispersions using a Specord M-40 spectrophotometer (Karl Zeiss Jena, Germany) equipped with a cuvette for turbid solutions. The effect of a background on the analytical signal was eliminated by the method heterochromatic extrapolation at two wavelengths [22].
PPA adsorption from aqueous solutions on the alumina surface was studied under static conditions. For this purpose, a solution (10 mL) of a studied acid with an appropriate concentration was mixed with the aqueous 0.2% dispersion (10 mL) of the adsorbent. A needed pH value was preset, and the mixture was stirred until equilibrium was established (2 h at 20°C). Then, the absorption spectrum (absorbance Аdisp) of the obtained Al2O3 dispersion was measured, because, at its concentration of 0.1%, it had a high sedimentation stability and a sufficient transparency. The equilibrium solution was separated by centrifugation (8000 rpm, 15 min) and its absorption spectrum А[С] was measured. Absorbances A s of cinnamic, coumaric, and ferulic acids adsorbed on alumina were determined as the following arithmetical differences:
A pure alumina dispersion subjected to all stages performed during the examination of the studied samples was used as a reference sample.
To determine the equilibrium PPA concentration, an HCl solution was added to the supernatant until a value of рН < 2.5–3.0 was reached (at which the light absorption of PPAs was almost independent of pH), the absorbance was measured, and the equilibrium acid concentration was calculated using corresponding molar extinction coefficient ε determined experimentally under the same conditions. For this purpose, the concentration dependences of the absorbance were preliminarily studied for the PPA solutions. The dependences were linearized by the least square method at correlation coefficients r 2 ≥ 0.9997. The found molar extinction coefficients (M–1 cm–1) were ε278 nm = 2.06 × 104 (cinnamic acid), ε310 nm = 1.97 × 104 (coumaric acid), and ε324 nm = 1.78 × 104 (ferulic acid).
Adsorption value а (mol/g) was calculated as а = (C – [C])V/m, where С and [C] are the initial and equilibrium concentrations of PPAs (M), respectively; V is the volume of a solution (L); and m is the mass of the adsorbent (g).
Adsorption isotherms were analyzed using the Langmuir equation
where a∞ is the maximum adsorption value (mol/g) and K is the equilibrium constant of the adsorption process (L/mol).
In the linearized form, Eq. (2) is as follows:
Being plotted in the [C]/a–[C] coordinates, the dependence is represented by a straight line, the parameters of which were used to calculate maximum adsorption value а∞ and equilibrium value K of the adsorption process.
RESULTS AND DISCUSSION
The acidity of a medium is an important factor of adsorption processes for compounds containing carboxyl and hydroxyl groups. When studying adsorption interactions in a heterogeneous alumina/3-phenylpropenic acid solution system, it is necessary to take into account all possible reactions occurring in an adsorbate solution and on the adsorbent surface. In aqueous solutions, monobasic cinnamic acid and dibasic coumaric and ferulic acids have molecular and/or deprotonated forms depending on solution pH. The distributions of these forms (Fig. 1, curves 1, 2) were calculated on the basis of the values of their first thermodynamic constants of dissociation (рКСООН = 4.5 ± 0.1) [23]. Since the dissociation constants of phenol hydroxyl groups in coumaric and ferulic acids are in a range of 8.7–9.5, actually no dianions are formed in the studied pH range of 2–7.
Distributions of (1) molecular and (2) deprotonated forms of PPAs in solutions and distributions of (3) protonated \( \equiv {\kern 1pt} {\text{AlOH}}_{2}^{ + }\), (4) nondissociated ≡AlOH and (5) deprotonated ≡AlO– groups on alumina surface [25, 26], as well as degrees of adsorption а/аmax of (6) cinnamic, (7) coumaric, and (8) ferulic acids, as depending on рН.
The surface of Al2O3 is amphoteric and, according to the notions of the complexation theory [24], its charge alters upon the interaction with protons of an aqueous solution. The equilibria of the protonation and deprotonation of surface ≡AlOH groups are described by equations
and characterized by constants pK1 and pK2, the values of which are, according to [25, 26], 6.8 and 9.2, respectively.
The obtained values of the constants were used to plot the distribution diagrams (Fig. 1) for relative fraction α of protonated \( \equiv {\kern 1pt} {\text{AlOH}}_{2}^{ + }\) (curve 3), nondissociated ≡AlOH (curve 4), and deprotonated ≡AlO– (curve 5) surface groups of alumina as depending on solution pH. It can be seen that the adsorption curves for cinnamic (6), coumaric (7), and ferulic (8) acids pass through maxima the positions of which in the pH scale nearly correspond to dissociation constants for the carboxyl groups of PPAs. The similar pattern of these dependences suggests that these acids are adsorbed via the same mechanism, which is possible only with involvement of −СООН groups. Adsorption of the PPAs on alumina grows as the pH increases from 2 to nearly 4.5. Within this pH range, protonated \( \equiv {\kern 1pt} {\text{AlOH}}_{2}^{ + }\) groups dominate on the adsorbent surface (curve 3), while the molecular forms of the acids prevail in a solution (curve 1). At рН > 4.5, the fraction of deprotonated carboxyl groups increases; however, in spite of this fact, the adsorption value decreases obviously due to a reduction in the amount of surface \( \equiv {\kern 1pt} {\text{AlOH}}_{2}^{ + }\) groups. At pH < 4, the contribution of hydrogen bonding between carbonyl groups of PPA molecules and neutral ≡AlOH groups on alumina surface (this contribution dominated in the case of PPA adsorption on cerium dioxide [23]) to the adsorption is relatively low, because the concentration of ≡AlOH groups on the alumina surface is too low in this pH range. As the pH increases above 4.5, the situation appears to be opposite: the concentration of neutral ≡AlOH groups increases, while the concentration of the molecular form of an acid in a solution decreases.
Additional information on the adsorption mechanism was obtained by comparatively analyzing the spectral characteristics of PPAs, in particular, positions of maxima λmax in their absorption spectra, which are sensitive markers for the acid-base equilibria of 3-phenylpropenic acids in solutions and on the alumina surface. It should be noted that the direct measurement of the absorbance of alumina dispersions in the presence of 3-phenylpropenic acids enables one to study the spectral characteristics of PPAs in solutions and on the adsorbent surface under the same conditions.
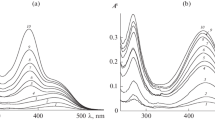
Figure 2 exemplifies the normalized absorption spectra of (a) cinnamic, (b) coumaric, and (c) ferulic acids adsorbed from solutions with pH 2 on the alumina surface (curves 1), the spectra of equilibrium solutions with pH 2 (curves 2), and PPA solutions with pH 6.5 (curves 3), in which the acids occur in the molecular and deprotonated forms, respectively.
As is seen in Fig. 2, when the adsorption is performed at рН < 4.5, analogous changes are observed in the absorption spectra of cinnamic and coumaric acids; namely, the spectra of the adsorbed acids undergo a hypsochromic shift relative to the spectra of their solutions. In the case of ferulic acid, the absorption maximum shifts from \(\lambda _{{{\text{max}}}}^{'} = 323\) nm to \(\lambda _{{{\text{max}}}}^{{"}} = 290\) nm (Fig. 2c, curve 1).
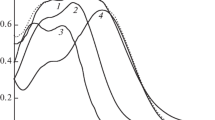
The influence of solution pH on wavelength difference Δλmax between the spectral maxima was studied for cinnamic (curve 1), coumaric (curve 2), and ferulic (curve 3) acids adsorbed on the surface of Al2O3 (\(\lambda _{{{\text{max}}}}^{{{{{\text{PPA}}} \mathord{\left/ {\vphantom {{{\text{PPA}}} {{\text{A}}{{{\text{l}}}_{{\text{2}}}}{{{\text{O}}}_{{\text{3}}}}}}} \right. \kern-0em} {{\text{A}}{{{\text{l}}}_{{\text{2}}}}{{{\text{O}}}_{{\text{3}}}}}}}}\)) and in corresponding solutions (\(\lambda _{{{\text{max}}}}^{{{\text{PPA}}}}\)) (Fig. 3).
Differences Δλmax between the wavelengths corresponding to the maxima in the spectra of (1) cinnamic, (2) coumaric, and (3) ferulic acids on Al2O3 surface (\(\lambda _{{{\text{max}}}}^{{{{{\text{PPA}}} \mathord{\left/ {\vphantom {{{\text{PPA}}} {{\text{A}}{{{\text{l}}}_{{\text{2}}}}{{{\text{O}}}_{{\text{3}}}}}}} \right. \kern-0em} {{\text{A}}{{{\text{l}}}_{{\text{2}}}}{{{\text{O}}}_{{\text{3}}}}}}}}\)) and in corresponding equilibrium solutions (\(\lambda _{{{\text{max}}}}^{{{\text{PPA}}}}\)) as functions of pH.
Analysis of the data presented in Fig. 3 has shown that, at рН < pKСООН, the λmax values of adsorbed PPAs correspond to λmax for these acids in solutions at markedly higher pH values. This indicates a larger fraction of a deprotonated acid on Al2O3 surface as compared with the solution of this acid with the same acidity. The maximum deviation of \(\lambda _{{{\text{max}}}}^{{{{{\text{PPA}}} \mathord{\left/ {\vphantom {{{\text{PPA}}} {{\text{A}}{{{\text{l}}}_{{\text{2}}}}{{{\text{O}}}_{{\text{3}}}}}}} \right. \kern-0em} {{\text{A}}{{{\text{l}}}_{{\text{2}}}}{{{\text{O}}}_{{\text{3}}}}}}}}\) from \(\lambda _{{{\text{max}}}}^{{{\text{PPA}}}}\) in the solutions is observed at pH 2–3, i.e., when protonated \( \equiv {\kern 1pt} {\text{AlOH}}_{2}^{ + }\) groups prevail on the adsorbent surface (Fig. 1, curve 3), while the molecular forms of the acids predominate in their solutions (Fig. 1, curve 1). It may be assumed that adsorption at pH 2–3.5 is accompanied by polarization of the bonds in PPA molecules (Scheme 1), which facilitates the detachment of protons from carboxyl groups and the bonding of anions with protonated surface \( \equiv {\kern 1pt} {\text{AlOH}}_{2}^{ + }\) groups of alumina (Scheme 2).

As рН grows above 3, the content of an acid in the anionic form increases in a solution, while shift Δλmax decreases down to zero at pH ≈ pKCOOH. At рН > 4.5, the values of λmax in the spectra of the PPAs in solutions and on alumina surface almost coincide with each other; i.e., no polarizing action of the sorbent surface groups on the adsorbed acids takes place, because the PPAs occur predominantly in the deprotonated form.
The quantitative parameters of PPA adsorption were studied at pH values corresponding to the maximum extraction of the acids. Figure 4 presents the adsorption isotherms obtained for cinnamic (curve 1), coumaric (curve 2), and ferulic (curve 3) acids at рН 4.5. The maximum PPA concentration in an initial solution was not higher than 1 × 10–3 M due to the limited solubility of these acids in water.
It follows from Fig. 4 that the adsorption isotherms of the acids are of the L type according to the Giles classification [27] and are linearized in the coordinates of the Langmuir equation. The corresponding calculated maximum adsorption values, equilibrium constants of the adsorption process, and correlation coefficients are presented in Table 1. It is seen that calculated maximum adsorption values а∞ of cinnamic, coumaric, and ferulic acids almost coincide with each other. This is possible only when they are bound to the surface by the same mechanism, namely, via carboxyl groups, the acidity of which does not depend on the presence of substituents (hydroxyl or methoxy groups) in benzene rings. This agrees with the above-presented data obtained when studying the effect of pH on PPA adsorption (Fig. 1).
It is worth noting that the studied PPAs are characterized by the presence of both hydrophobic (ethylene and phenyl radicals) and hydrophilic (carboxyl groups and phenyl hydroxyl groups) fragments in their molecules. Therefore, they may be bound to alumina surface due to electrostatic interactions and hydrogen bonding (hydrophilic contribution), as well as polarization and dispersion forces applied to aryl fragments (hydrophobic contribution). A generally accepted hydrophobicity parameter, which characterizes the lyophilic properties of substances, is their partition coefficient P in a water/n-octanol system (log P). The log P values presented in the literature [28] for cinnamic (log P = 2.13), coumaric (log P = 1.79), and ferulic (log P = 1.51) acids lead us to attribute these substances to moderately hydrophobic compounds (1 < log P < 3).
Figure 5 shows the dependence of the logarithmic values of the adsorption of cinnamic, coumaric, and ferulic acids by alumina on their log P parameters at the same equilibrium concentration of the PPAs in the solutions. The obtained dependence is linear (R = 0.980), thus suggesting the existence of a correlation between these two parameters, i.e., a noticeable contribution of hydrophobic interactions to the adsorption.
Thus, regularities have been determined for the adsorption of 3-phenylpropenic acids on the surface of highly dispersed alumina as depending on the chemical structure of the acids and the acidity of aqueous dispersions in a wide range of physiological pH values.
The coincidence of the maximum adsorption values for all studied PPAs and the positions of the maxima in the pH dependences of adsorption coinciding with the thermodynamic dissociation constants of the carboxyl groups of the acids suggest an identical binding of adsorbate molecules to the alumina surface predominantly via carboxyl groups.
The found regularities and parameters of adsorption make it possible to elaborate practical recommendations for the use of alumina as a sorbent for the isolation, concentration, and identification of 3-phenylpropenic acids.
REFERENCES
El-Seedi, H.R., El-Said, A.M., Khalifa, S.A., et al., J. Agric. Food Chem., 2012, vol. 60, p. 10877.
Balasundram, N., Sundram, K., and Samman, S., Food Chem., 2006, vol. 99, p. 191.
Razzaghi-Asl, N., Garrido, J., Khazraei, H., et al., Curr. Med. Chem., 2013, vol. 20, p. 4436.
Yashin, Ya.I., Ryzhnev, V.Yu., Yashin, A.Ya., and Chernousova, N.I., Prirodnye antioksidanty. Soderzhanie v pishchevykh produktakh i vliyanie ikh na zdo-rov’e i starenie cheloveka (Natural Antioxidants. Content in Food and Their Impact on Human Health and Aging), Moscow: TransLit, 2009.
Yashin, A.Ya., Sorbts. Khromatogr. Protsessy, 2014, vol. 14, p. 419.
Fizicheskaya khimiya lignina (Physical Chemistry of Lignin), Bogolitsyn, K.G. and Lunin, V.V., Eds., Arkhangelsk: Arkhang. Gos. Tekh. Univ., 2009.
Arceusz, A., Wesolowski, M., and Konieczynski, P., Nat. Prod. Commun., 2013, vol. 8, p. 1821.
Zeng, H., Liu, Z., Zhao, S., et al., J. Sep. Sci., 2016, vol. 39, p. 3806.
Shil’ko, E.A., Milevskaya, V.V., Temerdashev, Z.A., and Kiseleva, N.V., Anal. Kontrol, 2018, vol. 22, p. 303.
Silva, M., Castellanos, L., and Ottens, M., Ind. Eng. Chem. Res., 2018, vol. 57, p. 5359.
Dávila-Guzman, N.E., Cerino-Córdova, F.J., Diaz-Flores, P.E., et al., Chem. Eng. J., 2012, vol. 183, p. 112.
Simon, V., Thuret, A., Candy, L., et al., Chem. Eng. J., 2015, vol. 280, p. 748.
Thiel, A., Tippkötter, N., Suck, K., et al., Eng. Life Sci., 2013, vol. 13, p. 239.
Anastas, P.T. and Warner, J.C., Green Chemistry: Theory and Practice, Oxford: Oxford Univ. Press, 2000.
Eldin, A.B., Ismaiel, O.A., Hassan, W.E., and Shalaby, A.A., J. Anal. Chem., 2016, vol. 71, p. 861.
Pogorelyi, V.K., Barvinchenko, V.N., Pakhlov, E.M., and Smirnova, O.V., Colloid J., 2005, vol. 67, p. 172.
Dovbii, O.A., Kazakova, O.A., and Lipkovskaya, N.A., Colloid J., 2006, vol. 68, p. 707.
Pogorelyi, V.K., Kazakova, O.A., Barvinchenko, V.N., et al., Colloid J., 2007, vol. 69, p. 203.
Chukin, G.D., Stroenie oksida alyuminiya i katalizato-rov gidroobesserivaniya. Mekhanizmy reaktsii (Structure of Aluminum Oxide and Hydrodesulfurization Catalysts), Moscow: Printa, 2010.
Tikhomirova, T.I., Kubyshev, S.S, and Ivanov, A.V., Russ. J. Phys. Chem. A, 2013, vol. 87, p. 1357.
Jiang, L., Gao, L., and Liu, Y., Colloids Surf. A, 2002, vol. 211, p. 165.
Bernshtein, I.Ya. and Kaminskii, Yu.L., Spektrofotometricheskii analiz v organicheskoi khimii (Spectrophotometric Analysis in Organic Chemistry), Leningrad: Khimiya, 1986.
Barvinchenko, V.N., Lipkovskaya, N.A., Kulik, T.V., and Kartel’, N.T., Colloid J., 2019, vol. 81, p. 1.
Westall, J.C. and Hohl, H., Adv. Colloid Interface Sci., 1980, vol. 12, p. 265.
Tombácz, E. and Szekeres, M., Langmuir, 2001, vol. 17, p. 1411.
Tombácz, E., Szekeres, M., and Klumpp, E., Langmuir, 2001, vol. 17, p. 1420.
Giles, C.H., MacEwan, T.H., Nakhwa, S.N., and Smith, D., J. Chem. Soc., 1960, vol. 10, p. 3973.
Chemical Information Resources from the National Library of Medicine. http://sis.nlm.nih.gov/chemical.html.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Barvinchenko, V.N., Lipkovskaya, N.A. Adsorption of Natural 3-Phenylpropenic Acids on Highly Dispersed Alumina Surface. Colloid J 83, 183–188 (2021). https://doi.org/10.1134/S1061933X21020022
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X21020022