Abstract
Experimental data have been presented on the evaporation of water–alcohol solution droplets with different concentrations. The droplets have been suspended by a thread and applied onto a planar surface. The dynamics of variations in the geometric parameters of the evaporating water–alcohol solution droplets has been studied with the use of high-speed microphotography. Infrared thermography has been employed to confirm the three-stage variation in the surface temperature of the evaporating droplets, namely, an initial dramatic decrease in the temperature, the stage of a constant temperature, and its smooth increase up to the ambient air temperature. The experimental data have shown an essential influence of the solution concentration on the droplet evaporation process. The higher the ethanol concentration in a droplet, the closer the character of variations in the surface temperature and geometric parameters of the droplet to the variations in the same parameters of an alcohol droplet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The evaporation of water–alcohol solution droplets is of scientific interest and has a wide practical application. Thus, the majority of experimental works in this field deal with sessile droplets evaporating on substrates. At present, the effects of solution concentration [1–7], ambient conditions [8–10], and the thermal conductivity of substrates [11–14] on variations in the volumes, contact angles, and diameters of the contact spots of evaporating droplets have been studied in detail. The droplet temperature was experimentally measured with a thermocouple, and a decrease in evaporating droplet temperature relative to the ambient air temperature was recorded in [11, 12]. However, precise determination of the droplet temperature with a thermocouple is difficult, because, at contact measurements, the droplet evaporation is significantly affected by the heat supply through the thermocouple [15].
Using a noncontact method of infrared thermography [16–20], it has been shown that the surface temperature of evaporating droplets of water–alcohol solutions is nonuniform. When using infrared thermography, it is necessary to take into account the radiation properties of different liquids [21, 22]. As has been shown theoretically and experimentally in [23], the temperature of free evaporating water droplets varies in three stages. The analysis of variations in the geometric parameters of an evaporating sessile droplet of water performed by a calculation–experimental method under the assumption of the diffusion mechanism of evaporation [24] has resulted in the determination of the difference between the temperatures of the droplet and a substrate.
The analysis of the experimental studies of evaporating water–alcohol solution droplets has shown that the main attention was focused on variations in droplet geometric parameters. At the same time, the dynamics of time variations in the evaporating droplet temperature has remained to be studied.
The aim of this work was to study experimentally the changes in the geometric parameters and surface temperature of evaporating droplets of water–alcohol solutions with the use of advanced noncontact methods of measurement.
1 EXPERIMENTAL SETUP
In the experiments, the evaporation of water–alcohol solution droplets with ethanol concentrations of 0 (water), 25, 50, 75, and 92 wt % was studied at room temperature T = 24°C and air relative humidity φ = 24%. Droplets with a volume of 5 μL were formed using a single-channel dosimeter. To reduce and evaluate random errors, each experiment was repeated several times.
Two characteristic cases of droplet evaporation were considered in this work. In the first series of experiments, the evaporation of droplets suspended by a polypropylene thread 200 μm in diameter was investigated. Polypropylene (PP) has a relatively low thermal conductivity coefficient λ = 0.19 W/(m °C). Therefore, this experimental procedure enabled us to minimize the influence of the thread and to reach a good approximation to the evaporation conditions of free droplets. In the second series of the experiments, the evaporation of sessile droplets of water–alcohol solutions on a solid substrate surface was analyzed: the conditions of these experiments were described previously [25]. A Teflon plate (λ = 0.25 W/(m °C)) 3 mm thick was used as a substrate.
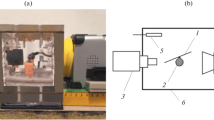
The evaporation of the droplets was examined on a special experimental stand [23]. A thermal imaging camera was placed in the vertical position over an evaporating droplet, and a digital microscope was placed horizontally on one side of a droplet. Variations in the shape of an evaporating droplet were recorded by the high-speed photography of the droplet profile with the use of the digital microscope. The noncontact recording of the temperature distribution over the droplet surface was performed with an accuracy of 0.1°C and a high space resolution using an NEC TH 7102WV thermal imaging camera equipped with a TH 71-377 microscope objective.
When determining the droplet surface temperature with the use of infrared thermography, the radiation coefficient of water was taken to be ε = 0.96 [26]. The ethanol radiation coefficient was determined in a series of experiments on the calibration of the measurement results using a chromel-alumel microthermocouple with a bead diameter of 50 μm, which provided a relatively low heat supply to the droplet. The performed measurements yielded a radiation coefficient for alcohol equal to 0.92, which agreed with the data obtained by other authors [27] for alcohol droplets with the same sizes.
2 EVAPORATION OF SUSPENDED DROPLETS
2.1 Variations in Geometric Parameters of Suspended Droplets
Micrographs taken during the evaporation of droplets of water–alcohol solution suspended by PP threads indicate that the droplets have a spherical shape throughout almost the entire the process. The data obtained on variations in the diameters of the suspended droplets of water–alcohol solutions by processing the micrographs are presented in Fig. 1. These data suggest that, in the course of evaporation, the alcohol droplet diameter decreases substantially faster than the water droplet diameter does. For droplets with different ethanol concentrations, an intermediate situation is observed: the higher the ethanol concentration in a solution droplet, the faster the decrease in its diameter.
The droplet size at which the surface tension is comparable with the gravitational force is characterized by the capillary constant of a liquid, while the Bond number is Bo ~ 1 [28]. If the diameter of the studied droplets does not exceed the capillary constant value, or Bo ⪡ 1, their shapes may be supposed to be spherical.
The data presented in Fig. 2 show that, for water and ethanol droplets, squared relative (dimensionless) droplet diameter (d/d0)2, where d0 is the initial droplet diameter, decreases linearly with evaporation time τ. For water–alcohol solution droplets, a situation intermediate between those for the alcohol and water droplets is observed. The higher the ethanol concentration in a droplet, the closer the character of time variations in parameter (d/d0)2 to that for the alcohol droplet, especially at the initial stage of evaporation. Therewith, the dependence of the squared dimensionless diameter on the evaporation time for water–alcohol droplets is nonlinear. Thus, it may be inferred that the linear dependence of the surface area of free droplets on evaporation time [29] is characteristic for only individual liquids—water and alcohol.
2.2 Variations in the Temperature of a Suspended Droplet Surface
The temperature of droplet surface was determined by infrared thermography. In the experiments performed with the use of a thermal imaging camera, thermograms of the temperature distribution over the droplet surface were recorded every 5 s. The processing of the obtained sequences of the thermograms has resulted in plotting the time dependences for the temperature of the surface of water–alcohol solution droplets suspended by a PP thread (Fig. 3).
At the initial stage of water droplet evaporation, the surface temperature decreased abruptly to 14.0°C. Then, the droplet temperature remained almost unchanged for 2700 s; after that, it sharply increased to the ambient air temperature. For alcohol droplets, the surface temperature also abruptly decreased to 9.5°C at the initial stage of evaporation. Then, the stage of the constant temperature was observed for approximately 460 s. After that, the temperature rapidly increased to the temperature of the ambient air at the final stage of the evaporation. It is seen that, under identical conditions of evaporation, the minimum temperatures of the surfaces of water and alcohol droplets are 14 and 9.5°C, respectively. The difference between the minimum surface temperatures of the evaporating water and alcohol droplets is due to the higher rate of ethanol evaporation as compared with water [6].
Under the experimental conditions, the temperatures of the adiabatic evaporation of water and alcohol were equal to 13.4 and 8.2°C, respectively. Thus, the obtained experimental data have shown that, upon the evaporation of water and alcohol droplets suspended by a PP thread, the minimum temperature values for both liquids are higher than the corresponding temperatures of the adiabatic evaporation. This is obviously caused by two main factors, of which the heat supply from the thread to the droplets is less significant because of the small diameter and low heat conductivity of the PP thread. In this case, the greatest effect results from the radiation heat exchange between a droplet and the environment [21, 22].
The analysis of the obtained results has shown that the dynamics of variations in the temperature of the suspended water–alcohol solution droplets depends essentially on ethanol concentration. For droplets with different ethanol concentrations, after a sharp decrease in the surface temperature, the stages of its smooth rise to the temperature of the adiabatic evaporation of water and the subsequent increase up the temperature of the ambient air are observed. The higher the ethanol concentration in a droplet, the more similar the variations in the droplet surface temperature to those of the alcohol droplet surface. At the initial stage of evaporation, the droplet temperature varies analogously to the temperature of the alcohol droplet; then, the character of the temperature variation for the solution droplet is similar to that observed for water droplets. This is presumably explained by the fact that, being a more volatile component, ethanol predominantly evaporates at the initial stage [6, 9, 10].
3 EVAPORATION OF SESSILE DROPLETS
3.1 Variations in Geometric Parameters of Sessile Droplets
Figure 4 shows the micrographs of water and alcohol sessile droplets on the surface of a Teflon plate at the initial stage of evaporation. The contact angles and contact spot diameters were obtained by processing the micrographs with the use of the Drop Shape Analysis software package. The initial values of the contact angle and contact spot diameter correspond to the onset of the contact between a droplet of a preset volume and the substrate. For alcohol and water droplets, the contact angles are of 30°–31.8° and 88.7°–91°, respectively. The presented micrographs show an essential difference between the shapes of the sessile water and alcohol droplets on the Teflon plate. Owing to its better wetting ability, the spreading of the alcohol droplet over the Teflon plate was stronger than the spreading of the water droplet. As a result, at the same droplet volume, the contact area and the area of evaporation surface are larger for the alcohol droplet than for the water droplet.
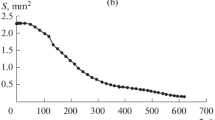
Figure 5 illustrates the results of the experimental determination of time variations in contact spot diameter d1 for sessile water–alcohol droplets evaporating on the Teflon plate with the use of a digital microscope.
The obtained results show that, for the water droplet, the contact spot diameter remains almost unchanged and the “pinning” regime is realized at the initial stage of evaporation. During the evaporation of the alcohol droplet, the contact spot diameter continuously varies; i.e., the evaporation is accompanied by the contact line movement toward the droplet center until it has completely evaporated. For droplets with different ethanol concentrations, a situation intermediate between those described above is observed. The higher ethanol concentration in a droplet, the shorter its evaporation time and the closer the character of variations in the contact spot diameter to that for the alcohol droplet. The data obtained on the variations in the geometric parameters of the droplets agree with the data of other researchers on the evaporation of sessile droplets of water–alcohol solutions [2, 3].
3.2 Variations in Surface Temperature of Sessile Droplets
The processing of the experimentally obtained sequences of thermograms has resulted in determining the time dependences for the surface temperature of sessile water–alcohol droplets evaporating on the Teflon plate (Fig. 6).
At the initial stage of evaporation, an abrupt decrease in the surface temperature of a water droplet to 21.5°C is observed. Thereafter, the droplet temperature remains almost unchanged for 700 s; then, it smoothly increases to the ambient air temperature. For an alcohol droplet, a sharp decrease in the temperature to 19.5°C, i.e., to a temperature lower than that for the evaporating water droplet, is observed at the initial stage of evaporation. After that, the stage of a constant temperature about 60 s long followed by the stage of its rapid increase to the ambient air temperature is observed.
It follows from the analysis of the data obtained that the dynamics of variations in the temperature of sessile evaporating droplets of a water–alcohol solution depends essentially on ethanol concentration. Analogously to the evaporation of the suspended droplets, the character of variations in the surface temperature for all evaporating droplets may be conventionally divided into three stages: the initial stage of an abrupt decrease in the temperature, the stage of a constant temperature, and the stage of its smooth increase to the ambient air temperature. However, in this case, the temperature values for the sessile water–alcohol solution droplets are higher than those for the suspended droplets of the corresponding concentrations. This is obviously due to the heat supply from the plate surface, which has a significant effect on the droplet evaporation, as has been noted in a number of works [11–14].
4 COMPARISON OF EVAPORATION PROCESSES OF SUSPENDED AND SESSILE DROPLETS
Let us compare the surface temperatures and evaporation times of suspended and sessile droplets.
For the comparison, Fig. 7 shows the time variations in the surface temperatures of 5-μL water (Fig. 7a) and alcohol (Fig. 7b) droplets evaporating at the same values of the temperature and humidity of the ambient air (T = 24°C, φ = 24%). The data are presented for both suspended droplets and sessile droplets on the Teflon plate surface. During evaporation, the surface temperature of the suspended water and alcohol droplets decreases to almost the adiabatic evaporation temperature. For the sessile droplets evaporating on the Teflon plate, the decrease in the surface temperature is significantly smaller. Therewith, the sessile droplets are evaporated more rapidly than the suspended ones. The ratios between the evaporation times of the suspended and sessile droplets are 1.5 and 2.4 for water and alcohol, respectively. Thus, the contact of the droplets with the surface intensifies the evaporation process.
The experimental data were used to plot the dependences of the minimum surface temperature of water–alcohol droplets on ethanol concentration (Fig. 8). The data shown in Fig. 8 suggest that the higher the concentration of the water–alcohol solution, the lower the minimum temperature of the droplet surface in the course of evaporation for both sessile and for suspended droplets. At the same time, the minimum surface temperatures of suspended droplets are lower than those of sessile droplets at the same ethanol concentrations.
The experimental data on the evaporation of water–alcohol solution droplets (Fig. 9) evidently show that the higher ethanol concentration, the shorter the evaporation time for both sessile and suspended droplets. Therewith, the suspended droplets are evaporated within a longer time period than the sessile droplets are.
5 CONCLUSIONS
The investigations that have been carried out have yielded experimental data on variations in the geometric parameters and surface temperatures of evaporating water–alcohol droplets with different ethanol concentrations. The microphotography has shown that the shape of the evaporating droplets suspended by a PP thread remains close to the spherical one almost throughout the evaporation time period. Linear dependences of the squared dimensionless diameter on the evaporation time are observed for both water and alcohol droplets. For water–alcohol droplets with different ethanol concentrations, the above dependences are nonlinear.
The results obtained by the method of infrared thermography have led to the conclusion that the dynamics of variations in the average surface temperature of the suspended water–alcohol droplets depends on ethanol concentration. During the evaporation of water and alcohol droplets, a rapid decrease in the surface temperature is followed by the stage of a constant temperature close to the temperature of adiabatic evaporation of a corresponding liquid. For droplets with different ethanol concentrations, a rapid decrease in their surface temperature is followed by the stages of a smooth rise in the temperature to the adiabatic evaporation temperature of water and the increase to the ambient air temperature. The higher the ethanol concentration in a droplet, the more similar the variations in the surface temperature of a solution droplet to those for an alcohol droplet.
The experimental data on sessile water–alcohol droplets have indicated that the shape of the evaporating droplets depends essentially on ethanol concentration. The higher ethanol concentration in a droplet, the larger is the area of the droplet contact with the surface of the Teflon plate and the higher the rate of droplet evaporation. The pattern of variations in the surface temperature of sessile water–alcohol droplets with different ethanol concentrations is characterized by three stages of evaporation similar to the stages observed for the suspended droplets. However, the minimum surface temperature of the sessile water–alcohol solution droplets is higher than that for the suspended droplets of solutions with the same concentrations. This is obviously explained by the heat supply to the droplets from the plate surface.
Moreover, the obtained data indicate that the suspended water–alcohol droplets are evaporated slower than the sessile droplets at the same thermal and humidity conditions. The evaporation time decreases for both sessile and suspended droplets with an increase in ethanol concentration. Therewith, the evaporation time is longer for the suspended droplets than that for the sessile ones at the same ethanol concentration.
REFERENCES
Chandra, S., Di Marzo, M., Qiao, Y.M., and Tartarini, P., Fire Saf. J., 1996, vol. 27, p. 141.
Sefiane, K., Tadrist, L., and Douglas, M., Int. J. Heat Mass Transfer, 2003, vol. 46, p. 4527.
Cheng, A.K.H., Soolaman, D.M., and Yu, H.Z., J. Phys. Chem. B, 2006, vol. 110, p. 11267.
Sefiane, K., David, S., and Shanahan, M.E.R., J. Phys. Chem. B, 2008, vol. 112, p. 11317.
Shi, L., Shen, P., Zhang, D., Lin, Q., and Jiang, Q., Surf. Interface Anal., 2009, vol. 41, p. 951.
Saverchenko, V.I., Fisenko, S.P., and Khodyko, Yu.A., Colloid J., 2015, vol. 77, p. 71.
Kuznetsov, G.V., Feoktistov, D.V., and Orlova, E.G., Teplofiz. Aeromekh., 2016, vol. 23, p. 17.
Kuchma, A.E., Esipova, N.E., Mikheev, A.A., Shchekin, A.K., and Itskov, S.V., Colloid J., 2017, vol. 79, p. 779.
Liu, C., Bonaccurso, E., and Butt, H.J., Phys. Chem. Chem. Phys., 2008, vol. 10, p. 7150.
Oztürk, T. and Erbil, H.Y., Colloids Surf. A, 2018, vol. 553, p. 327.
David, S., Sefiane, K., and Tadrist, L., Colloids Surf. A, 2007, vol. 298, p. 108.
Dunn, G.J., Wilson, S.K., Duffy, B.R., David, S., and Sefiane, K., J. Fluid Mech., 2009, vol. 623, p. 329.
Bazargan, V. and Stoeber, B., Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2016, vol. 94, p. 033103.
Borodulin, V.Y., Letushko, V.N., Nizovtsev, M.I., and Sterlyagov, A.N., MATEC Web of Conferences, 2017, vol. 115, p. 08005.
Han, K., Song, G., Ma, X., and Yang, B., Appl. Therm. Eng., 2016, vol. 101, p. 568.
Terekhov, V.I. and Shishkin, N.E., Polzunovsk. Vestn., 2010, no. 1, p. 55.
Sefiane, K., Moffat, J.R., Matar, O.K., and Craster, R.V., Appl. Phys. Lett., 2008, vol. 93, p. 074103.
Hamamoto, Y., Christy, J.R.E., and Sefiane, K., J. Therm. Sci. Technol., 2012, vol. 7, p. 425.
Nakoryakov, V.E., Misyura, S.Y., and Elistratov, L., J. Eng. Thermophys., 2013, vol. 22, p. 1.
Bochkareva, E.M., Terekhov, V.V., Nazarov, A.D., and Miskiv, N.B., J. Phys.: Conf. Ser., 2017, vol. 891, p. 012010.
Brutin, D., Sobac, B., Rigollet, F., and Le Niliot, C., Exp. Therm. Fluid Sci., 2011, vol. 35, p. 521.
Fedorets, A.A., Dombrovsky, L.A., and Medvedev, D.N., JETP Lett., 2015, vol. 102, p. 452.
Borodulin, V.Y., Letushko, V.N., Nizovtsev, M.I., and Sterlyagov, A.N., Int. J. Heat Mass Transfer, 2017, vol. 109, p. 609.
Kuchma, A.E., Shchekin, A.K., Esipova, N.E., Tat’yanenko, D.V., Itskov, S.V., and Savin, A.V., Colloid J., 2017, vol. 79, p. 353.
Sterlyagov, A.N., Letushko, V.N., Nizovtsev, M.I., and Borodulin, V.Yu., J. Phys.: Conf. Ser., 2018, vol. 1105, p. 012068.
Zolotarev, V.I. and Demin, V.A., Opt. Spektrosk., 1977, vol. 43, p. 271.
Brutin, D., Zhu, Z.Q., Rahli, O., Xie, J.C., Liu, Q.S., and Tadrist, L., Microgravity Sci. Technol., 2010, vol. 22, p. 387.
Lebedev-Stepanov, P.V., Vvedenie v samosborku ansamblei nanochastits (An Introduction to Self-Assembly of Nanoparticle Ensembles), Moscow: NIYaU MIFI, 2012.
Fuks, N.A., Rost i isparenie kapel’ v gazoobraznoi srede (Droplet Growth and Evaporation in Gaseous Medium), Moscow: Akad. Nauk SSSR, 1958.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Tkachenko
Rights and permissions
About this article
Cite this article
Borodulin, V.Y., Letushko, V.N., Nizovtsev, M.I. et al. The Experimental Study of Evaporation of Water–Alcohol Solution Droplets. Colloid J 81, 219–225 (2019). https://doi.org/10.1134/S1061933X19030049
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X19030049